근육수축의 기전 기초과학
http://www.blobs.org/science/article.php?article=38
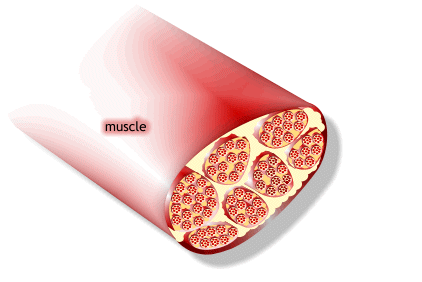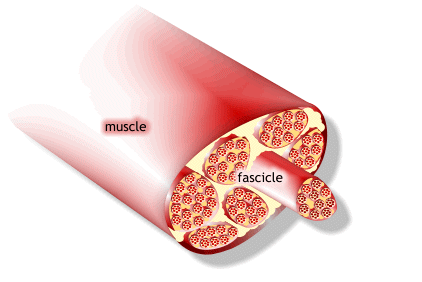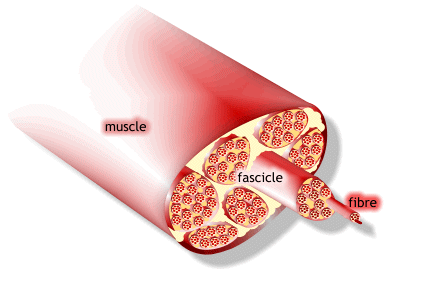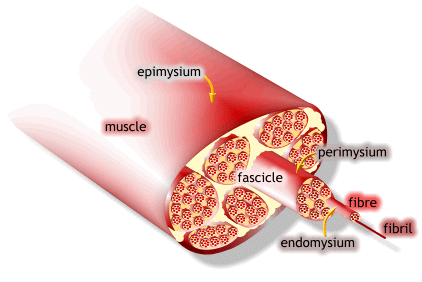
1) 근수축 최소단위는 muscle fiber
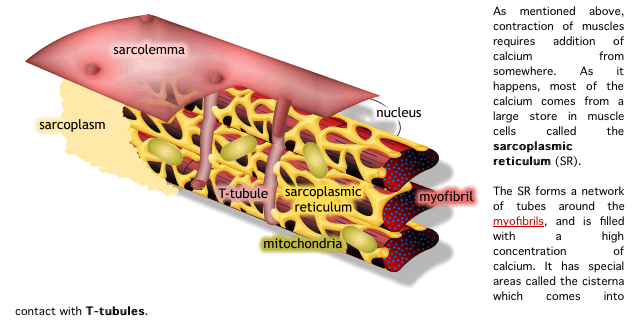
2) muscle fiber는 endomysium으로 둘러싸여 있고, 안쪽면에는 절연체(활동전위를 전달하는 단위)역할을 수행하는 근초(sarcolemma)가 있음
3) 척수의 알파 운동신경원-운동신경-운동종판을 잇는 운동단위(motor unit)가 있음.
4) 큰 움직임은 큰 운동단위, 작은 움직임은 작은 운동단위가 패턴을 가지고 동원함.
5) 감마 운동신경원은 길이, 장력을 구심전달하면서 감마 neuromuscular junction을 가지고 미세조절하는 근수축을 함.
6) .......
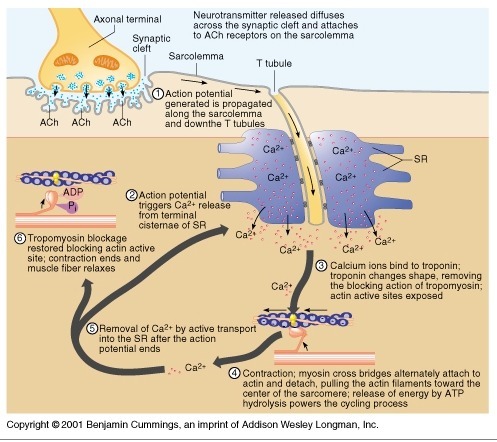
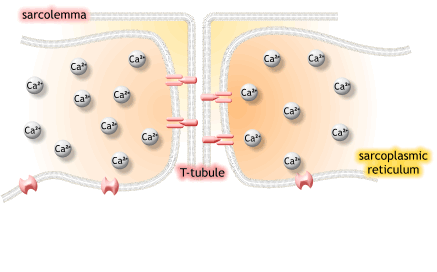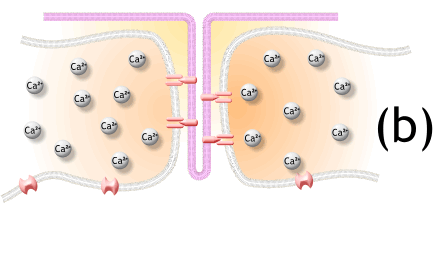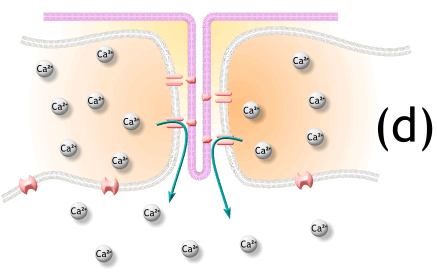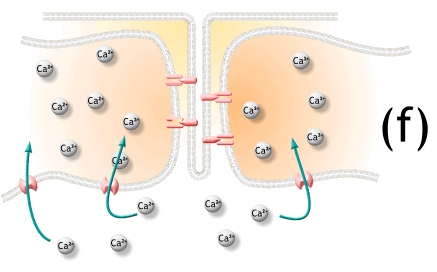
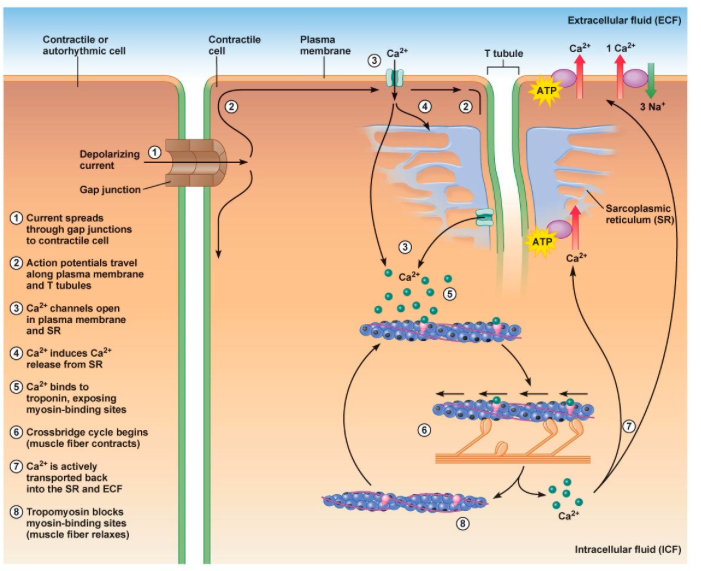
1) 생성된 활동전위가 근초와 T 튜블로 들어감.
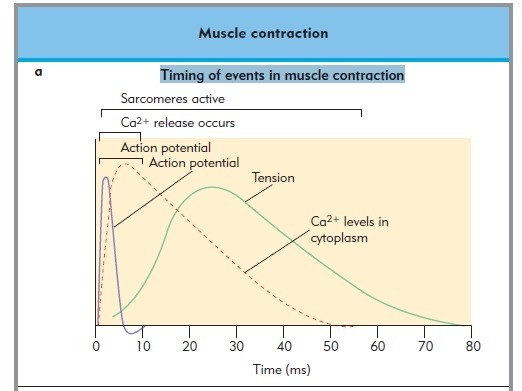
2) 활동전위는 SR의 terminal cisternae로부터 칼슘을 분비하도록 자극함
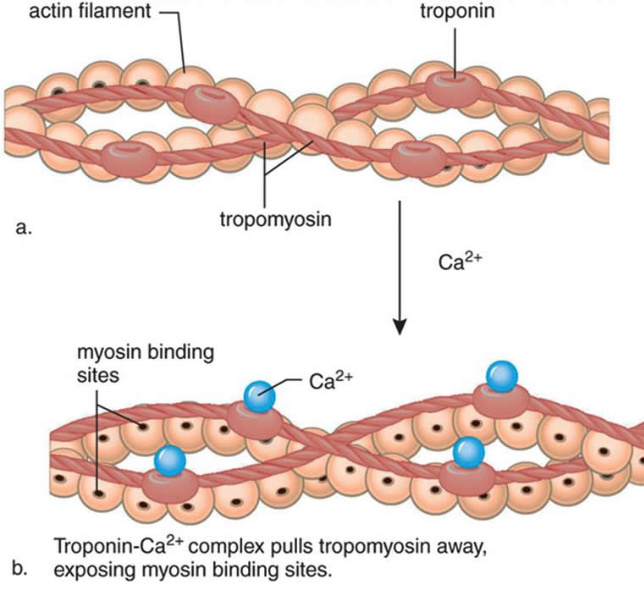
3) 칼슘이온이 트로포닌에 부착함. 트로포닌은 형태를 바꾸고 트로포마이오신의 액틴차단을 제거하고 액틴 활성위치가 열림
4) 근수축 : 마이오신이 액틴에 교차다리형태로 부착하여 액틴필라멘트를 끌어당김.
5) 활동전위가 끝나고 SR로 능동수동에 의해서 칼슘이 제거됨.
6) 트로포마이오신 블록은 액틴활성부위 차단을 회복하면 근수축이 끝나고 근육이 이완됨.
Molecular mechanisms of skeletal muscular function
Skeletal muscles contract according to the sliding filament model see also Excitation-contraction coupling
1. An action potential originating in the CNS reaches an alpha motor neuron, which then transmits an action potential down its own axon.
활동전위는 중추신경의 알파운동신경원에서 기원하여 운동신경의 축삭으로 활동전위가 전달됨.
2. The action potential propagates by activating voltage-gated sodium channels along the axon toward the neuromuscular junction. When it reaches the junction, it causes a calcium ion influx through voltage-gated calcium channels.
활동전위가 활성화된 전압-문 소듐채널(voltage-gated sodium channels)에 의해서 축삭을 따라 신경근접합으로 전달됨. 활동전위가 신경근접합에 전달되면 칼슘이온이 전압-문 칼슘채널을 따라서 유입됨.
3. The Ca2+ influx causes vesicles containing the neurotransmitter acetylcholine to fuse with the plasma membrane, releasing acetylcholine out into the extracellular space between the motor neuron terminal and the neuromuscular junction of the skeletal muscle fiber.
칼슘 유입은 아세틸콜린을 함유한 소포체가 세포막에 부착을 일으키고, 아세틸콜린은 골격근섬유의 세포외 공간인 신경말단과 신경근접합부에 분비됨.
4. The acetylcholine diffuses across the synapse and binds to and activates nicotinic acetylcholine receptors on the neuromuscular junction. Activation of the nicotinic receptor opens its intrinsic sodium/potassium channel, causing sodium to rush in and potassium to trickle out. Because the channel is more permeable to sodium, the charge difference between internal and external surfaces of the muscle fiber membrane becomes less negative, triggering an action potential.
아세틸콜린이 시냅스를 따라서 방사되고, 신경근접합부의 니코틴 아세틸콜린 수용체를 활성화함. 니코틴 수용체의 활성은 내부의 소듐/포타슘채널을 열고, 소듐이 들어가고, 포타슘이 새어나옴. 이 채널은 소듐에 좀더 투과성이 좋기 때문에 근섬유막의 내부와 외부의 전하차이가 좀더 음전하가 되고 활동전위를 일으킴.
5. The action potential spreads through the muscle fiber's network of T-tubules, depolarizing the inner portion of the muscle fiber.
활동전위는 근섬유의 t 튜불의 연결체로 퍼지고, 근섬유의 내부는 depolarizing됨.
6. The depolarization activates L-type voltage-dependent calcium channels (dihydropyridine receptors) in the T tubule membrane, which are in close proximity to calcium-release channels (ryanodine receptors) in the adjacent sarcoplasmic reticulum.
탈분극은 t튜불 내의 L-type voltage-dependent calcium channels을 활성화하고, 근처 SR에서 칼슘 분비채널을 닫음.
7. Activated voltage-gated calcium channels physically interact with calcium-release channels to activate them, causing the sarcoplasmic reticulum to release calcium.
활성화된 voltage-gated 칼슘채널은 물리적으로 칼슘분비채널과 상호작용하여 활성화되고, SR에서 칼슘이 분비됨.
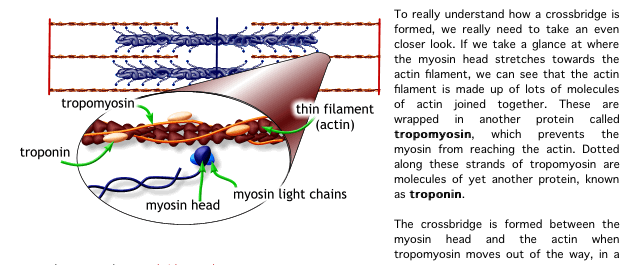
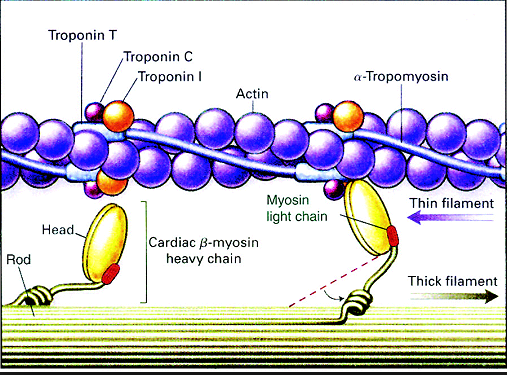
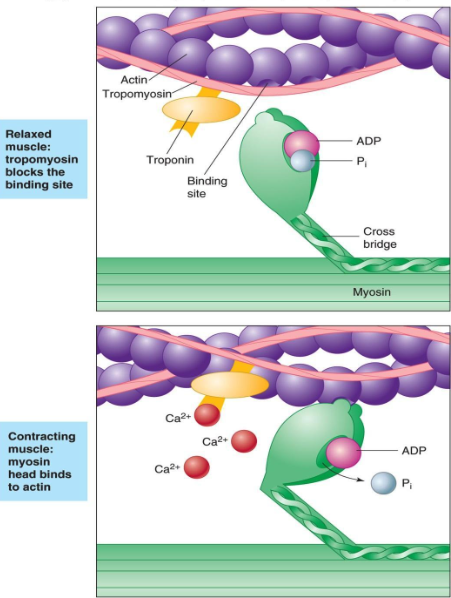
8. The calcium binds to the troponin C present on the actin-containing thin filaments of the myofibrils. The troponin then allosterically modulates the tropomyosin. Under normal circumstances, the tropomyosin sterically obstructs binding sites for myosin on the thin filament; once calcium binds to the troponin C and causes an allosteric change in the troponin protein, troponin T allows tropomyosin to move, unblocking the binding sites.
칼슘은 근원섬유의 액틴필라멘트에 있는 트로포닌 C에 부착함. 트로포닌은 알로스테릭하게 트로포마이오신을 조절함.
정상상황에서 트로포마이오신은 입체적으로 액틴위의 마이오신을 위한 부착부위를 막고 있음. 칼슘이 트로포닌 c에 부착하고 트로포닌 단백질에서 알로스테릭한 변화를 초래하면 트로포닌 t는 트로포마이오신이 움직이도록 허용하기 위해 부착부위를 열어줌.
9. Myosin (which has ADP and inorganic phosphate bound to its nucleotide binding pocket and is in a ready state) binds to the newly uncovered binding sites on the thin filament (binding to the thin filament is very tightly coupled to the release of inorganic phosphate). Myosin is now bound to actin in the strong binding state. The release of ADP and inorganic phosphate are tightly coupled to the power stroke (actin acts as acofactor in the release of inorganic phosphate, expediting the release). This will pull the Z-bands towards each other, thus shortening the sarcomere and the I-band.
마이오신은 액틴필라멘트위의 부착부위에 부착함. 마이오신은 강한 부착부위에서 액틴과 강하게 부착함. ADP 분비와
10. ATP binds to myosin, allowing it to release actin and be in the weak binding state (a lack of ATP makes this step impossible, resulting in the rigor state characteristic of rigor mortis). The myosin then hydrolyzes the ATP and uses the energy to move into the "cocked back" conformation. In general, evidence (predicted and in vivo) indicates that each skeletal muscle myosin head moves 10–12 nm each power stroke, however there is also evidence (in vitro) of variations (smaller and larger) that appear specific to the myosin isoform.
11. Steps 9 and 10 repeat as long as ATP is available and calcium is freely bound within the thin filaments.
12. While the above steps are occurring, calcium is actively pumped back into the sarcoplasmic reticulum. When calcium is no longer present on the thin filament, the tropomyosin changes conformation back to its previous state so as to block the binding sites again. The myosin ceases binding to the thin filament, and the contractions cease.
The calcium ions leave the troponin molecule in order to maintain the calcium ion concentration in the sarcoplasm. The active pumping of calcium ions into the sarcoplasmic reticulum creates a deficiency in the fluid around the myofibrils. This causes the removal of calcium ions from the troponin. Thus, the tropomyosin-troponin complex again covers the binding sites on the actin filaments and contraction ceases.
근수축에 관한 교과서
11.4 Behavior of Skeletal Muscle Fibers
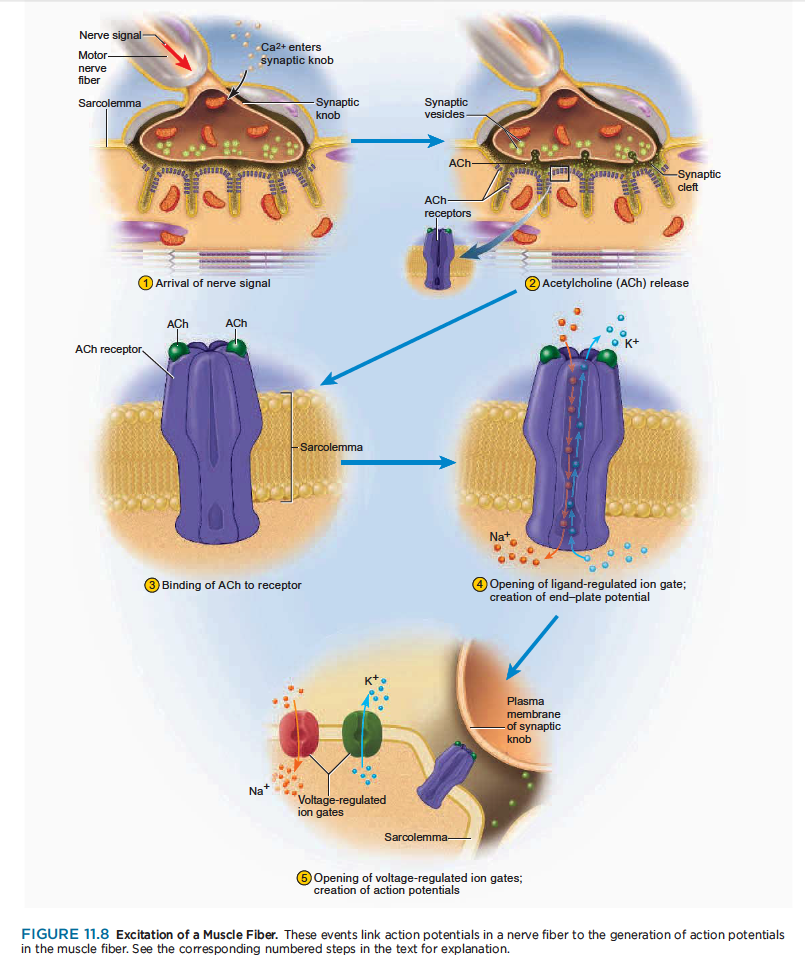
The process of muscle contraction and relaxation has four major phases: (1) excitation, (2) excitation–contraction coupling, (3) contraction, and (4) relaxation. Each phase occurs in several smaller steps, which we now examine in detail. The steps are numbered in the following descriptions to correspond to those in figures 11.8 to 11.11.
근육의 수축과 이완은 크게 네가지 단계
(1) excitation, (2) excitation–contraction coupling, (3) contraction, and (4) relaxation.
1) 흥분
2) 흥분-수축 동조
3) 수축
4) 이완
Excitation(흥분)
Excitation is the process in which action potentials in the nerve fiber lead to action potentials in the muscle fiber. The steps in excitation are shown in figure 11.8.
흥분과정은 신경섬유에서 발생한 활동전위가 근섬유로 전달되는 과정.
1 A nerve signal arrives at the synaptic knob and stimulates voltage-regulated calcium gates to open.
Calcium ions enter the synaptic knob.
신경신호는 시냅스 꼭지에 도달하여 voltage-조절칼슘 문을 자극하여 열어줌.
2 Calcium stimulates exocytosis of the synaptic vesicles, which release acetylcholine (ACh) into the
synaptic cleft. One action potential causes exocytosis of about 60 synaptic vesicles, and each vesicle
releases about 10,000 molecules of ACh.
칼슘은 시냅스 소포체의 방출을 자극하여 시냅스 간극에 아세틸콜린 분비. 하나의 활동전위는 60 시냅스소포체 방출을 야기하고, 각각 소포체는 아세틸콜린 1만개를 분비함.
3 ACh diffuses across the synaptic cleft and binds to receptor proteins on the sarcolemma.
아세틸콜린은 시냅스 간극을 따라 분비되어 근초위의 수용기 단백질에 부착함.
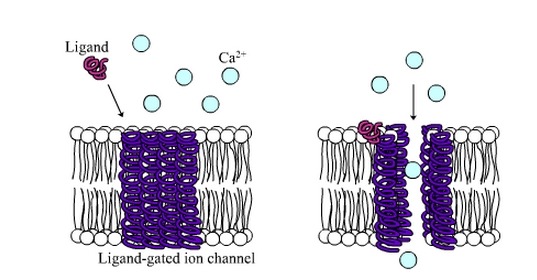
4 These receptors are ligand-regulated ion gates. Two ACh molecules must bind to each receptor to open
the gate. When this happens, Na diffuses quickly into the cell and K diffuses out. As a result of these
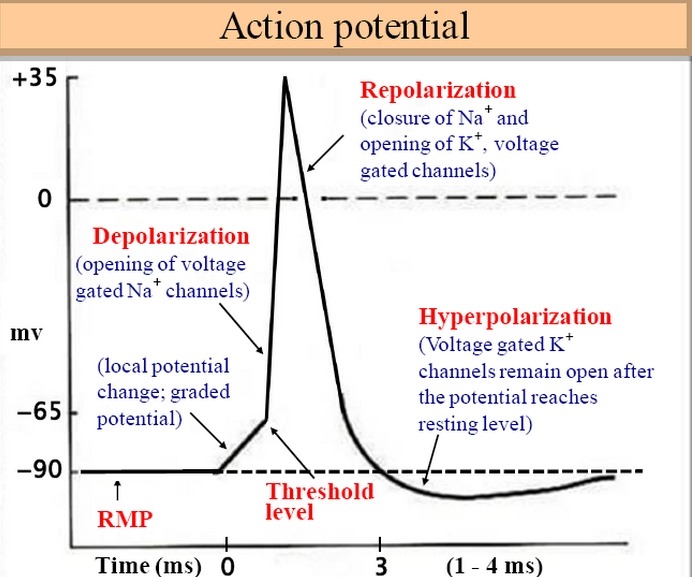
ion movements, the sarcolemma reverses polarity—its voltage quickly jumps from the RMP of 90 mV to
a peak of 75 mV as Na enters, and then falls back to a level close to the RMP as K diffuses out. This
rapid fluctuation in membrane voltage at the motor end plate is called the end-plate potential (EPP).
이 수용기는 ligand-regulated ion gate임. 두개 아세틸콜린 분자는 각각의 수용체에 부착해야만 세모막 문을 열수 있음. 그림과 같이 두개의 아세틸콜린 분자가 수용체에 부착하면 Na는 세포내로 들어가고, K는 밖으로 나감. 이러한 이온의 움직임 결과로 근초는 극성이 바뀌고.....
이러한 운동종판에서 세포막 전압의 빠른 변화는 운동종판 potential(EPP)라고 함.
참고) 단백질에 특이적으로 결합하는 물질. 효소에 결합하는 기질·보효소. 각종 리셉터와 결합하는 호르몬, 신경전달 물질 등은 모두 리간드
5 Areas of sarcolemma next to the motor end plate have voltage-regulated ion gates that open in
response to the EPP. Some of these are specific for Na and admit it to the cell, while others are specific
for K and allow it to leave. These ion movements create an action potential. The muscle fiber is now excited.
근초부위 운동종판은 volgate-regulated ion gates이고 EPP에 반응하여 열림. Na와 K의 위치가 바뀌고.. 이러한 이온움직임은 활동전위를 일으키고, 근섬유가 이제 흥분함.
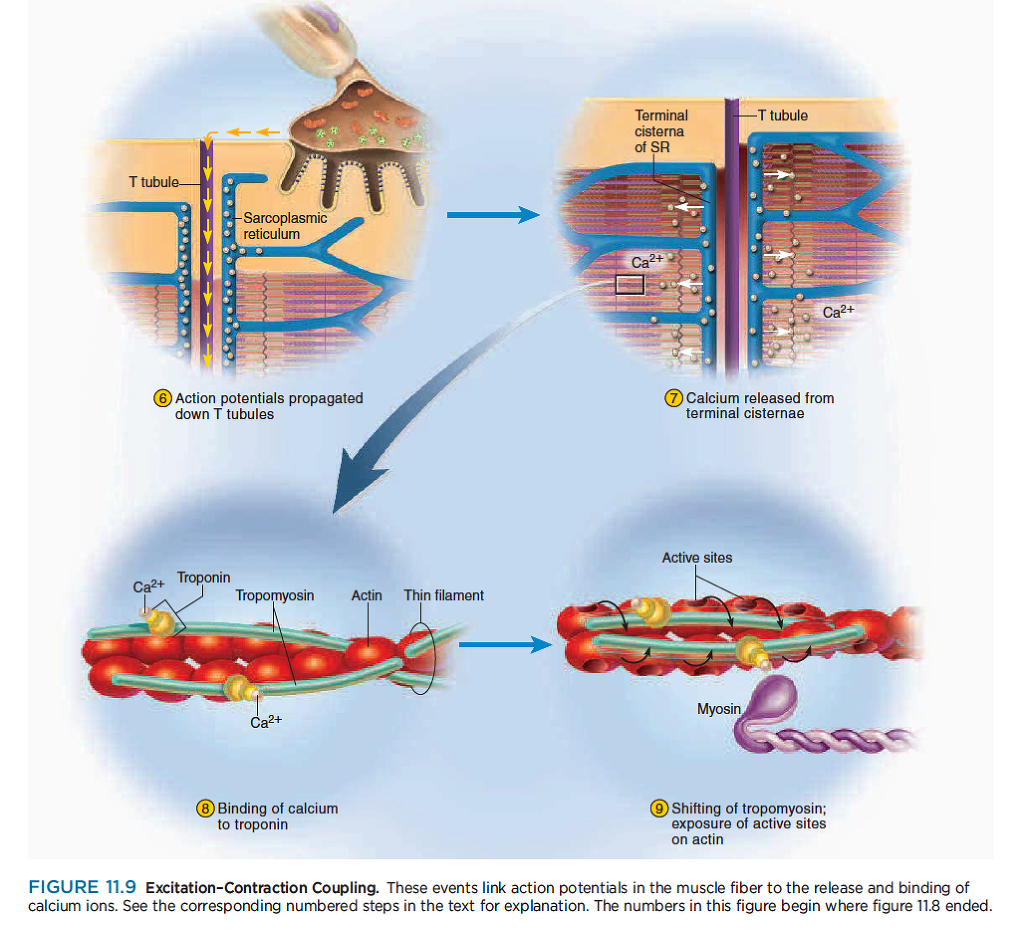
Excitation–Contraction Coupling
Excitation–contraction coupling refers to the events that link the action potentials on the sarcolemma to activation of the myofilaments, thereby preparing them to contract. The steps in the coupling process are shown in figure 11.9
흥분-수축 연결
흥분-수축 연결은 근초의 활동전위가 근원섬유의 활성과 연결되어 수축을 준비함.
6 A wave of action potentials spreads from the end plate in all directions, like ripples on a pond. When
this wave of excitation reaches the T tubules, it continues down them into the sarcoplasm.
운동종판으로부터 활동전위가 호수에 물결을 일으키는 것과 같은 파형을 만듬. 이 흥분 파형이 T 튜불에 도착할때, 근형질내로 이어짐....
7 Action potentials open voltage-regulated ion gates in the T tubules. These are physically linked to calcium
channels in the terminal cisternae of the sarcoplasmic reticulum (SR). Thus, gates in the SR open as
well and calcium diffuses out of the SR, down its concentration gradient and into the cytosol.
활동전위는 t 튜불내의 voltage-regulated ion gate를 염. 활동전위는 SR의 말단에서 물리적으로 칼슘채널과 연결됨. 그래서 SR에서 문이 열리고 칼슘이 Cytosol내로 빠져나가 cytosol내의 칼슘기울기가 변화함.
8 Calcium binds to the troponin of the thin filaments.
칼슘은 액틴 필라멘트의 트로포닌과 부착함.
9 The troponin–tropomyosin complex changes shape and sinks deeper into the groove of the thin filament.
This exposes the active sites on the actin filaments and makes them available for binding to myosin heads.
트로포닌-트로포마이오신 복합체는 액틴 필라멘트 구에 부착하도록 형태를 바꿈. 이것은 액틴 필라멘트의 활성화 부위를 마이오신 헤드의 부착부에 붙도록함.
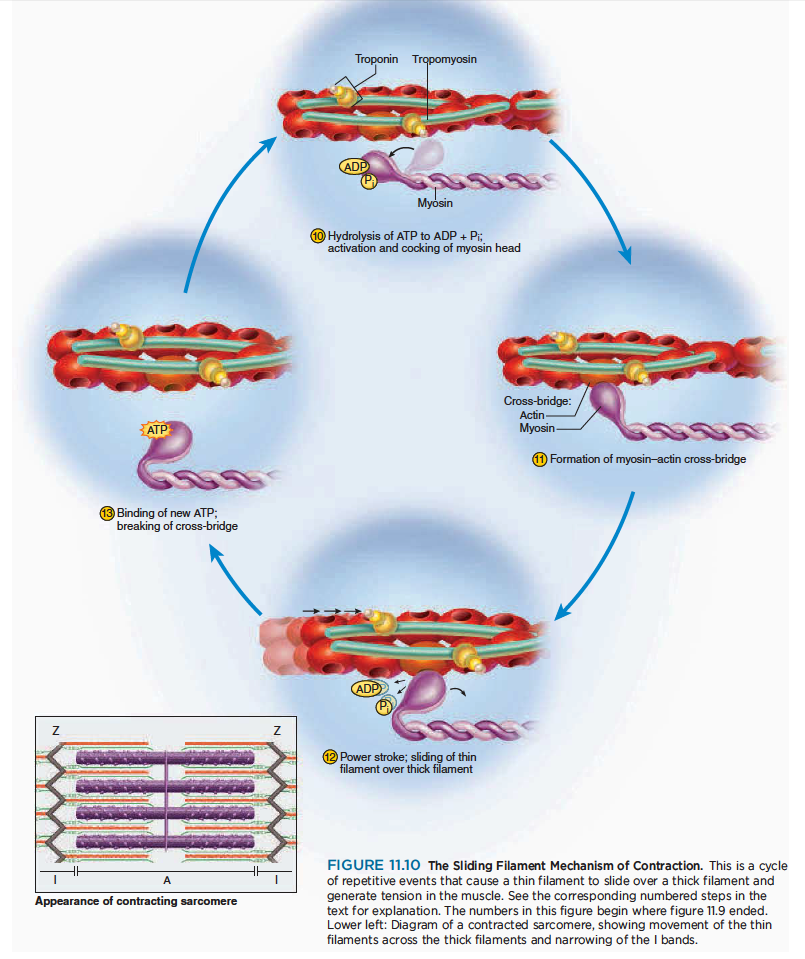
Contraction
Contraction is the step in which the muscle fiber develops tension and may shorten. (Muscles often “contract,” or develop tension, without shortening, as we see later.)
근수축은 근섬유가 장력이 발생하고 짧아지는 수축이 일어나는 과정
How a fiber shortens remained a mystery until sophisticated techniques in electron microscopy enabled cytologists to see the molecular organization of muscle fibers. In 1954, two researchers at the Massachusetts Institute of Technology—Jean Hanson and Hugh Huxley—reported convincing evidence for a model now called the sliding filament theory. This theory holds that the myofilaments do not become any shorter during contraction; rather, the thin filaments slide over the thick ones and pull the Z discs behind them, causing each sarcomere as a whole to shorten. The individual steps in this mechanism are shown in figure 11.10.
하지만 근섬유가 짧아지는 것은 여전히 미스테리로 남아있음. 존 핸슨과 헉슬리의 근활주가설이 유력한 근수축 기전.
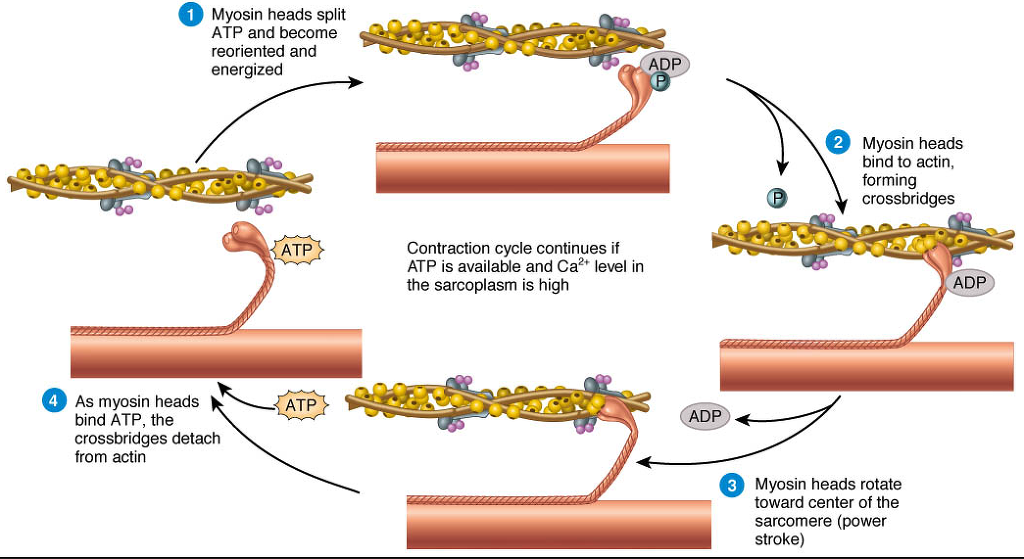
10 The myosin head must have an ATP molecule bound to it to initiate the contraction process. Myosin
ATPase, an enzyme in the head, hydrolyzes this ATP. The energy released by this process activates
the head, which “cocks” into an extended, high energy position. The head temporarily keeps the
ADP and phosphate group bound to it.
마이오신 헤드는 ATP분자를 가지고 있어 수축과정을 시작함. 마이오신 ATPase는 ATP를 가수분해함. 이과정에 의해서 만들어지는 에너지는 마이오신 헤드를 활성화하여 근육을 신장함.
11 The cocked myosin binds to an exposed active site on the thin filament, forming a cross-bridge between
the myosin and actin.
위로 젖혀진 마이오신은 액틴 필라멘트의 노출된 부위에 부착하고 마이오신과 액틴사이에 교차다리가 만들어짐.
12 Myosin releases the ADP and phosphate and flexes into a bent, low-energy position, tugging the thin
filament along with it. This is called the power stroke. The head remains bound to actin until it binds a new ATP.
마이오신은 ADP와 인을 분비하여 구부림. ... 파워 스트로크.. 마이오신 헤드는 새로운 ATP가 부착할때까지 부착함.
13 Upon binding more ATP, myosin releases the actin. It is now prepared to repeat the whole process—it
will hydrolyze the ATP, recock (the recovery stroke), attach to a new active site farther down the thin
filament, and produce another power stroke. It may seem as if releasing the thin filament at step would simply allow it to slide back to its previous position, so that nothing would have been accomplished. Think of the sliding filament mechanism, however, as being similar to the way you would pull in a boat anchor hand over hand. When the myosin head cocks, it is like your hand reaching out to grasp the anchor rope.
좀더 많은 ATP에 부착하면, 마이오신은 액틴과 떨어짐. 그것은 새로운 파워 스트로크를 만드는 준비임.
When it flexes back into the low-energy position, it is like your elbow flexing to pull on the rope and draw the anchor up a little bit. When you let go of the rope with one hand, you hold onto it with the other, alternating hands until the anchor is pulled in. Similarly, when one myosin head releases the actin in preparation for the recovery stroke, there are many other heads on the same thick filament holding onto the thin filament so that it doesn’t slide back.
At any given moment during contraction, about half of the heads are bound to the thin filament and the other half are extending forward to grasp the filament farther down. That is, the myosin heads of a thick filament do not all stroke at once but contract sequentially.
Each myosin head acts in a jerky manner, but hundreds of them working together produce a smooth, steady pull on the thin filament. This is similar to the locomotion of a millipede—a wormlike animal with a few hundred tiny legs. Each leg takes individual jerky steps, but all the legs working together produce a smooth gliding movement. Note that even though the muscle fiber contracts, the myofilaments do not become shorter any more than a rope becomes shorter as you pull in an anchor. The thin filaments slide over the thick ones, as the name of the theory implies.
A single cycle of power and recovery strokes by all the myosin heads in a muscle fiber would shorten the fiber by about 1%. A fiber, however, may shorten by as much as 40% of its resting length, so obviously the cycle of power and recovery must be repeated many times by each myosin head. Each head carries out about five strokes per second, and each stroke consumes one molecule of ATP.
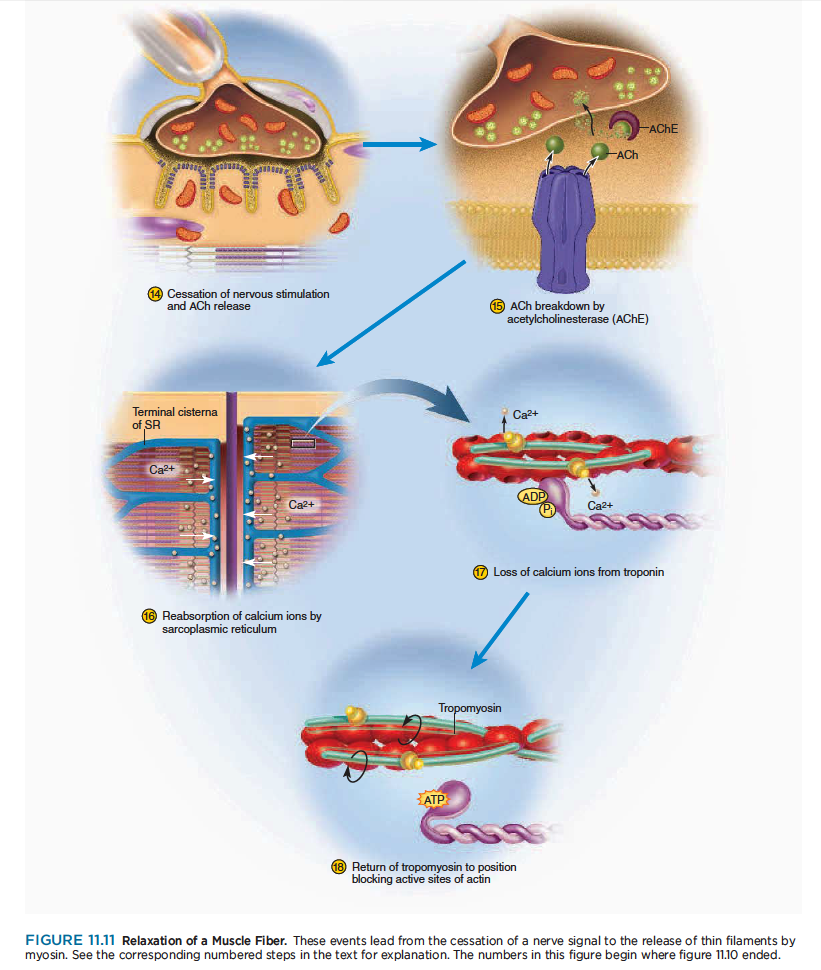
Relaxation
When its work is done, a muscle fiber relaxes and returns to its resting length. This is achieved by the steps shown in figure 11.11.
일이 끝나고 나면, 근섬유는 이완하고 안정길이로 되돌아감.
14 Nerve signals stop arriving at the neuromuscular junction, so the synaptic knob stops releasing ACh.
신경신호가 신경근접합부에서 멈추면 시냅스 꼭지에서 아세틸콜린 분비가 멈춤.
15 As ACh dissociates (separates) from its receptor, AChE breaks it down into fragments that cannot
stimulate the muscle. The synaptic knob reabsorbs these fragments for recycling. All of this happens
continually while the muscle is being stimulated, too, but when nerve signals stop, no new ACh is
released to replace that which is broken down. Therefore, stimulation of the muscle fiber by ACh ceases.
아세틸콜린이 수용체에서 떨어지고, 근육자극은 멈춤. 시냅스 꼭지는 아세틸콜린을 재흡수하여 원래자리로 되돌아감. 이 과정은 근육이 자극을 받는 동안 연속적으로 일어남. 하지만 신경신호가 멈출때, 새로운 아세틸콜린은 분비되지 않음. 그래서 아세틸콜린에 의해서 근섬유자극은 끝남.
16 Active transport pumps in the SR begin to pump Ca2 from the cytosol back into the cisternae. Here,
the calcium binds to a protein called calsequestrin (CAL-see-QUES-trin) and is stored until the fiber is
stimulated again. Since active transport requires ATP, you can see that ATP is needed for muscle relaxation as well as for muscle contraction (see Insight 11.2).
SR에서 능동수송 펌프는 칼슘을 cytosol로부터 되돌려 cisternae로 저장함. 단백질과 부착한 칼슘은 calsequestrin이라하고 근섬유가 다시 자극받을때가지 저장됨.
17 As calcium ions dissociate from troponin, they are pumped into the SR and are not replaced.
18 Tropomyosin moves back into the position where it blocks the active sites of the actin filament. Myosin
can no longer bind to actin, and the muscle fiber ceases to produce or maintain tension. A muscle returns to its resting length with the aid of two forces: (1) like a recoiling rubber band, its intracellular and perhaps extracellular elastic components stretch it; and (2) since muscles often occur in antagonistic pairs, the contraction of an antagonist lengthens the relaxed muscle. Contraction of the triceps brachii, for example,
extends the elbow and lengthens the biceps brachii.
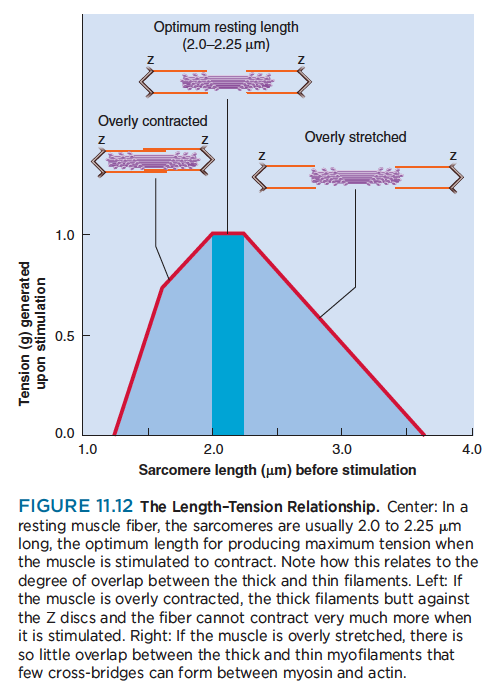
The Length–Tension Relationship and Muscle Tone
The amount of tension generated by a muscle, and therefore the force of its contraction, depends on how
stretched or contracted it was before it was stimulated, among other factors. This principle is called the length–tension relationship. The reasons for it can be seen in figure 11.12. If a fiber is overly contracted at rest, its thick filaments are rather close to the Z discs. The stimulated muscle may contract a little, but then the thick filaments butt against the Z discs and can go no farther. The contraction is therefore a weak one.
On the other hand, if a muscle fiber is too stretched before it is stimulated, there is relatively little overlap between its thick and thin filaments. When the muscle is stimulated, the myosin heads cannot “get a good grip” on the thin filaments, and again the contraction is weak. (As mentioned in chapter 10, this is one reason you should not bend at the waist to pick up a heavy object. Muscles of the back become overly
stretched and cannot contract effectively to straighten your spine against a heavy resistance.)
Between these extremes, there is an optimum resting length at which a muscle responds with the greatest force. The central nervous system continually monitors and adjusts the length of the resting muscles, maintaining a state of partial contraction called muscle tone. This maintains optimum length and makes the muscles ideally ready for action. The elastic filaments of the sarcomere also help to maintain enough myofilament overlap to ensure an effective contraction when the muscle is called into action.