![]() Tissue Stretch Induces Nuclear Remodeling in Connective Tis.pdf
Tissue Stretch Induces Nuclear Remodeling in Connective Tis.pdf
Tissue Stretch Induces Nuclear Remodeling in Connective Tissue Fibroblasts
- 조직 스트레치(늘림)은 결합조직 섬유아세포에서 핵재생을 유도함.
Abstract
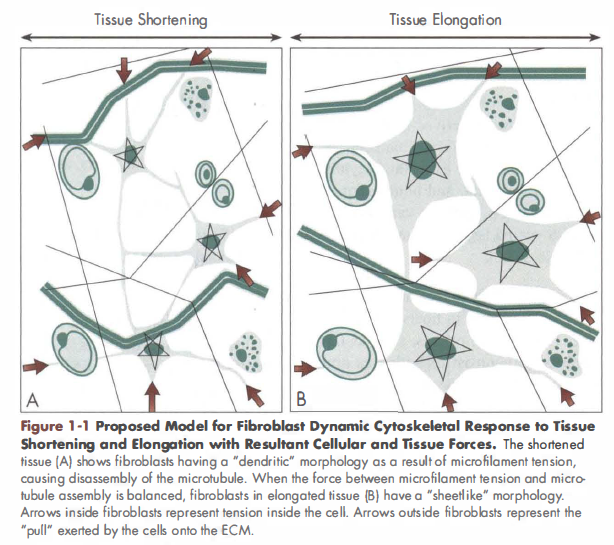
Studies in cultured cells have shown that nuclear shape is an important factor influencing nuclear function, and that mechanical forces applied to the cell can directly affect nuclear shape. In a previous study, we demonstrated that stretching of whole mouse subcutaneous tissue causes dynamic cytoskeletal remodeling with perinuclear redistribution of α-actin in fibroblasts within the tissue. We have further shown that the nuclei of these fibroblasts have deep invaginations containing α-actin. In the current study, we hypothesized that tissue stretch would cause nuclear remodeling with a reduced amount of nuclear invagination, measurable as a change in nuclear concavity. Subcutaneous areolar connective tissue samples were excised from 28 mice and randomized to either tissue stretch or no stretch for 30 min, then examined with histochemistry and confocal microscopy. In stretched tissue (vs. non-stretched), fibroblast nuclei had a larger cross-sectional area (P < 0.001), smaller thickness (P < 0.03) in the plane of the tissue, and smaller relative concavity (P < 0.005) indicating an increase in nuclear convexity.
The stretch-induced loss of invaginations may have important influences on gene expression, RNA trafficking and/or cell differentiation.
Alpha-Smooth Muscle Actin Expression Upregulates Fibroblast Contractile Activity
ABSTRACT
To evaluate whether α-smooth muscle actin (α-SMA) plays a role in fibroblast contractility, we first compared the contractile activity of rat subcutaneous fibroblasts (SCFs), expressing low levels of α-SMA, with that of lung fibroblasts (LFs), expressing high levels of α-SMA, with the use of silicone substrates of different stiffness degrees. On medium stiffness substrates the percentage of cells producing wrinkles was similar to that of α-SMA–positive cells in each fibroblast population. On high stiffness substrates, wrinkle production was limited to a subpopulation of LFs very positive for α-SMA. In a second approach, we measured the isotonic contraction of SCF- and LF-populated attached collagen lattices. SCFs exhibited 41% diameter reduction compared with 63% by LFs. TGFβ1 increased α-SMA expression and lattice contraction by SCFs to the levels of LFs; TGFβ-antagonizing agents reduced α-SMA expression and lattice contraction by LFs to the level of SCFs. Finally, 3T3 fibroblasts transiently or permanently transfected with α-SMA cDNA exhibited a significantly higher lattice contraction compared with wild-type 3T3 fibroblasts or to fibroblasts transfected with α-cardiac and β- or γ-cytoplasmic actin. This took place in the absence of any change in smooth muscle or nonmuscle myosin heavy-chain expression. Our results indicate that an increased α-SMA expression is sufficient to enhance fibroblast contractile activity.
댓글
댓글 리스트-
작성자문형철 작성자 본인 여부 작성자 작성시간 14.12.11 Tissue Stretch Induces Nuclear Remodeling in Connective Tissue Fibroblasts
-
작성자문형철 작성자 본인 여부 작성자 작성시간 16.11.13 섬유아세포에는 알파 actin이 있다.
. In a previous study, we demonstrated that stretching of whole mouse subcutaneous tissue causes dynamic cytoskeletal remodeling with perinuclear redistribution of α-actin in fibroblasts within the tissue. We have further shown that the nuclei of these fibroblasts have deep invaginations containing α-actin. -
작성자문형철 작성자 본인 여부 작성자 작성시간 16.11.13 To eval‎uate whether α-smooth muscle actin (α-SMA) plays a role in fibroblast contractility, we first compared the contractile activity of rat subcutaneous fibroblasts (SCFs), expressing low levels of α-SMA, with that of lung fibroblasts (LFs), expressing high levels of α-SMA, with the use of silicone substrates of different stiffness degrees.