Re:Feeding mitochondria: Potential role of nutritional components to Q12 improve critical illness convalescence
작성자문형철작성시간20.05.28조회수532 목록 댓글 0beyond reason
마음을 품고, 의도를 하고 찾으면 짠하고 나타나는 논문들!!
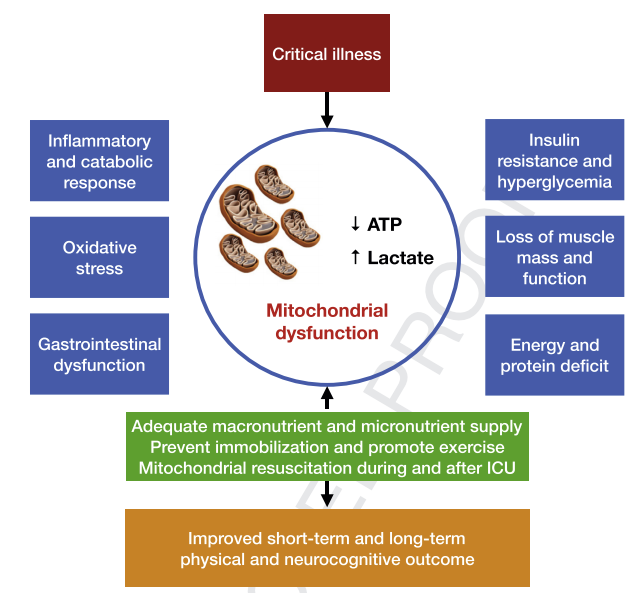
Fig. 1. Factors affecting mitochondrial function during and after critical illness. Mitochondrial function is essential to survive critical illness. Several factors are associated with mitochondrial dysfunction. Mitochondrial dysfunction is associated with decreased energy production reflected by lower ATP availability and increased lactate levels. Adequate nutrition during and after critical illness may improve mitochondrial function and result in better long-term physical and neurocognitive outcomes after critical illness. ATP: adenosinetriphosphate; GI-tract: Gastro-intestinal tract.
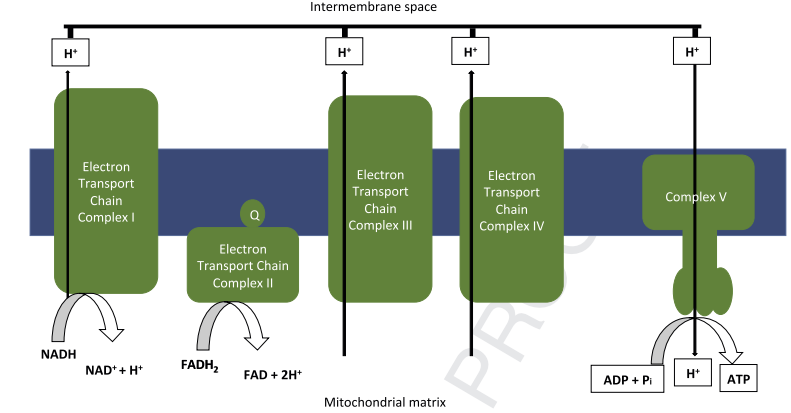
Fig. 2. Mitochondrial energy production. The oxidative phosphorylation (OXPHOS) system consists of five mitochondrial complexes and provides cellular energy by generating adenosinetriphospate (ATP) from adenosinediphosphate (ADP). The electron transport chain consists of the first four mitochondrial complexes. NADH and FADH2 are used as electron donors by the first and second complex. Mitochondria depend on the availability of reduced nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2), which are generated during the utilization of glucose, fatty acids and, to a lesser extent, amino acids [14]. The energy released during the electron transfer through the electron transport chain is used to pump protons (Hþ) in the mitochondrial matrix over the inner mitochondrial membrane into the intermembrane space. This process generates a proton gradient across this membrane. The energy stored in this proton gradient is used by THE FOF1-ATPase (complex V), which together with the electron transport chain forms the OXPHOS system, to generate ATP from ADP and inorganic phosphate [14].
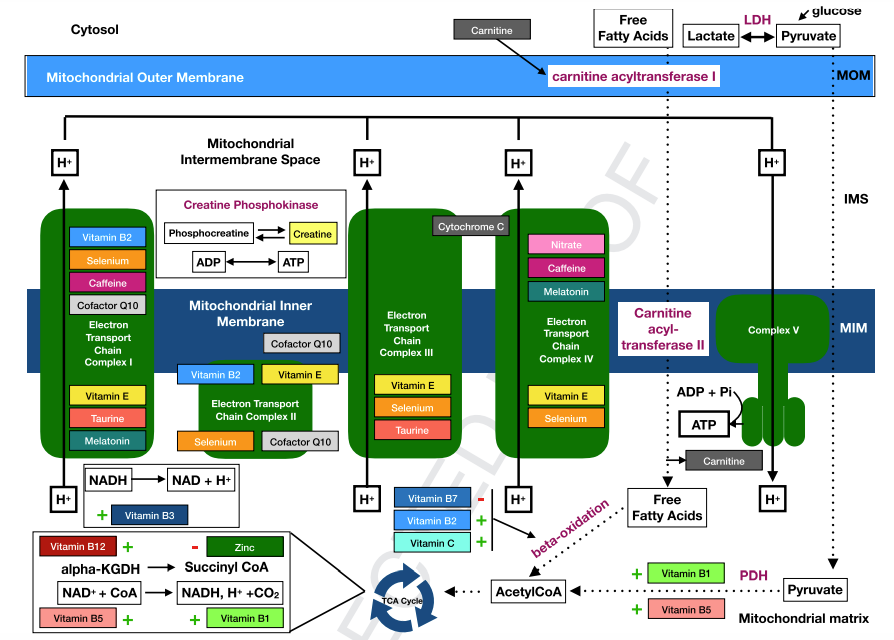
Fig. 3. Overview of relevant nutrients in bioenergetic mitochondrial processes. Several nutrients are involved in the formation of acetyl CoA, which is essential in energy production as it is the starting point of the TCA cycle. Thiamine (vitamin B1) is essential for the conversion of pyruvate to acetyl-coA. Furthermore, high levels of zinc were found to inhibit the glycolysis and TCA cycle. Carnitine is essential in beta-oxidation of free fatty acids. In addition to the formation of acetyl CoA, several nutrients have an direct effect on the TCA cycle. Pantothenic acid (vitamin B5) is the precursor of CoA. Vitamin B 12 is an essential cofactor in the formation of succinyl-CoA, an important metabolite of the TCA cycle. Besides, several nutrients influences the activity of the electron transport chain. Niacin (vitamin B3) is the precursor of NADþ, which has a crucial role in the formation of NADH, which on turn plays a crucial role in the electron transport chain. Complex I and IV activity is decreased during critical illness, but several nutrients positively affect complex I and IV performance. Complex I and IV may be stimulated by selenium, caffeine and melatonin. Complex I and II are also stimulated by CoQ10. Taurine depletion is associated with impaired activity of complexes I and III. Whether the effect of vitamin E on the complexes I and IV is stimulating or inhibiting has not yet been revealed. Nitrate probably inhibits complex IV activity. Riboflavin (vitamin B2) is an important building block for complexes I and II and involved in fatty acid oxidation in the TCA cycle. a-KGDH: alpha-ketoglutarate dehydrogenase; ATP: adenosine triphosphate; CoA: coenzyme A; CO2: carbon dioxide; CoQ: coenzyme Q; NAD(H): Nicotinamide adenine dinucleotide (reduced); PDH: pyruvate dehydrogenase; Vit: vitamin.
summary
Persistent physical impairment is frequently encountered after critical illness. Recent data point towards mitochondrial dysfunction as an important determinant of this phenomenon. This narrative review provides a comprehensive overview of the present knowledge of mitochondrial function during and after critical illness and the role and potential therapeutic applications of specific micronutrients to restore mitochondrial function.
Increased lactate levels and decreased mitochondrial ATP-production are common findings during critical illness and considered to be associated with decreased activity of muscle mitochondrial complexes in the electron transfer system. Adequate nutrient levels are essential for mitochondrial function as several specific micronutrients play crucial roles in energy metabolism and ATP-production.
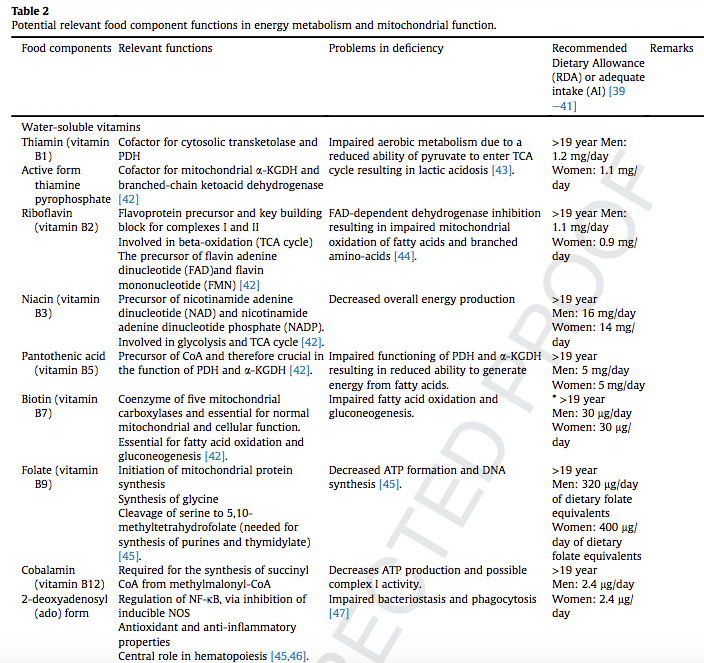
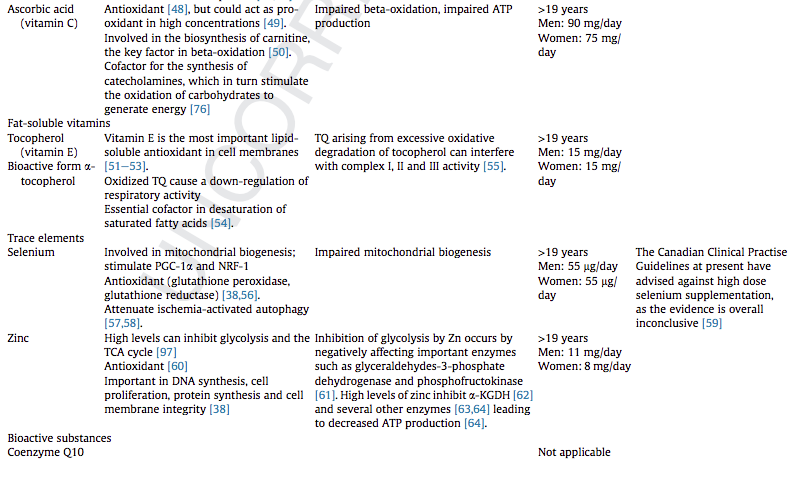
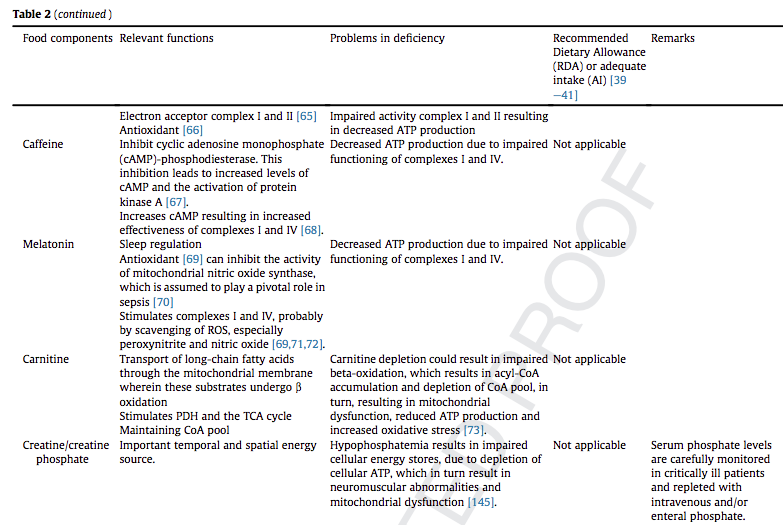
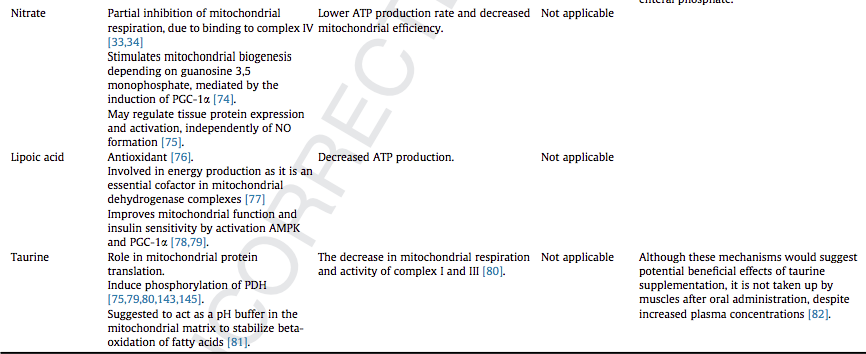
We have addressed the role of B vitamins, ascorbic acid, a-tocopherol, selenium, zinc, coenzyme Q10, caffeine, melatonin, carnitine, nitrate, lipoic acid and taurine in mitochondrial function. B vitamins and lipoic acid are essential in the tricarboxylic acid cycle, while selenium, a-tocopherol, Coenzyme Q10, caffeine, and melatonin are suggested to boost the electron transfer system function. Carnitine is essential for fatty acid beta-oxidation. Selenium is involved in mitochondrial biogenesis.
Notwithstanding the documented importance of several nutritional components for optimal mitochondrial function, at present, there are no studies providing directions for optimal requirements during or after critical illness although deficiencies of these specific micronutrients involved in mitochondrial metabolism are common. Considering the interplay between these specific micronutrients, future research should pay more attention to their combined supply to provide guidance for use in clinical practise.