Allergy Asthma Proc. 2019 Mar; 40(2): 84–92.
PMCID: PMC6399565
PMID: 30819278
Pathophysiology of atopic dermatitis: Clinical implications
Jihyun Kim, M.D., Ph.D.,1,–,3 Byung Eui Kim, M.D., Ph.D.,2 and Donald Y. M. Leung, M.D., Ph.D.

2
Author information Copyright and License information PMC Disclaimer
Abstract
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease. Genetic predisposition, epidermal barrier disruption, and dysregulation of the immune system are some of the critical components of AD. An impaired skin barrier may be the initial step in the development of the atopic march as well as AD, which leads to further skin inflammation and allergic sensitization. Type 2 cytokines as well as interleukin 17 and interleukin 22 contribute to skin barrier dysfunction and the development of AD. New insights into the pathophysiology of AD have focused on epidermal lipid profiles, neuroimmune interactions, and microbial dysbiosis. Newer therapeutic strategies focus on improving skin barrier function and targeting polarized immune pathways found in AD. Further understanding of AD pathophysiology will allow us to achieve a more precision medicine approach to the prevention and the treatment of AD.
아토피 피부염(AD)은
가장 흔한 만성 염증성 피부 질환입니다.
유전적 소인,
표피 장벽 파괴,
면역 체계의 조절 장애는
아토피 피부염의 중요한 구성 요소 중 일부입니다.
Genetic predisposition, epidermal barrier disruption, and dysregulation of the immune system
손상된 피부 장벽은
아토피뿐만 아니라
AD의 초기 단계일 수 있으며,
이는 추가적인 피부 염증과 알레르기 감작으로 이어집니다.
제2형 사이토카인과
인터루킨 17 및
인터루킨 22는
피부 장벽 기능 장애와 AD의 발병에 기여합니다.
AD의 병태생리에 대한 새로운 통찰력은
표피 지질 프로파일,
신경 면역 상호 작용 및 미생물 이상증식에 초점을 맞추고 있습니다.
새로운 치료 전략은
피부 장벽 기능을 개선하고
AD에서 발견되는 양극화된 면역 경로를 표적으로 삼는 데 중점을 둡니다.
AD 병태생리에 대한 이해가 더 깊어지면
AD의 예방과 치료에 대한
정밀 의학 접근법을 더욱 발전시킬 수 있을 것입니다.
Keywords: Atopic dermatitis, epidermal barrier, immune dysregulation, microbiome, skin lipid
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease.1 The U.S. prevalence of AD was reported to be 11.3–12.7% and 6.9–7.6% in children and in adults, respectively.2 The Hanifin and Rajka criteria and the American Academy of Dermatology Consensus Criteria are useful diagnostic tools based on features of AD.3,4 AD severity can be assessed by using validated methods such as Scoring Atopic Dermatitis or the Eczema Area and Severity Index.5
Although the pathophysiology of AD is not completely understood, numerous studies demonstrated that skin barrier dysfunction and immune dysregulation contribute to the pathobiology of AD.6–8 The epidermis plays a crucial role as a physical and functional barrier, and skin barrier defects are the most significant pathologic findings in AD skin.1,9,10 Filaggrin (FLG), transglutaminases, keratins, and intercellular proteins are key proteins responsible for epidermal function. Defects in these proteins facilitate allergen and microbial penetration into the skin.9–11
Skin barrier dysfunction has been considered to be the first step in the development of atopic march as well as AD.7,12 However, it is also now evident that immune dysregulation, including the activation of type 2 immune responses, results in impairment of the epidermal barrier.13–16 Recently, new insights into the pathophysiology of the development of AD focused on an important role of abnormalities in epidermal lipid layer as well as neuroimmune interactions and microbial dysbiosis.17–20 These factors have been used to develop novel therapeutic and preventative strategies of AD. This review addressed recent insights into the pathophysiologic mechanism of AD and the clinical application of these factors for improved treatment and prevention of AD. This work was supported by National Institutes of Health (grant AR41256). J. Kim and B. Eui Kim contributed equally to the article.
아토피 피부염(AD)은
가장 흔한 만성 염증성 피부 질환입니다.1
미국의 AD 유병률은
어린이와 성인에서
각각 11.3-12.7%, 6.9-7.6%로 보고되었습니다.2
하니핀 및 라즈카 기준과 미국피부과학회 합의 기준은 AD의 특징에 근거한 유용한 진단 도구입니다.3,4 AD 중증도는 아토피 피부염 점수 또는 습진 면적 및 중증도 지수 등의 검증된 방법을 사용하여 평가할 수 있습니다.5
AD의 병태생리가 완전히 이해되지는 않았지만,
수많은 연구에서
피부 장벽 기능 장애와
면역 조절 장애가
AD의 병태생리에 기여한다는 사실이 입증되었습니다.6-8
표피는
물리적 및 기능적 장벽으로서 중요한 역할을 하며,
피부 장벽 결함은 AD 피부에서 가장 중요한 병리학적 소견입니다.1,9,10
필라그린(FLG),
트랜스글루타미나제,
케라틴 및 세포 간 단백질은
표피 기능을 담당하는 핵심 단백질입니다.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9140947/

이러한
단백질의 결함은
알레르겐과
미생물의 피부 침투를 용이하게 합니다.9-11
피부 장벽 기능 장애는
아토피뿐만 아니라
아토피성 행진 발병의 첫 단계로 간주되어 왔습니다.7,12
그러나
이제
제2형 면역 반응의 활성화를 포함한
면역 조절 장애가 표피 장벽의 손상을 초래한다는 사실도
분명해졌습니다.13 -16
최근에는
AD 발병의 병태생리에 대한 새로운 통찰력이
표피 지질층의 이상과
신경 면역 상호 작용 및
미생물 이상 생물의 중요한 역할에 초점을 맞추고 있습니다.17-20
이러한 요인들은
AD의 새로운 치료 및 예방 전략을 개발하는 데 사용되었습니다.
이 리뷰에서는
AD의 병태생리학적 메커니즘에 대한
최근의 통찰과 AD의 치료 및 예방 개선을 위한
이러한 인자들의 임상적 적용에 대해 다룹니다.
이 연구는 미국 국립보건원(AR41256 보조금)의 지원을 받았습니다. 이 논문에는 J. Kim과 B. Eui Kim이 동등하게 기여했습니다.
GENETICS
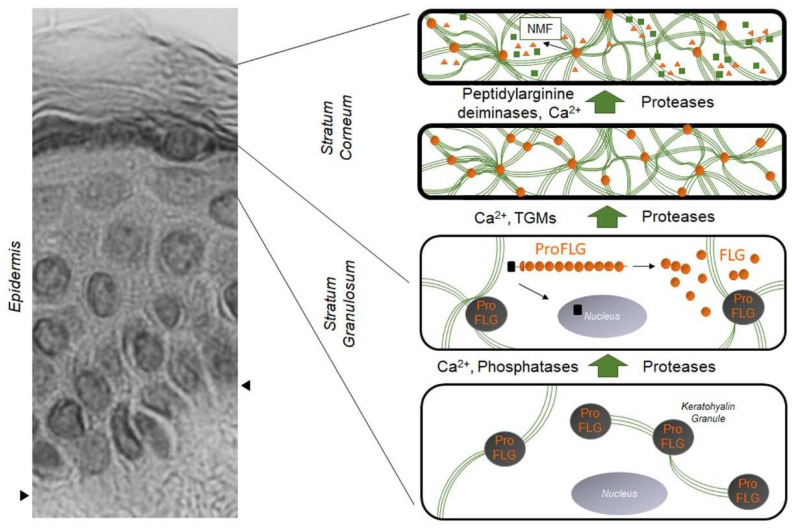
The filaggrin (FLG) gene is located on chromosome 1q2, and encodes FLG (filaggrin protein), which is a major structural protein in the stratum corneum (SC).21 Pro-FLG polymers are proteolytically cleaved and dephosphorylated into FLG monomers, which are associated with the aggregation of keratin filaments and the formation of SC.13 The generation of FLG degradation products, urocanic acid and pyrrolidine carboxylic acid, contributes to SC hydration and acidic pH of skin.14 It is well known that FLG null mutations impair skin barrier function and increase the risk of AD.21,22 FLG mutations, particularly homozygous mutations, are associated with an increased risk of severe AD with earlier onset, longer persistence, and skin infections.8,22,23 Approximately 10% of European populations are heterozygous carriers of FLG mutations, which results in a 50% reduction in expressed protein.22 However, the pathophysiology of AD goes far beyond FLG mutations. For example, Japanese and Korean patients have a lower frequency of FLG mutations than do patients in Western populations.13,24 Furthermore, ∼40% of subjects with FLG-null alleles do not show characteristics of AD, and most of the patients with AD and with FLG mutations eventually outgrow the disease.25
Polymorphisms of various immune pathway genes are associated with an increased risk of AD through alternations in the T-helper (Th) type 2 signaling pathway.21,26 Upregulation of interleukin (IL) 4 and IL-13 lowers FLG expression, which leads to skin barrier defects.27,28 A gain of functional polymorphisms of type 2 cytokine receptors (IL-4R and IL-13R) are also implicated in AD pathogenesis.28,29 Other immune-related genes that contribute to the development of AD include IL-31, IL-33, signal transducer and activator of transcription (STAT) 6, thymic stromal lymphopoietin (TSLP) and its receptors (IL-7R and TSLPR), interferon regulatory factor 2, Toll-like receptor 2, and high-affinity IgE receptor (FcεRI) α gene in specific populations.21,26,30–33 Additionally, recent studies demonstrated that vitamin D receptor polymorphisms and cytochrome P450 family 27 subfamily A member 1 (CYP27A1) variant are associated with AD.34,35 CYP27A1 is known to be involved in the metabolism of vitamin D3, which plays an essential role in immune modulation.34
Epigenetic mechanisms are heritable and can regulate gene expression without changing the DNA sequence.13 There is increasing evidence that demonstrates that environmental exposures induce epigenetic changes and AD through DNA modification and micro-RNA–mediated posttranscriptional regulation.26,36 A recent study provided evidence for the importance of DNA methylation and showed the relationship between umbilical cord blood methylation at 5′-C-phosphate-G-3′ sites of IL-4R and the development of AD at 1 year of age.37 DNA methylation in one adjacent CpG site of FLG was reported to have a significant interaction with FLG sequence variants and association with the increased risk of eczema,38 whereas another study, which used buccal cells, could not show the relationship between methylation of the FLG promoter and gene expression and allergic diseases.39 Furthermore, hypomethylation of TSLP and FcεRI γ promoters contributes to gene overexpression in patients with AD.26
필라그린(FLG) 유전자는
염색체 1q2에 위치하며
각질층(SC)의 주요 구조 단백질인 FLG(필라그린 단백질)를 암호화합니다.21
Pro-FLG 폴리머는
단백질 분해적으로 절단되어
FLG 모노머로 탈인산화되며,
이는 각질 필라멘트 응집과 SC 형성에 관여합니다.13
FLG 분해 산물인
우로칸산과 피롤리딘 카르복실산의 생성은
피부의 SC 수화 및 산성 pH에 기여합니다.14
FLG 결핍 돌연변이는
피부 장벽 기능을 손상시키고
AD의 위험을 증가시키는 것으로 잘 알려져 있습니다.21,22
FLG 돌연변이,
특히 동형 접합 돌연변이는
조기 발병, 지속 기간 연장 및 피부 감염과 함께
중증 AD의 위험 증가와 관련이 있습니다.8,22,23
유럽 인구의 약 10%가
FLG 돌연변이의 이형 접합 보인자로,
발현 단백질이 50% 감소합니다.22
그러나
AD의 병리 생리학은
FLG 돌연변이를 훨씬 넘어서고 있습니다.
예를 들어,
일본인과 한국인 환자는
서양인 환자보다 FLG 돌연변이 빈도가 낮습니다.13,24
또한,
FLG 대립유전자를 가진 대상자의 ∼40%는
AD의 특징을 보이지 않으며,
AD 환자 및 FLG 돌연변이를 가진 환자의 대부분은
결국 질병이 진행되지 않습니다.25
다양한 면역 경로 유전자의 다형성은
T-헬퍼(Th) 2형 신호 경로의 교대를 통해
AD 위험 증가와 관련이 있습니다.21,26
인터루킨(IL) 4 및 IL-13의 상향 조절은
FLG 발현을 낮추어
피부 장벽 결함을 유발합니다.27,28
2형 사이토카인 수용체(IL-4R 및 IL-13R)의 기능 다형성 증가도
AD 발병에 관여합니다.28,29
AD 발병에 기여하는 다른 면역 관련 유전자로는
IL-31, IL-33, 신호 전달 및
전사 활성화 인자(STAT) 6,
흉선 기질 림프포이에틴(TSLP) 및
그 수용체(IL-7R 및 TSLPR),
인터페론 조절 인자 2,
톨 유사 수용체 2,
특정 집단에서 고친화성 IgE 수용체(FcεRI) α 유전자가 있습니다.21,26,30 -
또한, 최근 연구에 따르면
비타민 D 수용체 다형성과
사이토크롬 P450 패밀리 27 서브 패밀리 A 멤버 1(CYP27A1) 변이가
AD와 관련이 있는 것으로 나타났습니다.34,35
CYP27A1은
면역 조절에 필수적인 역할을 하는
비타민 D3의 대사에 관여하는 것으로 알려져 있습니다.34
후성유전학적 메커니즘은
유전적이며
DNA 서열을 변경하지 않고도
유전자 발현을 조절할 수 있습니다.13
환경 노출이 DNA 변형 및 마이크로-RNA 매개 전사 후 조절을 통해 후성유전학적 변화와 AD를 유도한다는 증거가 증가하고 있습니다.26,36 최근 연구에서는 DNA 메틸화의 중요성에 대한 증거를 제공하고 IL-4R의 5′-C-인산염-G-3′ 부위에서 제대혈 메틸화와 1세 때 AD 발병 사이의 관계를 보여주었습니다.37 FLG의 한 인접 CpG 부위에서 DNA 메틸화는 FLG 서열 변이와 유의미한 상호 작용 및 습진 위험 증가와 연관성이 있는 것으로 보고된 반면,38 협측 세포를 사용한 다른 연구에서는 FLG 프로모터의 메틸화와 유전자 발현 및 알레르기 질환 사이의 관계를 보여주지 못했습니다.39 또한, TSLP 및 FcεRI γ 프로모터의 저메틸화는 AD 환자에서 유전자 과발현에 기여합니다.26
IMMUNE DYSREGULATION
Previous studies showed that type 2 immune cytokines, e.g., IL-4 and IL-13, play important roles in chemokine production, skin barrier dysfunction, suppression of antimicrobial peptides (AMP), and allergic inflammation.15,40 Interestingly, IL-31 was reported to enhance the release and production of brain-derived natriuretic peptide and to coordinate cytokine and chemokine release from skin cells, thereby inducing itch in patients with AD.41 In addition, TSLP is highly expressed in the epidermis of patients with AD, and its production is triggered by exposure to environmental factors such as allergens, microorganisms, diesel exhaust, cigarette smoke, and chemical irritants.13,42,43 When using skin tape samples, a Korean birth cohort study showed elevated expression of TSLP in the skin of 2 month-old infants before the development of clinical AD at 24 months of age.44
Although blockade of type 2–driven inflammation improves AD symptoms, the pathogenesis of AD is not exclusively explained by Th2 immunity. In this regard, IL-17 has been reported to reduce expression of FLG and involucrin.45,46 More prominent Th17 activation was observed in blood and acute AD skin lesions in Asian patients compared with European-American patients.47 In addition, AD is classified as the extrinsic and the intrinsic type, and production of IL-17 cytokine is higher in intrinsic AD with normal immunoglobulin E levels than in extrinsic AD.48 IL-22 is also highly upregulated in the skin of patients with AD and is associated with skin barrier dysfunction and abnormal epidermal markers, such as keratin 6 and keratin 16.49–51 In particular, transition to the chronic phase is manifested by the start of Th1-cell activation as well as the sustained activation of Th2 and Th22 cells (Fig. 1).52,53 Of interest, tumor necrosis factor α in combination with Th2 cytokines altered the expression of early and terminal differentiation products and reduced the level of long-chain free fatty acids (FFA) and ester linked ω-hydroxy (EO) ceramides.17,20
이전 연구에 따르면
제2형 면역 사이토카인(예: IL-4 및 IL-13)은
케모카인 생성,
피부 장벽 기능 장애,
항균 펩타이드(AMP) 억제 및 알레르기 염증에 중요한 역할을 합니다.15,40
흥미롭게도
IL-31은
뇌 유래 나트륨이뇨펩타이드의 방출과 생산을 촉진하고
피부 세포에서 사이토카인 및 케모카인 방출을 조정하여
AD 환자에서 가려움을 유발하는 것으로 보고되었습니다.41
또한,
TSLP는
AD 환자의 표피에서 높게 발현되며,
알레르겐, 미생물, 디젤 배기가스, 담배 연기 및 화학 자극제와 같은 환경 요인에 노출되면
그 생성이 촉발됩니다.13,42,43
피부 테이프 샘플을 사용한 한국인 출생 코호트 연구에서 24개월에 임상 AD가 발병하기 전 2개월 된 영아의 피부에서 TSLP의 발현이 증가한 것으로 나타났습니다.44
2형 염증을 차단하면
AD 증상이 개선되지만,
AD의 발병 기전이 Th2 면역으로만 설명되는 것은 아닙니다.
이와 관련하여
IL-17은
FLG 및 인베루크린의 발현을 감소시키는 것으로 보고되었습니다.45,46
유럽계 미국인 환자에 비해 아시아 환자의 혈액 및 급성 AD 피부 병변에서 더 두드러진 Th17 활성화가 관찰되었습니다.47 또한 AD는 외인성 및 내인성 유형으로 분류되며 면역 글로불린 E 수준이 정상인 내인성 AD에서 IL-17 사이토카인의 생산이 외인성 AD보다 높습니다.48 IL-22는 또한 AD 환자의 피부에서 고도로 상향 조절되며 피부 장벽 기능 장애 및 케라틴 6 및 케라틴 16과 같은 비정상 표피 마커와 관련이 있습니다 .49-51 특히 만성기로의 전환은 Th1 세포 활성화의 시작과 Th2 및 Th22 세포의 지속적인 활성화에 의해 나타납니다 (그림. 1).52,53 종양괴사인자 α는 Th2 사이토카인과 결합하여 초기 및 말기 분화 산물의 발현을 변화시키고 장쇄 유리 지방산(FFA)과 에스테르 연결된 ω-하이드록시(EO) 세라마이드의 수준을 감소시켰습니다.17,20
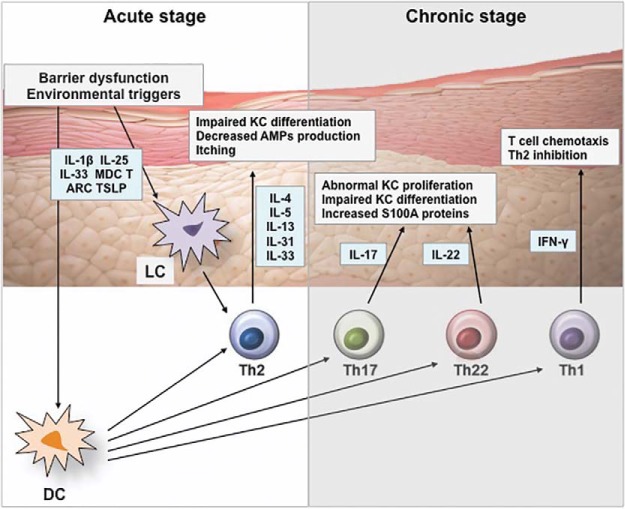
Effects of cytokines on epidermis in AD.
Disrupted epidermal barrier and environmental triggers stimulate keratinocytes to release IL-1β, IL-25, IL-33, MDC, TARC, and TSLP, which activate dendritic cells and Langerhans cells. Activated dendritic cells stimulate Th2 cells to produce IL-4, IL-5, IL-13, IL-31, and IL-33, which leads to barrier dysfunction, decreased AMP production, impaired keratinocyte differentiation, and itch symptoms. Chronic AD is characterized by recruitment of Th1, Th22, and Th17 subsets, which results in epidermal thickening and abnormal keratinocyte proliferation. AD = atopic dermatitis; AMP = antimicrobial peptide; DC = dendric cell; IFN = interferon; IL = interleukin; KC = keratinocyte; LC = Langerhans cell; MDC = macrophage-derived chemokine; S100A = S100 calcium-binding protein A; Th = T-helper type; TARC = thymus and activation-regulated chemokine; TSLP = thymic stromal lymphopoietin.
Recent studies showed that skin-resident group 2 innate lymphoid cells (ILC2) play a role in the pathogenesis of AD. ILC2s were found to produce IL-5 and IL-13, which result in the development of an AD-like skin lesion.54,55 Similarly, human skin ILC2s are highly enriched in lesional skin of patients with AD and activated by the epithelial cell–derived cytokines such as IL-25, IL-33, and/or TSLP.55,56 This leads to the production of type 2 cytokines and skin allergic inflammation.55,56 In contrast, epidermal ILC2s are inhibited by E-cadherin, and its downregulation recent studies showed that skin-resident.56
사이토카인이 AD에서 표피에 미치는 영향.
표피 장벽과 환경적 유발 요인은
각질 세포를 자극하여
수지상 세포와 랑게르한스 세포를 활성화하는
IL-1β, IL-25, IL-33, MDC, TARC 및 TSLP를 방출합니다.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9782496/

활성화된 수지상 세포는
Th2 세포를 자극하여 IL-4, IL-5, IL-13, IL-31 및 IL-33을 생성하여
장벽 기능 장애,
AMP 생성 감소,
각질 세포 분화 장애 및 가려움증 증상을 유발합니다.
만성 AD는
Th1, Th22 및 Th17 하위 집합의 모집이 특징이며,
이로 인해 표피가 두꺼워지고 비정상적인 각질 세포 증식이 발생합니다.
AD = 아토피 피부염; AMP = 항균 펩타이드; DC = 수지상 세포; IFN = 인터페론; IL = 인터루킨; KC = 각질 세포; LC = 랑게르한스 세포; MDC = 대식세포 유래 케모카인; S100A = S100 칼슘 결합 단백질 A; Th = T-헬퍼 유형; TARC = 흉선 및 활성화 조절 케모카인; TSLP = 흉선간질 림프포이에틴.
최근 연구에 따르면
피부 상주 2군 선천성 림프구 세포(ILC2)가
AD 발병에 중요한 역할을 하는 것으로 나타났습니다.
ILC2는
IL-5와 IL-13을 생성하여
AD와 유사한 피부 병변을 일으키는 것으로 밝혀졌습니다.54,55
마찬가지로, 사람의 피부 ILC2는 AD 환자의 병변 피부에서 매우 풍부하며 상피 세포 유래 사이토카인인 IL-25, IL-33 및/또는 TSLP에 의해 활성화됩니다.55,56 이는 제2형 사이토카인과 피부 알레르기 염증의 생성으로 이어집니다.55,56 대조적으로 표피 ILC2는 E-카데린에 의해 억제되며, 최근 연구에 따르면 피부 상주.56
NEUROIMMUNOLOGIC MECHANISMS
A subset of sensory neurons that express histamine H1 receptor and histamine H4 receptor is activated by histamine, which can cause itch as well as allergic inflammation.57 H1 antihistamines have been widely used for the treatment of itch due to urticaria, but its effects are limited in the treatment of chronic itch in patients with AD. Recently, much interest has focused on the role of histamine-independent itch signaling pathways in which TSLP and type 2 cytokines, such as IL-4, IL-13, and IL-31, stimulate neurons expressing transient receptor potential cation channel subfamily A member 1 and afferent neurons via its receptors and Janus kinase (JAK) family, respectively.19 Of note, IL-31 induces sensory nerve elongation and branching, which supports its role that involves sensitivity to minimal stimuli and sustained itch in patients with AD.58 In addition, the activation of STAT3 in the astrocytes of the spinal dorsal horn has been reported to be involved in chronic pruritus via the generation of lipocalin-2.59
히스타민 H1 수용체와 히스타민 H4 수용체를 발현하는
감각 뉴런의 하위 집합은 히스타민에 의해 활성화되어
가려움증과 알레르기 염증을 유발할 수 있습니다.57
H1 항히스타민제는
두드러기로 인한 가려움증 치료에 널리 사용되어 왔지만
AD 환자의 만성 가려움증 치료에는 그 효과가 제한적입니다.
최근에는
히스타민 독립적인 가려움증 신호 전달 경로의 역할에
많은 관심이 집중되고 있는데,
TSLP와 IL-4, IL-13, IL-31과 같은
제2형 사이토카인은 각각 그 수용체와
야누스 키나아제(JAK) 계열을 통해
과도 수용체 잠재 양이온 채널 서브 패밀리 A 멤버 1을 발현하는 뉴런과
구 심성 뉴런을 자극합니다.19
특히 IL-31은
감각 신경의 신장과 분지를 유도하여
최소 자극에 대한 민감성과 AD 환자의 지속적인 가려움증과 관련된 역할을 뒷받침합니다.58
또한 척추 등돌기 성상교세포에서 STAT3의 활성화는 리포칼린-2의 생성을 통해 만성 소양증에 관여하는 것으로 보고되고 있습니다.59
EPIDERMAL DYSFUNCTION
IL-4, IL-13, IL-31, IL-33, and high-mobility group box 1 downregulate the production of epidermal barrier proteins, including FLG, keratins, loricrin, involucrin, and cell adhesion molecules.14,15,60–62 A damaged epidermal barrier not only leads to the development of AD but also heightens sensitization to allergens and contributes to the risk of Food allergy (FA) and airway hyperreactivity.7,12 Impairment of skin barrier function at birth and at 2 months, as evaluated by transepidermal water loss (TEWL), can precede clinical AD by 12 months of age.63 Moreover, increased TEWL in the early newborn period is associated with a higher incidence of FA at 2 years of age, which supports the concept of transcutaneous allergen sensitization.64 Defects in epidermal barrier proteins, such as FLG, transglutaminases, keratins, and intercellular proteins, facilitate dysregulated immune responses to external antigens and drive skin and systemic inflammatory responses (Table 1).9,10 FLG is highly downregulated in both lesional and nonlesional skin of patients with AD.65
IL-4, IL-13, IL-31, IL-33 및
고이동성 그룹 박스 1은
FLG, 케라틴, 로리크린, 인베루크린 및 세포 부착 분자를 포함한
표피 장벽 단백질의 생성을 하향 조절합니다.14,15,60 -62
손상된 표피 장벽은
AD 발병을 유발할 뿐만 아니라
알레르겐에 대한 민감성을 높이고
음식 알레르기(FA) 및 기도 과민 반응의 위험에 기여합니다.7,12
경피 수분 손실(TEWL)로 평가한 출생 시와 2개월 시점의
피부 장벽 기능 손상은
생후 12개월까지
임상 AD에 선행할 수 있습니다.63
또한 신생아 초기에 TEWL이 증가하면
2세 때 FA 발생률이 높아지는 것과 관련이 있으며,
이는 경피 알레르기 항원 감작의 개념을 뒷받침합니다.64
FLG, 트랜스글루타미나제, 케라틴 및 세포 간 단백질과 같은 표피 장벽 단백질의 결함은 외부 항원에 대한 조절 장애 면역 반응을 촉진하고 피부 및 전신 염증 반응을 유도합니다(표 1).9,10 FLG는 AD 환자의 병변 피부와 비 병변 피부 모두에서 크게 하향 조절되어 있습니다.65
Table 1
Epithelial skin dysfunction in atopic dermatitis
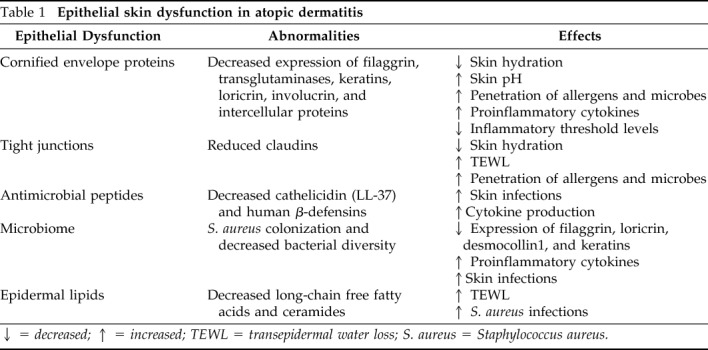
↓ = decreased; ↑ = increased; TEWL = transepidermal water loss; S. aureus = Staphylococcus aureus.
Recently, McAleer et al.66 demonstrated that FLG breakdown products in the first year of life are lowest in the cheek compared with the elbow and the nasal tip, and the slowest to achieve maturity levels, which supports the importance of FLG on the pathogenesis of infantile AD. In that study, FLG processing enzymes such as bleomycin hydrolase and calpain-1 were also increased at cheek skin by 1 month of age.64 This may explain the predilection for AD at the cheeks initially in early childhood. Epidermal FLG levels are also reduced by environmental factors, including low humidity, sunburns, diesel exhaust particles, and skin irritants.67,68 In addition, loricrin and involucrin are downregulated by overexpression of Th2 cytokines through a STAT6-dependent mechanism in AD skin.69 Corneodesmosin (CDSN) and tight junctions play a central role by supporting the adhesion between corneocytes and the integrity of the skin barrier as an intercellular protein.9,70 A recent study showed that CDSN was downregulated by IL-4, IL-13, IL-22, IL-25, and IL-31 in human keratinocytes, and the penetration of vaccinia virus was enhanced in a CDSN-deficient skin model.69 In addition, claudin 1–deficient mice were reported to die within 1 day of birth with wrinkled skin appearance and severe dehydration, which provides good evidence for the essential role of claudin for the skin barrier function.72
AMPs, including cathelicidin (LL-37) and human β-defensins, are produced by keratinocytes and play a pivotal role for host defense as well as control of host physiologic functions, such as inflammation and wound healing.73 AMP expressions are inhibited by Th2 cytokines, which are highly produced in AD skin.74 The decreased expression of AMPs is associated with a higher predisposition to Staphylococcus aureus colonization, which can aggravate AD.75 It has been reported that human β-defensins and LL-37 are chemoattractants for T lymphocytes, monocytes, dendritic cells, and neutrophils, and can induce cytokine production by monocytes and epithelial cells.76,77 These immunomodulatory properties of AMPs have important roles for host defense against infections through activation of immune cells as well as their direct antimicrobial activity.
최근 McAleer 등66은
생후 첫해의 FLG 분해 산물이 팔꿈치와 코끝에 비해
뺨에서 가장 낮고 성숙 수준에 도달하는 데
가장 느리다는 것을 입증하여 영
아 AD의 발병 기전에서 FLG의 중요성을 뒷받침했습니다.
이 연구에서는 생후 1개월이 되면 뺨 피부에서 블레오마이신 가수분해효소 및 칼파인-1과 같은 FLG 처리 효소도 증가했습니다.64 이는 유아기 초기에 뺨에서 AD가 발생하는 이유를 설명할 수 있습니다. 낮은 습도, 일광 화상, 디젤 배기가스 입자, 피부 자극제 등 환경적 요인에 의해서도 표피 FLG 수치가 감소합니다.67,68 또한 로리크린과 인베루크린은 AD 피부에서 STAT6 의존 메커니즘을 통해 Th2 사이토카인의 과발현으로 하향 조절됩니다.69 코르네오드모신(CDSN)과 단단한 접합은 세포 간 단백질로서 각질 세포 사이의 접착과 피부 장벽의 완전성을 지원함으로써 중심적인 역할을 합니다.9,70 최근 연구에 따르면 CDSN은 인간 각질 세포에서 IL-4, IL-13, IL-22, IL-25, IL-31에 의해 하향 조절되며, CDSN 결핍 피부 모델에서 백시니아 바이러스의 침투가 강화되었습니다.69 또한 클라우딘 1 결핍 마우스는 생후 1일 이내에 주름진 피부와 심한 탈수로 사망하는 것으로 보고되어 피부 장벽 기능에 클라우딘의 필수적인 역할에 대한 좋은 증거를 제공합니다.72
카테리시딘(LL-37) 및 인간 β-디펜신을 포함한 AMP는 각질 세포에서 생성되며 염증 및 상처 치유와 같은 숙주 생리 기능 조절뿐만 아니라 숙주 방어에 중추적인 역할을 합니다.73 AMP 발현은 AD 피부에서 많이 생성되는 Th2 사이토카인에 의해 억제됩니다.74 AMP의 발현 감소는 황색 포도상구균 군집화 경향이 높아져 AD를 악화시킬 수 있는 것과 연관성이 있습니다.75 인간 β-디펜신과 LL-37은 T 림프구, 단핵구, 수지상 세포 및 호중구의 화학 유인 물질이며 단핵구와 상피 세포의 사이토카인 생산을 유도할 수 있다고 보고되었습니다.76,77 이러한 AMP의 면역 조절 특성은 직접적인 항균 활성뿐만 아니라 면역 세포의 활성화를 통해 감염에 대한 호스트 방어에 중요한 역할을 합니다.
LIPIDS
Lipids, such as ceramides, long-chain FFAs, and cholesterol, constitute the lipid matrix that is organized in lamellar bodies and located between corneocytes.78 During epidermal differentiation, precursor lipids are stored in lamellar bodies within the upper cell layers of the epidermis and extruded into the extracellular domain.79 Subsequent enzymic processing produces the major lipid classes, which are necessary to maintain the integrity of the epidermal barrier. Altered lipid composition is observed in lesional and nonlesional AD skin.20 In particular, long-chain EO ceramides are essential because they are covalently bound to cornified-envelope proteins and cover the surface of each corneocyte.79 Th2 cytokines reduce levels of long-chain FFAs and EO ceramides with a STAT6-dependent manner.17,18,20 The levels of long-chain ceramides were decreased in patients with AD and who were colonized with S. aureus when compared with those who were not colonized. TEWL was negatively correlated with levels of these ceramides.80
세라마이드, 장쇄 FFA, 콜레스테롤과 같은 지질은
라멜라 체에 조직되어 각질 세포 사이에 위치한
지질 매트릭스를 구성합니다.78
표피 분화 과정에서
전구 지질은
표피 상부 세포층 내의 라멜라 체에 저장되어
세포 외 영역으로 배출됩니다.79
이후 효소 처리로 표피 장벽의 완전성을 유지하는 데 필요한 주요 지질 종류가 만들어집니다. 병변성 및 비병변성 AD 피부에서 변화된 지질 구성이 관찰됩니다.20 특히, 장쇄 EO 세라마이드는 각질화 외피 단백질에 공유 결합되어 각 각질 세포의 표면을 덮기 때문에 필수적입니다.79 Th2 사이토카인은 STAT6 의존적으로 장쇄 FFA 및 EO 세라마이드의 수준을 감소시킵니다.17,18,20 장쇄 세라마이드 수준은 식민지가 없는 환자에 비해 식민지가 있는 AD 환자에게서 감소했습니다. TEWL은 이러한 세라마이드 수치와 음의 상관관계가 있었습니다.80
MICROBIOME
AD skin has decreased bacterial diversity associated with increased Staphylococcus, Corynebacterium, and with reduced Streptococcus, Propionibacterium, Acinetobacter, Corynebacterium, and Propionibacterium during AD flares.81,82 Greater bacterial diversity with increased abundance of Staphylococcus epidermidis and Streptococcus, Corynebacterium, and Propionibacterium species was observed after AD treatment and reduced eczema.82 Species-level investigation of AD has shown a higher predominance of S. aureus in patients with more-severe disease and an abundance of S. epidermidis in patients with less-severe disease.83 S. aureus colonizes AD skin and has pivotal roles in the development and exacerbation of AD.84 S. aureus can induce T-cell–independent B-cell expansion; upregulate proinflammatory cytokines, such as TSLP, IL-4, IL-12, and IL-22; and stimulate mast cell degranulation, which results in Th2 skewing and skin inflammation.85–88
A recent study demonstrated that epidermal thickening and expansion of cutaneous Th2 and Th17 cells were induced when mice were exposed to S. aureus isolates from patients with AD.83 Of note, methicillin-resistant S. aureus colonization on AD skin is associated with lower microbial diversity and a more profound reduction in the composition of commensal bacteria, such as Streptococcus and Propionibacterium, than methicillin-sensitive S. aureus colonization.89 It is presumed that the differences and shifts in skin microbiome according to AD status are associated with the production of bacteriocins and AMPs from commensal bacteria.90,91 In addition, a recent study showed a positive correlation between the abundance of propionibacteria and corynebacteria on epidermis and long-chain unsaturated FFAs, such as FA20:1, FA20:2, FA22:1, and FA24:1.92 These findings highlight the importance of the balance between S. aureus and commensal bacteria.
Patients with AD have significantly lower numbers of intestinal commensal Bifidobacterium and higher numbers of Staphylococcus than healthy control subjects.93 Overgrowth of pathogenic bacteria, such as Escherichia coli and Clostridium difficile, is postulated as being associated with a decrease in beneficial bacteria, reduced induction of regulatory T (Treg) cells, loss of immune tolerance, and increased intestinal permeability.94,95 These observations support the hypothesis that specific microbial composition in the gut prevented Th2-shifted immunity and stimulated regulatory immunity, producing regulatory dendritic cells and Treg cells.96,97 However, further studies are necessary to elucidate how dysbiosis affects epidermal barrier function and the development of AD.
AD 피부는
AD 발적 동안
황색포도상구균, 코리네박테리움의 증가와
스트렙토코커스, 프로피오니박테리움, 아시네토박터, 코리네박테리움 및 프로피오니박테리움의 감소와 함께
박테리아 다양성이 감소했습니다.81,82
AD 치료 후
표피상구균과 스트렙토코커스, 코리네박테리움 및 프로피오니박테리아 종이 풍부해지고
습진이 감소하는
박테리아 다양성이 증가하는 것이 관찰되었습니다.82
AD에 대한 종 수준 조사에 따르면
질병이 더 심한 환자에서는
황색포도상구균이 더 우세하고
질병이 덜 심한 환자에서는 표피포도상구균이 더 많은 것으로 나타났습니다.83
S. aureus는
AD 피부에 서식하며
AD의 발병과 악화에 중추적인 역할을 합니다.84
황색포도상구균은
T세포와 무관한 B세포 확장을 유도하고,
TSLP, IL-4, IL-12, IL-22와 같은
전 염증성 사이토카인을 상향 조절하며,
비만세포 탈과립화를 자극하여
Th2 왜곡과 피부 염증을 유발할 수 있습니다.85-88
최근 연구에 따르면
생쥐를 AD 환자에서 분리한 황색포도상구균에 노출시켰을 때
표피 비후와 피부 Th2 및 Th17 세포의 확장이 유도되는 것으로 나타났습니다.83
주목할 점은, AD 피부의 메티실린 내성 황색포도상구균 집락은 메티실린 감수성 황색포도상구균 집락보다 미생물 다양성이 낮고 스트렙토코커스 및 프로피온니박테리움 같은 공생균 구성이 더 심하게 감소하는 것과 관련이 있다는 점입니다.89 AD 상태에 따른 피부 마이크로바이옴의 차이와 변화는 공생 박테리아의 박테리오신 및 AMP 생산과 관련이 있는 것으로 추정됩니다.90,91 또한 최근 연구에 따르면 표피의 프로피오니박테리아와 코리네박테리아의 풍부함과 FA20:1, FA20:2, FA22:1, FA24:1과 같은 장쇄 불포화 FFA 사이에 양의 상관관계가 있는 것으로 나타났습니다.92 이러한 결과는 황색포도상구균과 공생 세균 사이의 균형이 중요하다는 것을 강조합니다.
AD 환자는 건강한 대조군에 비해 장내 공생 비피도박테리움의 수가 현저히 낮고 포도상구균의 수가 높습니다.93 대장균 및 클로스트리디움 디피실리와 같은 병원성 박테리아의 과증식은 유익균의 감소, 조절 T(Treg) 세포의 유도 감소, 면역 내성 손실, 장 투과성 증가와 관련이 있는 것으로 추정되고 있습니다.94,95 이러한 관찰은 장내 특정 미생물 구성이 Th2 전환 면역을 예방하고 조절 면역을 자극하여 조절 수지상 세포와 Treg 세포를 생성한다는 가설을 뒷받침합니다.96,97 그러나 장내 미생물 이상증이 표피 장벽 기능 및 AD 발병에 미치는 영향을 규명하기 위해서는 추가 연구가 필요합니다.
CLINICAL APPLICATION
Frequent application of appropriate moisturizers, such as physiologic lipid mixtures and ceramide-dominant lipid, is known to help reduce TEWL, enhance skin hydration, decrease bacterial colonization, and improve skin barrier function, which leads to decreased need for topical corticosteroid.1,98,99 Petrolatum application has been reported to upregulate AMPs; induce key barrier differentiation markers, e.g., FLG; and reduces T-cell infiltration in AD skin.98 Of note, regular application of emollients has been reported to reduce the risk of AD development as a primary prevention strategy in infants at high risk.100,101 In addition, a recent study demonstrated that topical application of a liver X receptor agonist (VTP-38543) improved epidermal differentiation and lipids in patients with mild-to-moderate AD.102
Topical calcineurin inhibitors, such as tacrolimus and pimecrolimus, inhibit calcineurin-dependent T-cell activation, which leads to downregulation of proinflammatory cytokines.99 Systemic immunosuppressants, including cyclosporine, methotrexate, and azathioprine, are used in patients with severe and difficult-to-treat symptoms.99 However, these drugs have limitations and adverse reactions. Therefore, various biologics to target polarized immune pathways have been newly developed for patients with moderate-to-severe AD. Although omalizumab did not show beneficial effects to treat AD,103,104 dupilumab, a humanized monoclonal antibody (mAb) to block IL-4 and IL-13, has been approved by the Food and Drug Administration.105,106 Clinical efficacy of dupilumab occurred without significant safety concerns in adult patients with AD.105,106 Clinical trials are also underway with dupilumab in pediatric populations (NCT02407756, NCT02612454, NCT03054428, NCT03346434, NCT03345914). Because the upregulation of Th17 and Th22 cytokines have been identified in patients with AD, the blockade of these pathways is being investigated by using secukinumab and a human monoclonal antibody against interleukin-22 (ILV-094; NCT02594098, NCT01941537). Moreover, Guttman-Yassky et al.107 reported that an anti-IL-22 mAb (fezakinumab) showed clinical improvement in patients with severe AD.107
A recent study also showed clear trends of therapeutic effects of ustekinumab, which is an IL-12/IL-23p40 antagonist, to suppress Th1, Th17, and Th22 immune activation in adults with moderate-to-severe AD.108 However, there was no significant difference between treatment and placebo groups in that study.108 Another Japanese study also did not demonstrate meaningful efficacy of ustekinumab on AD,109 although it is known to be effective for psoriasis.110 Nemolizumab (anti–IL-31R mAb), lebrikizumab (anti–IL-13 mAb), and tralokinumab (anti–IL-13 mAb) revealed promising results.106 Other biologic agents, such as Bristol-Myers Squibb-981164 (anti–IL-31 mAb), Tezepelumab (anti–TSLP mAb), and MK-8226 (anti-TSLP receptor mAb), are studied and may offer a range of new therapeutic options of AD. In addition, topical tofacitinib (JAK1/JAK 3 inhibitor) and oral baricitinib (JAK1/ JAK2 inhibitor) were reported to have reduced skin inflammation and pruritus in patients with AD.111,112
Although topical and systemic antibiotics have been used to eradicate bacteria from AD skin, long-term use has limitations due to the induction of resistant microorganisms and the negative impact on host commensal bacteria. Recent studies reported that a bleach bath is effective for the restoration of skin microbiome and the treatment of AD.113,114 However, a recent meta-analysis did not show its additional benefits compared with water bath alone.115 Interestingly, Nakatsuji et al.116 found targeted autologous skin microbiome transplantation of S. hominis and S. epidermidis decreased S. aureus from AD skin. Another recent study showed that the topical transplantation with Roseomonas mucosa improved AD severity and reduced Staphylococcus aureus colonization.117
Recent studies demonstrated that appropriate probiotics are beneficial in the prevention and treatment of AD through the modulation of host immune responses.96,118,119 However, there have still been controversies regarding these clinical effects of probiotics in patients with AD, which might be due to a difference in the strains of probiotics and the characteristics of the host. It is noteworthy that the response to probiotics is greater in patients with an immunologically active state characterized by high total immunoglobulin E levels and increased expression of transforming growth factor β and Treg cells.96 Analysis of these emerging data indicated that identification of adequate AD phenotypes for the specific therapeutic option could be a key to achieve a good clinical outcome (Fig. 2).
생리적 지질 혼합물 및
세라마이드 우세 지질과 같은
적절한 보습제를 자주 바르면
TEWL을 줄이고 피부 보습을 강화하며
박테리아 군집을 줄이고
피부 장벽 기능을 개선하여
국소 코르티코스테로이드의 필요성을 감소시키는 것으로 알려져 있습니다.1,98,99
physiologic lipid mixtures and ceramide-dominant lipid

바셀린 도포는
AMP를 상향 조절하고
주요 장벽 분화 마커, 예를 들어 예를 들어, FLG; 및 AD 피부의 T 세포 침윤을 감소시킵니다.98
특히, 고위험군 유아의 일차 예방 전략으로 연화제를 정기적으로 바르면 AD 발병 위험을 줄이는 것으로 보고되었습니다.100,101 또한 최근 연구에 따르면 간 X 수용체 작용제(VTP-38543)의 국소 도포가 경증에서 중등도 AD 환자의 표피 분화 및 지질을 개선하는 것으로 입증되었습니다.102
타크로리무스 및 피메크로리무스와 같은 국소 칼시뉴린 억제제는 칼시뉴린 의존성 T 세포 활성화를 억제하여 염증성 사이토카인의 하향 조절을 유도합니다.99 사이클로스포린, 메토트렉세이트 및 아자티오프린을 포함한 전신 면역 억제제는 증상이 심하고 치료하기 어려운 환자에게 사용됩니다.99 그러나 이러한 약물에는 한계와 이상 반응이 있습니다. 따라서 중등도에서 중증의 알츠하이머병 환자를 위해 양극화된 면역 경로를 표적으로 하는 다양한 생물학적 제제가 새롭게 개발되고 있습니다. 오말리주맙은 AD 치료에 유익한 효과를 보이지 못했지만,103,104 IL-4와 IL-13을 차단하는 인간화 단일클론항체(mAb)인 두필루맙이 식품의약품안전처의 승인을 받았습니다.105,106 두필루맙의 임상적 효능은 성인 AD 환자에서 심각한 안전성 문제 없이 나타났습니다.105,106 두필루맙에 대한 소아 대상 임상시험도 진행 중입니다(NCT02407756, NCT02612454, NCT03054428, NCT03346434, NCT03345914). AD 환자에서 Th17 및 Th22 사이토카인의 상향 조절이 확인되었으므로 세쿠키누맙과 인터루킨-22에 대한 인간 단일 클론 항체(ILV-094, NCT02594098, NCT01941537)를 사용하여 이러한 경로의 차단을 연구하고 있습니다. 또한, 구트만-야스키 등.107은 항 IL-22 mAb(페자키누맙)가 중증 AD 환자에서 임상적 개선을 보였다고 보고했습니다.107
최근의 한 연구에서도 중등도에서 중증의 성인 AD 환자에서 IL-12/IL-23p40 길항제인 우스테키누맙이 Th1, Th17, Th22 면역 활성화를 억제하는 치료 효과의 명확한 경향을 보여주었습니다.108 그러나 해당 연구에서는 치료군과 위약군 간에 유의미한 차이가 없었습니다.108 또 다른 일본 연구에서도 건선에 효과적인 것으로 알려져 있지만 우스테키누맙이 AD에 유의미한 효과를 보여주지는 못했습니다.109 건선에 대한 효과는 입증되지 않았습니다.110 네몰리주맙(항 IL-31R mAb), 레브리키주맙(항 IL-13 mAb), 트랄로키누맙(항 IL-13 mAb)은 유망한 결과를 보여주었습니다.106 브리스톨-마이어스 스퀴브-981164(항 IL-31 mAb), 테제펠루맙(항 TSLP mAb), MK-8226(항 TSLP 수용체 mAb) 등 다른 생물학적 제제도 연구되고 있으며 AD의 새로운 치료 옵션을 다양하게 제공할 수 있는 것으로 알려져 있습니다. 또한 국소 토파시티닙(JAK1/JAK 3 억제제)과 경구 바리시티닙(JAK1/JAK2 억제제)은 AD 환자의 피부 염증과 소양증을 감소시키는 것으로 보고되었습니다.111,112
국소 및 전신 항생제가 AD 피부에서 박테리아를 박멸하는 데 사용되었지만, 내성 미생물의 유도 및 숙주 공생 박테리아에 대한 부정적인 영향으로 인해 장기 사용에는 한계가 있습니다. 최근 연구에 따르면 표백제 목욕이 피부 마이크로바이옴의 회복과 AD 치료에 효과적이라고 합니다.113,114 그러나 최근 메타 분석에서는 물 목욕만 하는 것에 비해 추가적인 이점을 보여주지 못했습니다.115 흥미롭게도 나카츠지 등.116은 S. 호미니스와 S. 에피더미디스의 표적 자가 피부 마이크로바이옴 이식이 AD 피부에서 S. 아우레우스 감소를 발견했습니다. 또 다른 최근 연구에 따르면 로조모나스 점막을 국소 이식하면 AD 중증도가 개선되고 황색포도상구균 집락이 감소했습니다.117
최근 연구에 따르면 적절한 프로바이오틱스는 숙주 면역 반응의 조절을 통해 AD 예방 및 치료에 도움이 되는 것으로 나타났습니다.96,118,119 그러나 AD 환자에서 프로바이오틱스의 이러한 임상 효과에 대해서는 여전히 논란이 있으며, 이는 프로바이오틱스의 균주 및 숙주의 특성에 차이가 있기 때문일 수 있습니다. 프로바이오틱스에 대한 반응은 총 면역글로불린 E 수치가 높고 형질전환 성장인자 β 및 Treg 세포의 발현이 증가하는 면역학적 활성 상태를 특징으로 하는 환자에서 더 큰 것으로 나타났습니다.96 이러한 새로운 데이터의 분석은 특정 치료 옵션에 적합한 AD 표현형을 확인하는 것이 좋은 임상 결과를 달성하는 데 핵심이 될 수 있음을 보여주었습니다(그림 2).
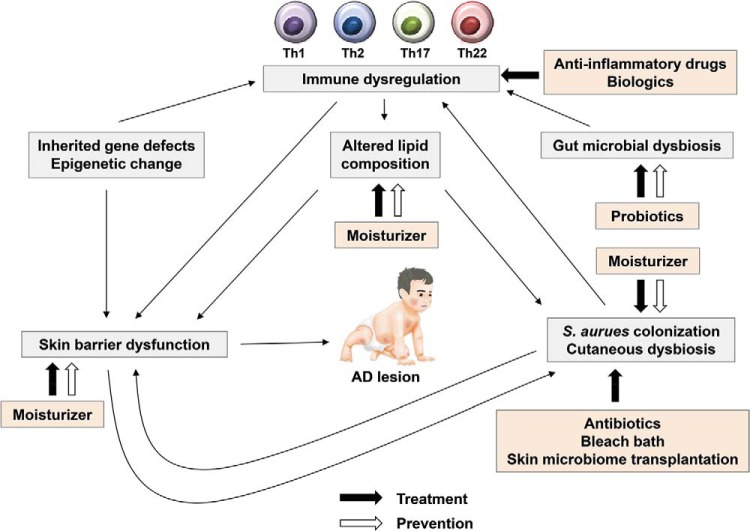
Prevention and treatment of AD. Skin barrier defects are the initial steps in the development of AD. Moisturizer prevents skin barrier defects and inhibits Staphylococcus aureus colonization in the skin. Oral probiotics may prevent the development of AD and correct gut microbial dysbiosis. Various biologics, e.g., dupilumab, target immune dysregulation. Antibiotics, bleach batch, and skin microbiome transplantation inhibit S. aureus colonization and improve cutaneous dysbiosis. AD = atopic dermatitis; Th = T helper.
아토피 피부염의 예방과 치료
피부 장벽 결함은 AD 발병의 초기 단계입니다. 보습제는 피부 장벽 결함을 예방하고 피부의 황색포도상구균 군집을 억제합니다. 경구용 프로바이오틱스는 AD의 발병을 예방하고 장내 미생물 이상균총을 교정할 수 있습니다. 두필루맙과 같은 다양한 생물학적 제제는 면역 조절 장애를 표적으로 합니다. 항생제, 표백제 배치, 피부 마이크로바이옴 이식은 황색포도상구균의 군집화를 억제하고 피부 미생물 이상증식을 개선합니다. AD = 아토피 피부염; Th = T 헬퍼.특정 치료 옵션은 좋은 임상 결과를 얻기 위한 열쇠가 될 수 있습니다(그림 2).
CONCLUSION
Multiple factors, including epidermal gene mutations, skin barrier dysfunction, immune dysregulation, neuroinflammation, altered lipid composition, and microbial imbalance, can contribute to the development of AD. Various strategies have been used to restore skin barrier function and control skin inflammation in patients with AD. To overcome limitations of topical anti-inflammatory drugs and systemic immunosuppressants, substantial effort has been committed to the development of new therapeutic options, including biologics and microbiome transplantation. In addition, moisturizers and probiotics may prevent the development of AD in infants at high risk. Further advances in our understanding of AD pathophysiology will allow us to achieve a precision medicine approach to the prevention and the treatment of AD.
표피 유전자 돌연변이, 피부 장벽 기능 장애, 면역 조절 장애, 신경 염증, 지질 구성 변화, 미생물 불균형 등 다양한 요인이 AD의 발병에 기여할 수 있습니다. AD 환자의 피부 장벽 기능을 회복하고 피부 염증을 조절하기 위해 다양한 전략이 사용되어 왔습니다. 국소 항염증제와 전신 면역 억제제의 한계를 극복하기 위해 생물학적 제제 및 마이크로바이옴 이식을 포함한 새로운 치료 옵션 개발에 상당한 노력을 기울이고 있습니다. 또한 보습제와 프로바이오틱스는 고위험군 유아의 AD 발병을 예방할 수 있습니다. AD 병리 생리학에 대한 이해가 더욱 발전하면 AD 예방과 치료에 대한 정밀 의학 접근법을 달성할 수 있을 것입니다.
ACKNOWLEDGMENTS
We thank Samsung Medical Information and Media Services, Samsung Medical Center for the preparation of figures for this article.
Footnotes
This work was supported by NIH grant AR41256. This study was also supported by Ministry of Environment, Republic of Korea.
Presented at the Eastern Allergy Conference, June 2, 2018, Palm Beach Florida
D.Y.M. Leung has consulted for Regeneron, Sanofi, Novartis and Genzyme. The remaining authors have no conflicts of interest to declare pertaining to this article
No external funding sources reported
REFERENCES
1. Kim BE, Leung DYM. Significance of skin barrier dysfunction in atopic dermatitis. Allergy Asthma Immunol Res. 2018; 10:207–215. [PMC free article] [PubMed] [Google Scholar]
2. Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017; 35:283–289. [PubMed] [Google Scholar]
3. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh) 1980; 92:44–47. [Google Scholar]
4. Eichenfield LF. Consensus guidelines in diagnosis and treatment of atopic dermatitis. Allergy 2004; 59 (Suppl 78):86–92, 2004. [PubMed] [Google Scholar]
5. Schmitt J, Langan S, Deckert S, et al. Assessment of clinical signs of atopic dermatitis: a systematic review and recommendation. J Allergy Clin Immunol. 2013; 132:1337–1347. [PubMed] [Google Scholar]
6. Brunner PM, Leung DYM, Guttman-Yassky E. Immunologic, microbial, and epithelial interactions in atopic dermatitis. Ann Allergy Asthma Immunol. 2018; 120:34–41. [PMC free article] [PubMed] [Google Scholar]
7. Lowe AJ, Leung DYM, Tang MLK, Su JC, Allen KJ. The skin as a target for prevention of the atopic march. Ann Allergy Asthma Immunol. 2018; 120:145–151. [PubMed] [Google Scholar]
8. Kim BE, Leung DY. Epidermal barrier in atopic dermatitis. Allergy Asthma Immunol Res. 2012; 4:12–16. [PMC free article] [PubMed] [Google Scholar]
9. Egawa G, Kabashima K. Multifactorial skin barrier deficiency and atopic dermatitis: Essential topics to prevent the atopic march. J Allergy Clin Immunol. 2016; 138:350–358.e1. [PubMed] [Google Scholar]
10. Schleimer RP, Berdnikovs S. Etiology of epithelial barrier dysfunction in patients with type 2 inflammatory diseases. J Allergy Clin Immunol. 2017; 139:1752–1761. [PMC free article] [PubMed] [Google Scholar]
11. Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Disruption of the stratum corneum allows potent epicutaneous immunization with protein antigens resulting in a dominant systemic Th2 response. Eur J Immunol. 2004; 34:2100–2109. [PubMed] [Google Scholar]
12. Dharmage SC, Lowe AJ, Matheson MC, Burgess JA, Allen KJ, Abramson MJ. Atopic dermatitis and the atopic march revisited. Allergy. 2014; 69:17–27. [PubMed] [Google Scholar]
13. Thyssen JP, Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 2014; 134:792–799. [PubMed] [Google Scholar]
14. Egawa G, Kabashima K. Barrier dysfunction in the skin allergy. Allergol Int. 2018; 67:3–11. [PubMed] [Google Scholar]
15. Howell MD, Kim BE, Gao P, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2009; 124:R7–R12. [PubMed] [Google Scholar]
16. Leonardi S, Rotolo N, Vitaliti G, Spicuzza L, La Rosa M. IgE values and T-lymphocyte subsets in children with atopic eczema/dermatitis syndrome. Allergy Asthma Proc. 2007; 28:529–534. [PubMed] [Google Scholar]
17. Danso MO, van Drongelen V, Mulder A, et al. TNF-α and Th2 cytokines induce atopic dermatitis-like features on epidermal differentiation proteins and stratum corneum lipids in human skin equivalents. J Invest Dermatol. 2014; 134:1941–1950. [PubMed] [Google Scholar]
18. Janssens M, van Smeden J, Gooris GS, et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J Lipid Res. 2012; 53:2755–2766. [PMC free article] [PubMed] [Google Scholar]
19. Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. 2018; 4:1. [PubMed] [Google Scholar]
20. Berdyshev E, Goleva E, Bronova I, et al. Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI Insight. 2018; 3 pii: 98006 [Epub ahead of print]. [PMC free article] [PubMed] [Google Scholar]
21. Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups-Variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018; 27:340–357. [PubMed] [Google Scholar]
22. Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011; 365:1315–1327. [PubMed] [Google Scholar]
23. Brown SJ, McLean WH. One remarkable molecule: filaggrin. J Invest Dermatol. 2012; 132:751–762. [PMC free article] [PubMed] [Google Scholar]
24. Yu HS, Kang MJ, Jung YH, et al. Mutations in the filaggrin are predisposing factor in Korean children with atopic dermatitis. Allergy Asthma Immunol Res. 2013; 5:211–215. [PMC free article] [PubMed] [Google Scholar]
25. O'Regan GM, Sandilands A, McLean WH, McLean WHI, Irvine AD. Filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2008; 122:689–693. [PubMed] [Google Scholar]
26. Bin L, Leung DY. Genetic and epigenetic studies of atopic dermatitis. Allergy Asthma Clin Immunol. 2016; 12:52. [PMC free article] [PubMed] [Google Scholar]
27. Howell MD, Kim BE, Gao P, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007; 120:150–155. [PMC free article] [PubMed] [Google Scholar]
28. Hussein YM, Shalaby SM, Nassar A, Alzahrani SS, Alharbi AS, Nouh M. Association between genes encoding components of the IL-4/IL-4 receptor pathway and dermatitis in children. Gene. 2014; 545:276–281. [PubMed] [Google Scholar]