
An Bras Dermatol. 2016 Jul-Aug; 91(4): 472–478.
doi: 10.1590/abd1806-4841.20164412
PMCID: PMC4999106
PMID: 27579743
Skin barrier in atopic dermatitis: beyond filaggrin*
Mariana Colombini Zaniboni,1 Luciana Paula Samorano,1 Raquel Leão Orfali,1 and Valéria Aoki1
Author information Article notes Copyright and License information PMC Disclaimer
Abstract
Atopic dermatitis is a chronic inflammatory skin disease with a complex pathogenesis, where changes in skin barrier and imbalance of the immune system are relevant factors. The skin forms a mechanic and immune barrier, regulating water loss from the internal to the external environment, and protecting the individual from external aggressions, such as microorganisms, ultraviolet radiation and physical trauma. Main components of the skin barrier are located in the outer layers of the epidermis (such as filaggrin), the proteins that form the tight junction (TJ) and components of the innate immune system. Recent data involving skin barrier reveal new information regarding its structure and its role in the mechanic-immunological defense; atopic dermatitis (AD) is an example of a disease related to dysfunctions associated with this complex.
아토피 피부염은
피부 장벽의 변화와 면역 체계의 불균형이 관련 요인으로 작용하는
복잡한 발병 기전을 가진 만성 염증성 피부 질환입니다.
피부는
기계적이고
면역적인 장벽을 형성하여
내부 환경에서 외부 환경으로의 수분 손실을 조절하고
미생물,
자외선 및
물리적 외상과 같은 외부 공격으로부터 개인을 보호합니다.
피부 장벽의 주요 구성 요소는
표피의 바깥층(예: 필라그린)에 위치하며,
타이트 접합부(TJ)를 형성하는 단백질과
천성 면역 체계의 구성 요소입니다.
https://www.nature.com/articles/s41598-020-58718-9

피부 장벽과 관련된 최근 데이터는
피부 장벽의 구조와 기계 면역학적 방어에서의 역할에 관한 새로운 정보를 제공하며,
아토피성 피부염(AD)은 이 복합체와 관련된 기능 장애와 관련된 질병의 한 예입니다.
Keywords: Antimicrobial cationic peptides, Claudins, Dermatitis, atopic, Immunity, innate
INTRODUCTION
Atopic dermatitis (AD) is a highly prevalent dermatosis in the population, especially in children. It has a chronic, inflammatory and pruriginous nature and progresses with periods of exacerbation. Its increasing prevalence in recent decad ranges from 10% to 20% in children and reaches 3% in adults. 1,2 AD can be associated with other manifestations of atopic disease such as asthma and rhinitis, which occur more frequently in patients with recalcitrant AD.1
For many years, the altered immune response in AD has been considered the main mechanism for inflammation and changes in skin permeability (inside-ouside theory). However, the outside-inside theory was then conceived, and the skin barrier defects in AD proved to exert a key role in the pathogenesis of AD.3,4
This review aims to focus on dysfunction of proteins of the skin barrier (filaggrin and claudins 1 and 4) and of components of the innate immune system (pattern recognition receptors, secretory elements, predominant cells of the innate immune system and skin microbiota) in AD patients, which contribute to the constant AD phenotype of xerosis, inflammation and susceptibility to infections.
아토피 피부염(AD)은 인구 중,
특히 어린이에게 매우 흔한 피부병입니다.
만성적이고 염증성이며
가려움증을 동반하며
악화와 호전을 반복하며 진행됩니다.
최근 10년간 유병률은 소아에서
10%에서 20%까지 증가했으며
성인에서는 3%에 이릅니다. 1,2
아토피는
천식 및 비염과 같은 아토피 질환의 다른 증상과 연관될 수 있으며,
이는 난치성 아토피 환자에서 더 자주 발생합니다.1
수년 동안 AD의 변화된 면역 반응은
염증과 피부 투과성 변화의 주요 메커니즘으로 여겨져 왔습니다(내부-외부 이론).
inside-ouside theory
그러나 이후
외부-내부 이론이 고안되었고,
AD의 피부 장벽 결함이
AD 발병에 핵심적인 역할을 하는 것으로 입증되었습니다.3,4
이 리뷰에서는
AD 환자의
피부 장벽 단백질(필라그린 및 클라우딘 1과 4)과
선천성 면역계 구성 요소(패턴 인식 수용체, 분비 요소, 선천성 면역계의 우세 세포 및 피부 미생물총)의 기능 장애에
초점을 맞추고,
이는
건조증,
증 및 감염에 대한 감수성이라는
지속적인 AD 표현형에 기여하는 것으로 보고 있습니다.
SKIN BARRIER
Protection and defense are the main functions of the skin. Regulation of the transepidermal water loss (TEWL), defense against the action of external physico-chemical agents and aggression of microorganisms are part of the skin barrier. The stratum corneum (SC) is the main component of such barrier, and is based on the "brick and mortar" structure. Its filmogenic feature is due to the association of SC with surface lipids.5
Filaggrin and proteins of the tight junctions (TJs) have been the most studied components of the skin barrier. Filaggrin, after hydrolyzed, contributes to the formation of relevant components for pH maintenance, moisture and skin protection against microbial agents. TJ protein with active expression, on the other hand, are important to control the selective permeability of the epidermis to build the barrier against the external environment, therefore promoting recognition of the cell "territory".6
보호와 방어는
피부의 주요 기능입니다.
경표피 수분 손실(TEWL)의 조절, 외
부 물리 화학 물질의 작용 및
미생물의 공격에 대한 방어는 피부 장벽의 일부입니다.
각질층(SC)은
이러한 장벽의 주요 구성 요소이며
"벽돌과 몰타르" brick and mortar 구조를 기반으로 합니다.

https://www.mdpi.com/2079-9284/6/3/52




필름 형성 기능은
SC와 표면 지질의 연관성 때문입니다.5
필라그린과
타이트 접합부(TJ)의 단백질은
피부 장벽에서 가장 많이 연구된 구성 요소입니다.
필라그린은
가수분해된 후 pH 유지,
수분 및 미생물로부터 피부를 보호하는
관련 성분의 형성에 기여합니다.
반면에
발현이 활성화된 TJ 단백질은
표피의 선택적 투과성을 조절하여
외부 환경에 대한 장벽을 구축함으로써 세포 "영역"의 인식을 촉진하는 데 중요합니다.6
Skin barrier proteins with functional relevance
Filaggrin
Filaggrin is a protein originated from pro-filaggrin, produced by keratinocytes. It is the main component of keratohyalin granules, visualized by light microscopy within the granular layer. Conversion of pro-filaggrin into filaggrin, both intracellular proteins, occurs through dephosphorylation and proteolysis by serine proteases, releasing multiple active monomers of filaggrin.7 With the decrease of the water gradient in the outer layers of the epidermis, filaggrin hydrolysis occurs in hygroscopic amino acids.8,9 Factors such as age, ultraviolet B radiation, relative humidity and hypoxia affect this process.10,11
Hygroscopic amino acids, especially arginine, glutamine and histidine are detected within the intercellular space.10 They generate the natural moisturizing factors (NMF), responsible for the maintenance of SC and pH hydration for the production of urocanic acid (UCA) in its cis and trans forms, as well as 5- pyrrolidone carboxylic acid (PCA).10,12,13 Furthermore, these two byproducts filaggrin have inhibitory effects on the Staphylococcus aureus (S. aureus) growth.14
Changes in barrier proteins, such as decreased expression of filaggrin in the skin, and mutations with loss of function in filaggrin gene (FLG), such as those found in ichthyosis vulgaris, have been described in AD.10,15 These mutations lead to increased risk of early onset of the disease, respiratory atopy, allergies, elevated IgE serum levels and persistence of AD in adulthood.16 Moreover, there is a significant relationship between AD with FLG mutation and peanut allergy mediated by IgE, indicating an increased skin permeability and consequent enhanced exposure to allergens.17
Interleukins (IL) 4 and 13, detected in AD lesions, also lead to decreased expression of filaggrin in keratinocytes.18 The family of IL-1 has relevant pro-inflammatory features, such as IL-1α, suggesting that the onset of inflammation may occur due to changes in the skin barrier. Morevoer, there are reports of decrease of NMF in the SC of individuals with AD and mutations in the FLG gene, with increase of IL-1 family cytokines in non-inflammed skin.19
Therefore, patients with AD and deficiency in FLG expression have decreased SC hydration, increased TEWL, and higher pH than non-atopic individuals, with an augmented risk of developing allergies, asthma and rhinitis.20 Changes in the skin barrier due to filaggrin deficiency may also lead to inflammation, and reduced protein expression in keratinocytes (Chart 1).
필라그린은
각질 세포에서 생성되는
프로 필라그린에서 유래한 단백질입니다.
이는 과립층 내에서
광학 현미경으로 시각화되는
케라토히알린 과립의 주성분입니다.
세포 내 단백질인
프로-필라그린이
필라그린으로 전환되는 것은
세린 프로테아제에 의한 탈인산화 및
단백질 분해를 통해 일어나며,
여러 활성 필라그린 단량체가 방출됩니다.7
표피 외층의 수분 구배가 감소하면
흡습성 아미노산에서
필라그린 가수 분해가 발생합니다.8,9
나이,
자외선 B 복사,
상대 습도 및 저산소증 같은 요인이
이 과정에 영향을 미칩니다.10,11
흡습성 아미노산 Hygroscopic amino acids ,
특히 아르기닌,
글루타민 및 히스티딘은
세포 간 공간에서 검출됩니다.10
이들은
시스 및 트랜스 형태의 우로카닉산(UCA)과
5-피롤리돈 카르복실산(PCA)의 생산을 위한
SC 및 pH 수화 유지를 담당하는
천연 보습 인자(NMF)를 생성합니다.10,12,13

또한 이 두 부산물 필라그린은
황색 포도상구균(S. aureus) 성장에
억제 효과를 가지고 있습니다.14
피부에서
필라그린의 발현 감소와 같은
장벽 단백질의 변화와
한선증에서 발견되는 것과 같은 필라그린 유전자(FLG)의 기능 상실 돌연변이가
AD에서 설명되었습니다.10,15
이러한
돌연변이는
질병의 조기 발병,
호흡기 아토피,
알레르기,
IgE 혈청 수치 상승 및 성인기 AD의 지속성 위험을 증가시킵니다.16
또한,
FLG 돌연변이가 있는 AD와
IgE가 매개하는 땅콩 알레르기 사이에는
상당한 관계가 있으며
이는 피부 투과성이 증가하고
결과적으로 알레르겐에 대한 노출이 강화되었음을 나타냅니다.17
AD 병변에서 검출되는
인터루킨(IL) 4 및 13은
각질 세포에서 필라그린의 발현을 감소시킵니다.18
IL-1 계열은 IL-1α와 같은
전 염증성 특징을 가지고 있어
피부 장벽의 변화로 인해 염증이 시작될 수 있음을 시사합니다.
또한, 염증이 없는 피부에서
IL-1 계열 사이토카인의 증가와 함께
AD 및 FLG 유전자의 돌연변이가 있는
개인의 SC에서
NMF가 감소한다는 보고가 있습니다.19
따라서
아토피 피부염이 있고
FLG 발현이 결핍된 환자는
아토피가 없는 사람보다 SC 수분이 감소하고
TEWL이 증가하며
pH가 높아 알레르기, 천식 및 비염이 발생할 위험이 증가합니다.20
필라그린 결핍으로 인한
피부 장벽의 변화는 또한 염증을 유발하고
각질 세포의 단백질 발현을 감소시킬 수 있습니다(도표 1).
Chart 1
Key topics on filaggrin
ATOPIC DERMATITIS AND FILAGGRIN:
| • Filaggrin gene (FLG) mutation |
| • Decreased filaggrin expression: |
| • higher risk of early onset of the disease |
| • persistence of atopic dermatitis in adulthood |
| • increased risk of allergies by percutaneous sensitization |
| • association with high serum levels of IgE and other manifestations of atopy |
Tight junctions
TJ are formed by a complex of transmembrane and intracellular proteins found in simple and stratified mammalian epithelia. In normal skin, they are detected in the granular layer, and its expression rapidly increases after injury. They are essential for cell differentiation and keratinization of epidermal cells.6 In skin diseases with altered keratinization, such as psoriasis and ichthyosis vulgaris, they are present even in the deeper layers of the epidermis.21
TJ play an important role in epidermal selective permeability, controlling intercellular flow of substances such as hormones, cytokines and electrolytes, functioning as "gates". This permeability depends directly on the size and ionic specificity of the molecule. In addition to the intercellular permeability function, these structures also act as markers of the cell "territory".22
The intracellular portion of TJ binds to cytoskeletal plasma proteins, while the extracellular portion forms a "loop" in the intercellular space, connecting with the adjacent cell loop. TJ are formed by occludin, claudin, zonula occludens 1 (ZO1) and 2 (ZO2), junctional adhesion molecule-1 (JAM1) and the multi-PDZ-1 protein (MUPP1).23
In 2002, Tsukita and Furuse showed that claudin 1 deficiency in mice led to high TEWL and liver abnormalities, culminating with death.22 These animals showed no structural abnormalitie, but significant loss of function of the skin barrier. A similar clinical condition of claudin 1 deficiency was described in human neonates (ichthyosis-sclerosis-cholangitis syndrome).24
TJ proteins also play an important role in the invasion of some viruses (e.g.: herpes simplex) and bacteria. Some of these organisms use claudin 1 as receptors; others modulate the structure of TJ, inserting effectors, activating signals or even directly connecting to them, resulting in their partial break.25,26 Its expression rapidly increases via activation of toll-like receptor 2 (TLR2).27
The lesional skin of atopic patients contains significant decreased claudin 1 expression, but no claudin 4 reduction, when compared to the skin of non-atopic individuals (Figure 1).28-30 Reduced claudin 1 appears to be related to increased risk of infection by herpes virus type 1 (HSV1) in individuals with AD.25 There is also an inverse relation between the expression of claudin 1 and the presence of the immune response markers Th2, suggesting that this protein affects the immune response to potential environmental allergens (Chart 2).28
TJ는
단순하고 층화된 포유류 상피에서 발견되는
막 통과 단백질과
세포 내 단백질의 복합체에 의해 형성됩니다.
정상 피부에서는
과립층에서 발견되며
손상 후 발현이 급격히 증가합니다.
이들은
세포 분화와 표피 세포의 각질화에 필수적입니다.6
건선이나
한선증과 같이 각질화에 변화가 있는 피부 질환에서는
표피의 더 깊은 층에도 존재합니다.21
TJ는
호르몬,
사이토카인 및 전해질과 같은
물질의 세포 간 흐름을 제어하여
"게이트" 역할을 하는 표피 선택적 투과성에서 중요한 역할을 합니다.
이 투과성은 분자의 크기와 이온 특이성에 직접적으로 의존합니다.
세포 간 투과성 기능 외에도 이러한 구조는 세포 "영역"의 마커 역할도 합니다.22
TJ의 세포 내 부분은
세포 골격질 단백질에 결합하고,
세포 외 부분은 세포 간 공간에서 "루프"를 형성하여
인접한 세포 루프와 연결됩니다.
https://www.mdpi.com/1422-0067/20/16/3869#

TJ는
오클루딘,
클라우딘,
조눌라 오클루덴스 1(ZO1) 및 2(ZO2),
접합 접착 분자-1(JAM1) 및
다중 PDZ-1 단백질(MUPP1)에 의해 형성됩니다.23
2002년에 츠키타와 후루세는
생쥐에서 클라우딘 1 결핍이 높은
TEWL과 간 이상으로 이어져
사망에 이른다는 것을 보여주었습니다.22
이 동물들은 구조적 이상은 없었지만 피부 장벽의 기능이 크게 상실된 것으로 나타났습니다. 클라우딘 1 결핍의 유사한 임상 상태가 인간 신생아(소양증-경화증-담관염 증후군)에서도 설명되었습니다.24
TJ 단백질은
또한 일부 바이러스(예: 단순 포진)와
박테리아의 침입에 중요한 역할을 합니다.
이러한 유기체 중 일부는
클라우딘 1을 수용체로 사용하고,
다른 유기체는 이펙터를 삽입하거나 신호를 활성화하거나
직접 연결하여 TJ의 구조를 조절하여
부분적으로 파괴합니다.25,26
Toll 유사 수용체 2(TLR2)의 활성화를 통해
발현이 급격하게 증가합니다.27
아토피 환자의 병변 피부는
비아토피 환자의 피부와 비교했을 때
클라우딘 1의 발현은 현저히 감소하지만
클라우딘 4의 감소는 없습니다(그림 1).28-30
클라우딘 1의 감소는
AD 환자의 헤르페스 바이러스 1형(HSV1) 감염 위험 증가와 관련이 있는 것으로 보입니다.25
클라우딘 1의 발현과
면역 반응 마커 Th2의 존재 사이에도 역관계가 있어
이 단백질이 잠재 환경 알레르기 항원에 대한 면역 반응에 영향을 준다고 합니다(도표 2).28
따라서
아토피 피부염이 있고
FLG 발현이 결핍된 환자는
아토피가 없는 사람보다 SC 수분이 감소하고
TEWL이 증가하며
pH가 높아
알레르기, 천식 및 비염이 발생할 위험이 증가합니다.20
필라그린 결핍으로 인한 피부 장벽의 변화는
또한 염증을 유발하고
각질 세포의 단백질 발현을 감소시킬 수 있습니다(도표 1).
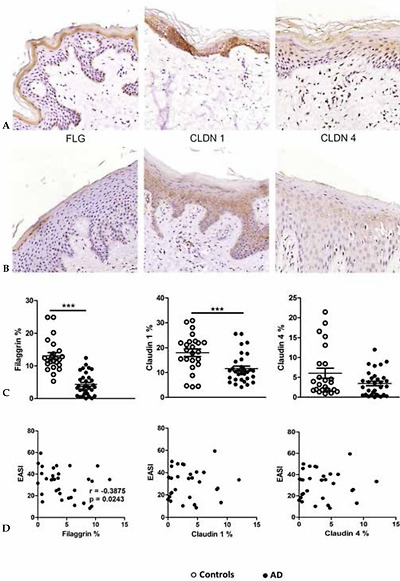
Expression of filaggrin (FLG), claudin 1 (CLDN1) and claudin 4 (CLDN4) in skin fragments of adults with atopic dermatitis (AD) stained by immunohistochemistry. (A) Skin fragments of healthy controls: FLG, CLDN1 and CLDN4. (B) Skin fragments of patients with AD, showing reduced expression of FLG, CLDN1 and CLDN4. (C) Expression of FLG, CLDN1 and CLDN 4 (area percentage) in the control group without AD (n=33) compared with patients with AD (n=25). (D) Correlation between disease severity (EASI) and the expression of the proteins in the skin barrier. The line represents the arithmetic mean of the expression of proteins in the skin barrier (percentage area). ** p≤0.01 and *** p≤0.001
Adapted from Batista, et al. 2015.29
Chart 2
Key topics on tight junctions
ATOPIC DERMATITIS AND TIGHT JUNCTIONS:
| • Decreased expression on the skin without injury |
| • Favors infection by herpes-virus type 1 and other viral skin infections |
| • Increased paracellular permeability |
| • Increased risk of allergy to large molecules |
| • Proteins with active expression: |
| • Increase with activation via Toll-like receptor 2 |
| • Decrease with increased Th2 citokynes |
Innate immune system
The innate immune system represents the initial and non-specific response of the human body to external aggressions. This response does not derive or result from target-oriented immune memory, but has an essential role in protecting the individual against potential pathogens. An intact skin barrier is needed, with proper maintenance of its cycle, pH and microbiota. Other componenents of such defense system includes secretory elements, cell receptors, such as pattern recognition receptors (PRR), immune cells and the skin microbiota (Figure 2).
선천성 면역 체계는
외부의 공격에 대한 인체의 초기 비특이적 반응을 나타냅니다.
이 반응은
표적 지향 면역 기억에서 파생되거나
그 결과물이 아니지만
잠재적인 병원체로부터 개인을 보호하는 데 필수적인 역할을 합니다.
이를 위해서는
피부 장벽의 주기,
pH,
미생물총을 적절히 유지하여
온전한 피부 장벽이 필요합니다.
이러한 방어 시스템의 다른 구성 요소로는
분비 요소,
패턴 인식 수용체(PRR)와 같은 세포 수용체,
면역 세포 및
피부 미생물이 있습니다(그림 2).
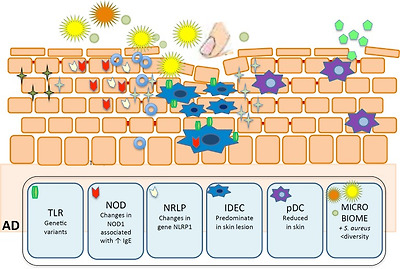
Main components of innate immune system in epidermis and their role in atopic dermatitis (AD).
Defects in Toll-like receptor 2 contribute to increased colonization and infection by S. aureus. Decreased AD expression of AMP (catelicidin-LL37 and β-defensin) also favors skin infections. Reduced plasmacytoid dendritic cells in skin injured areas by AD, facilitating certain viral skin infections. Reduced NK cells in AD. S. epidermidis increase the expression of human β-defensin by human keratinocytes through TLR2 signaling pathway. IDEC are increased in AD skin lesion. NOD1 changes are associated with elevated IgE levels in AD individuals. Changes in expression of NLRP1 gene were associated to AD severity
톨 유사 수용체 2의 결함은
황색포도상구균의 군집과
감염 증가에 기여합니다.
AMP(카탈리시딘-LL37 및 β-디펜신)의
AD 발현 감소도
피부 감염에 유리합니다.
AD에 의한 피부 손상 부위의 형질세포 수지상 세포 감소는
특정 바이러스성 피부 감염을 촉진합니다.
AD에서 NK 세포 감소.
S. 표피는 TLR2 신호 전달 경로를 통해 인간 각질 세포에 의한 인간 β- 데펜신의 발현을 증가시킵니다.
IDEC는 AD 피부 병변에서 증가합니다.
NOD1의 변화는 AD 환자의 IgE 수치 상승과 관련이 있습니다.
NLRP1 유전자의 발현 변화는 AD 중증도와 관련이 있습니다.

Staphylococcus aureus (S. aureus),

Staphylococcus epidermidis (S. epidermidis),

other bacteria,

staphylococcal enterotox in,

virus,

Toll-like receptors (TLR),

nucleotide-binding oligomerization domain-containing protein (NOD)

NOD-like receptor protein (NLRP)

β-defensina 1,

HBD-2,3 e LL37,

inflammatory dendritic epidermal cells (IDEC),

plasmacytoid DC (pDC).
PATTERN RECOGNITION RECEPTORS (PRR)
The arsenal of PRR comprises members of the toll-like receptors (TLR), nucleotide-binding oligomerization domain-containing protein (NOD-like receptors or NLR), retinoic acid-inducible gene, C-type lectin receptors (CLR) and PGLYRPs (peptidoglycan recognition proteins).31,32
패턴 인식 수용체(PRR)
PRR은
toll 유사 수용체(TLR),
뉴클레오티드 결합 올리고머화 도메인 함유 단백질(NOD 유사 수용체 또는 NLR),
레티노산 유도 유전자,
C형 렉틴 수용체(CLR) 및
PGLYRP(펩티도글리칸 인식 단백질)의 구성원으로 이루어져 있습니다.31,32
TLR family
TLRs are well-known transmembrane proteins that play as innate receptors. In humans, TLR1-10 have been described and they have the ability to recognize pathogen-associated molecular patterns (PAMPs). TLR1, 2, 4-6,10 are in charge of such recognition on the cell surface, whereas TLR3, 7-9 are found in the endosomes.33 They also recognize endogenous ligands in response to tissue damage, contributing to the maintenance of skin barrier.34,35 TLRs are usually expressed both by innate immune cells, such as DC, NK and macrophages, as well as adaptive immune cells, including T and B cells. Activation of TLR triggers the release of proinflammatory cytokines, therefore modulating the immune response against pathogens. 33
TLR2 is the receptor that recognizes a broad spectrum of PAMPs, including lipopeptides from Gram-positive bacteria, among others.33 There are reports on genetic variants of TLRs that are associated with AD; however, there is emphasis on TLR2, which is capable of recognizing products of the cell wall of S. aureus. AD individuals are more colonized and infected by S. aureus than non-atopic groups, suggesting that mutations of TLR2 may facilitate such susceptibility.36
TLR은
선천성 수용체 역할을 하는 잘 알려진
막 통과 단백질입니다.
인간에게는
TLR1-10이 알려져 있으며
병원체 관련 분자 패턴(PAMP)을 인식하는 능력이 있습니다.
TLR1, 2, 4-6,10은
세포 표면에서 이러한 인식을 담당하는 반면,
TLR3, 7-9는 엔도솜에서 발견됩니다.33
또한 조직 손상에 대한 반응으로
내인성 리간드를 인식하여
피부 장벽 유지에 기여합니다.34,35
TLR은
일반적으로
수지상 세포 DC,
자연 살해세포 NK,
대식세포와 같은 선천 면역 세포와
T 및 B 세포를 포함한 적응 면역 세포에서 모두 발현됩니다.

TLR의 활성화는
염증성 사이토카인의 방출을 촉발하여
병원균에 대한 면역 반응을 조절합니다. 33
TLR2는
그람 양성 박테리아의 리포펩타이드를 포함한
광범위한 PAMP를 인식하는 수용체입니다.33
AD와 관련된
TLR의 유전적 변이에 대한 보고가 있지만,
황색포도상구균의 세포벽 생성물을 인식할 수 있는
TLR2에 중점을 두고 있습니다.
AD 환자는
비아토피 그룹보다
황색포도상구균에 의해 더 많이 집락화되고 감염되며,
이는 TLR2의 돌연변이가
이러한 감수성을 촉진할 수 있음을 시사합니다.36
NLR family
The NLR (NOD like receptors) family has three distinct subfamilies: the NODs (nucleotide-binding oligomerization domain-containing protein), NLRPs (NOD-like receptor protein) and IPAF (ice protease-activating factor).37
NOD receptors are intracellular receptors that respond to a diversity of microbial products.38 NOD1 (also known as CARD4 - caspase activation and recruitment domain 4), selectively respond to Gram-negative bacteria, and NOD2 recognizes a fragment common to all bacteria. NOD1 changes are associated with elevated IgE levels in AD individuals, and are important indicative factors of atopy susceptibility.39 NOD2 mutations that might result in inappropriate immunomodulation, are not only associated with autoimmune diseases but also with AD.40
NRLPs respond to a large variety of ligands, such as DAMPs (damage-associated molecular patterns), ATP and urate crystals, and exogenous agents, such as asbestos and silica. These receptors form a multiprotein complex, named inflammasome, which leads to the production of IL-1β and IL-18 by activation of caspase 1.37,41 There are four main subclasses of inflammasomes: NRLP3, NLRP1, IPAF (also known as NLRC4-NLR Family, CARD Domain Containing 4) and AIM2 (absent in melanoma 2).37
Changes in expression of NLRP1 gene were associated to AD severity.42 Impaired NLRP3 expression may partially explain how skin colonization and infection with S. aureus can contribute to chronic skin inflammation in AD.43 Increased epidermal expression of IL-1β cytokine has been observed in AD patients presenting FLG mutations.19 It was demonstrated enhanced levels of IL-18 both in sera and culture supernatants under staphylococcal enterotoxin A stimuli in AD patients.44
NLR(NOD 유사 수용체) 계열에는
NOD(뉴클레오티드 결합 올리고머화 도메인 함유 단백질),
NLRP(NOD 유사 수용체 단백질),
IPAF(아이스 프로테아제 활성화 인자)의
세 가지 하위 계열이 있습니다.37
NLRPs (NOD-like receptor protein) and
IPAF (ice protease-activating factor).
NOD 수용체는
다양한 미생물 생성물에 반응하는
세포 내 수용체입니다.38
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8564530/


NOD1(CARD4 - 카스파제 활성화 및 모집 도메인 4라고도 함)은
그람 음성 박테리아에 선택적으로 반응하고
NOD2는
모든 박테리아에 공통적인 조각을 인식합니다.
NOD1의 변화는
아토피 환자의 IgE 수치 상승과 관련이 있으며
아토피 감수성의 중요한 지표 인자입니다.39
부적절한 면역 조절을 초래할 수 있는 NOD2 돌연변이는
자가 면역 질환뿐만 아니라
AD와도 관련이 있습니다.40
NRLP는
DAMP(손상 관련 분자 패턴),
ATP 및 요산염 결정,
석면 및 실리카 같은 외인성 물질과 같은 다양한 리간드에 반응합니다.
이러한 수용체는
인플라마좀이라는
다중 단백질 복합체를 형성하여
카스파제 1을 활성화하여
IL-1β 및 IL-18을 생성합니다.37,41
인플라마좀에는 네 가지 주요 하위 클래스가 있습니다:
NRLP3,
NLRP1,
IPAF(NLRC4-NLR 계열, 카드 도메인 함유 4라고도 함) 및
AIM2(흑색종 2에는 없음).37
NLRP1 유전자의 발현 변화는
AD 중증도와 관련이 있습니다.42
NLRP3 발현 장애는
피부 집락화와
황색 포도상구균 감염이
어떻게 AD의 만성 피부 염증에 기여할 수 있는지
부분적으로 설명할 수 있습니다.43
FLG 변이가 있는 AD 환자에서
IL-1β 사이토카인의 표피 발현 증가가 관찰되었습니다.19
AD 환자에서
포도구균 장독소 A 자극 하에서 혈청 및 배양 상청 모두에서
IL-18 수준이 향상되는 것으로 입증되었습니다.44
CLR family
C-Type Lectin Receptors (CLRs) contain C-type lectin-domains, therefore recognizing sugars present in microorganisms. KACL (keratinocyte-associated C-type lectin), expressed by human keratinocytes, is highlighted in this group. It triggers cytolytic activity of Natural killer (NK) cells and cytokine secretion; despite changes in the expression and function of this receptor have not been described in AD, atopic patients exhibit defective cytotoxicity of NK cells.45
C형 렉틴 수용체(CLR)는
C형 렉틴 도메인을 포함하고 있어
미생물에 존재하는 당을 인식합니다.
이 그룹에서는
인간 각질 세포에서 발현되는
KACL(각질 세포 관련 C형 렉틴)이 강조 표시됩니다.
이 수용체의 발현과 기능의 변화는
AD에서 설명되지 않았지만,
아토피 환자는
자연살해(NK) 세포의 세포 용해 활성과
사이토카인 분비를 촉발합니다.45
Antimicrobial peptides (AMP)
AMPs play an important role in the skin innate immunity acting as endogenous antibiotics. Cathelicidin (LL37) and β-defensin family are the main AMPs, but other keratinocyte products are also recognized for their anti-microbial functions, such as ribonuclease (RNase), S100 family, dermcidin and regenerating islet-derived (REG3α).46
While human β-defensin 1 (HDB1) is expressed by normal human keratinocytes, dermal inflammation induces expression of HBD2, HBD3 and LL37. AD skin lesions have is significantly lower levels of AMPs than psoriatic lesions. Reduced expression and secretion of AMPs may contribute to increased susceptibility to skin infections by viruses, bacteria and fungi in AD patients (Chart 3).32
항균 펩타이드(AMP)
AMP는
내인성 항생제 역할을 하는
피부 선천 면역에 중요한 역할을 합니다.
카텔리시딘(LL37)과
β-데펜신 계열이
주요 AMP이지만
리보뉴클레아제(RNase),
100 계열,
더르시딘 및 재
생 섬 유래(REG3α) 등
른 각질 세포 제품도 항균 기능으로 인정받고 있습니다.46
인간 β-디펜신 1(HDB1)은
정상적인 인간 각질 세포에서 발현되는 반면,
피부 염증은
HBD2, HBD3 및 LL37의 발현을 유도합니다.
아토피 피부 병변은
건선 병변보다
AMP 수치가 현저히 낮습니다.
AMP의 발현 및 분비 감소는
AD 환자의 바이러스,
박테리아 및 곰팡이에 의한 피부 감염에 대한 감수성 증가에 기여할 수 있습니다(도표 3).32
Chart 3
Key topics on the innate immune system (part 1)
Atopic dermatits and innate immune system
| • Changes in pathogen recognition receptors |
| • Defects in Toll-like receptor 2 contribute to increased |
| colonization and infection by S. aureus |
| • Anti-mycobians peptides (AMPs) |
| • Main AMPs: catelicidin (LL37) and β-defensin |
| • Decreased AD expression. Favors skin infections |
Dendritic cells (DC)
DC belong to the family of antigen-presenting cells, and are known as sentinels of the immune system, recognizing and presenting antigens, leading to T cell activation.47,48 They lack other markers of leukocyte lineages (CD3, 14, 16, 19, 20, and 56), and express high levels of MHC class II (HLA-DR) molecules.49 A DC lineage-specific marker has not yet been identified, and the subsets of DC in humans and mice are therefore currently defined by lineage − MHC II+ cells, in combination with various cell surface markers.50
There are two major human DC subsets: CD11c+ myeloid DC (mDC) and CD123+ plasmacytoid DC (pDC); mDC are efficient in the uptake, processing, and presentation of foreign antigens, and under Toll-like receptor (TLR) stimulation, induce secretion of tumor necrosis factor α (TNF-α) and proinflammatory cytokines, such as IL-12. Conversely, pDC are less effective in these processes and mainly known for their function in antiviral immunity.50 The pDC are a critical source for the antiviral type I IFNs (IFNα and IFNβ), and a reduction of these cells in AD skin, facilitate viral skin infections such as eczema herpeticum.38,51
In AD, a single population of inflammatory DC is well described, which belongs to mDC group. They were initially named inflammatory dendritic epidermal cells (IDEC) based on flow cytometry analysis of cells from epidermal suspensions 52-54. IDEC were defined by the following: HLA-DR+LIN-CD11c+CD1a+ and co-express CD206, CD36, FceRI, IgE, CD1b/c, CD11b, among others.55 Yet, IDECs can be modulated by calcineurin inhibitors and topical corticosteroids.51,56
DC는
항원 제시 세포군에 속하며,
항원을 인식하고 제시하여
T 세포 활성화를 유도하는 면역계의 파수꾼으로 알려져 있습니다.47,48
이들은 백혈구 계통의 다른 마커(CD3, 14, 16, 19, 20, 56)가 부족하고
높은 수준의 MHC 클래스 II(HLA-DR) 분자를 발현합니다.49
DC 계통 특이적 마커는
아직 확인되지 않았으며,
따라서 현재 인간과 마우스의 DC 하위 집합은 다양한 세포 표면 마커와 결합된 계통인 MHC II+ 세포에 의해 정의됩니다.50
인간의 DC 하위 집합에는
크게 두 가지가 있습니다:
CD11c+ 골수성 DC(mDC)와
CD123+ 형질세포성 DC(pDC);
mDC는 외부 항원의 흡수, 처리 및 제시에서 효율적이며 Toll 유사 수용체(TLR) 자극 시 종양 괴사인자 α(TNF-α)와 IL-12와 같은 염증성 사이토카인의 분비를 유도합니다. 반대로, pDC는 이러한 과정에서 덜 효과적이며 주로 항바이러스 면역 기능으로 알려져 있습니다.50 pDC는 항바이러스 I형 IFN(IFNα 및 IFNβ)의 중요한 공급원이며, AD 피부에서 이러한 세포가 감소하면 헤르페스 한포진과 같은 바이러스성 피부 감염이 촉진됩니다.38,51
AD에서는 염증성 DC의 단일 집단이 잘 설명되어 있으며, 이는 mDC 그룹에 속합니다. 이들은 처음에 표피 현탁액 세포의 유세포 분석에 기초하여 염증성 수지상 표피 세포(IDEC)로 명명되었습니다(52-54). IDEC는 다음과 같이 정의되었습니다: HLA-DR+LIN-CD11c+CD1a+ 및 CD206, CD36, FceRI, IgE, CD1b/c, CD11b 등을 공동 발현합니다.55 그러나 IDEC는 칼시뉴린 억제제 및 국소 코르티코스테로이드에 의해 조절될 수 있습니다.51,56
Natural killer cells (NK)
NK cells are capable of destroying cells infected by microorganisms and tumor cells, without previous activation by reconizing the lack of MHC-I espression on the surface of such cells. They release perforins and protease granzime, promoting target cell lysis, and produce a large variety of cytokines, such as TNF-α, IFN-γ, GMCSF, IL-5 and IL-8.51,57 In AD, there is a reduced number of both and in situ and circulating NK.51 In the affected AD tissue, NK cells are in close contact with dendritic cells, indicating that NK cells in direct contact with activated monocytes are ideal targets for apoptosis; this would lead to reduced Th1 cytokine production, and enhanced Th2 immune response, favoring microbial infection.58 Cytokines derived from the keratinocyte, such as TSLP (thymic stromal lymphopoietin), activate NK cells and induce Th2-prone response.59
Regulatory T lymphocytes (Treg)
In patients with AD, circulating regulatory T cells (Treg) (CD4+CD25+FoxP3+ phenotype) are detected in greater numbers and with unchanged immunosuppressive activity.60 These Tregs seem to lose their immunosuppressive activity after stimulation with superantigens, suggesting an increase of effector T cell activation in such individuals.61 Furthermore, the innate immune system produces cytokines inducers of T cells differentiation into Th2, Th17 and Th22.60,61
Other cells of the innate immune system
The innate lymphoid cells (ILCs) group comprises NK cells and ILCs non-NK cells (ILC1, ILC2 and ILC3). They are morphologically very similar to lymphocytes, but lack expression of conventional markers (non-T and non-B cells). They depend on the common γ chain of IL-2 receptor for their development, and on ID2 transcription factor.61 ILC2 has been found in gastrointestinal, skin and lung tissue in humans. Epithelial cytokines IL-25, IL-3 and TSLP, as well as leukotriene D4, activate ILC2 under specific conditions. Studies in animal models of asthma and AD suggest a role of ILC2 in inflammation (Chart 4).62
자연 살해 세포(NK)
NK 세포는
미생물이나 종양 세포에 감염된 세포를 파괴할 수 있으며,
이러한 세포 표면의 MHC-I 발현이 부족하다는 것을 인식하여
사전 활성화 없이도 세포를 파괴할 수 있습니다.
이들은
퍼포린과 프로테아제 그랜짐을 방출하여
표적 세포 용해를 촉진하고 T
NF-α, IFN-γ, GMCSF, IL-5 및 IL-8과 같은
다양한 사이토카인을 생성합니다.51,57
AD에서는
두 가지 모두와 현장 및 순환하는 NK의 수가 감소합니다.51
영향을 받은 AD 조직에서
NK 세포는 수지상 세포와 밀접하게 접촉하여
활성화된 단핵구와 직접 접촉하는 NK 세포가
세포 사멸의 이상적인 표적임을 나타내며,
이는 Th1 사이토카인 생성을 감소시키고
Th2 면역 반응을 강화하여
미생물 감염에 유리합니다.58
TSLP(흉선 기질 림포포이에틴)와 같은 각질 세포에서 유래한 사이토카인은
NK 세포를 활성화하고
h2 취약 반응을 유도합니다.59
조절 T 림프구(Treg)
AD 환자에서는 순환 조절 T 세포(Treg)(CD4+CD25+FoxP3+ 표현형)가 더 많이 검출되고 면역 억제 활동은 변하지 않습니다.60 이러한 Treg는 초항원 자극 후 면역 억제 활동을 잃는 것으로 보이며, 이는 이러한 환자에서 효과 T 세포 활성화가 증가함을 시사합니다.61 또한 선천 면역계는 Th2, Th17 및 Th22로 분화하는 T 세포 유도 사이토카인을 생산합니다.60,61
선천 면역계의 다른 세포
선천성 림프구 세포(ILC) 그룹은 NK 세포와 비NK 세포(ILC1, ILC2 및 ILC3)로 구성됩니다. 이들은 형태학적으로 림프구와 매우 유사하지만 기존 마커(비-T 및 비-B 세포)의 발현이 부족합니다. 이들은 발달을 위해 IL-2 수용체의 공통 γ 사슬과 ID2 전사인자에 의존합니다.61 ILC2는 사람의 위장관, 피부 및 폐 조직에서 발견되었습니다. 상피 사이토카인 IL-25, IL-3 및 TSLP와 류코트리엔 D4는 특정 조건에서 ILC2를 활성화합니다. 천식 및 AD 동물 모델 연구에서는 염증에서 ILC2의 역할을 시사합니다(도표 4).62
Chart 4
Key topics on the innate immune system (part 2)
Atopic dermatitis and innate immune system (2):
| • Dendritic cells (DCs) |
| • Reduced plasmacytoid dendritic cells in skin injured areas by AD, facilitating certain viral skin infections |
| • IDECs can be modulated by calcineurin inhibitors and topical corticosteroids |
| • Natural killer cells (NK) |
| • Reduced NK cells in AD |
| • TSLP (thymic stromal lymphopoietin) activates NK cells and induces Th2 cytokines secretion |
| • Regulatory T cells (Tregs) |
| • Increased circulating Treg |
| • Tregs lose their immunosuppressive activity with superantigens of S. aureus |
| • Non-NK innate lymphoid cells (ILC) |
| • Inflammation-promoting role of ILC-2 in animal |
| models of asthma and AD |
Skin Microbiome
There is a wide group of microorganisms that colonize the skin; rather than passive inhabitants, they actively interact with host cells and influence the innate immune response.63 There is poor bacterial diversity in active lesions of AD, with predominance of S. aureus; once the patient reaches control, the bacterial milieu is then at least partially recovered. Interestingly, the number of commensal bacteria (Staphylococcus epidermidis) increases during exacerbations of AD, suggesting a compensatory mechanism to control S. aureus.64 S. epidermidis produces two AMP (phenol-soluble modulins γ and δ), which are selective for skin pathogens, such as S. aureus, group A Streptococcus, and Escherichia coli, but do not combat S. epidermidis.65 Furthermore, LTA released by S. epidermidis inhibits skin inflammation during tissue damage, through a TLR2-dependent mechanism.66 Finally, small molecules secreted by S. epidermidis increase the expression of human β-defensin by human keratinocytes through TLR2 signaling pathway. These findings evidence a potential inhibition of the skin microflora on survival of cutaneous pathogens, while promoting recovery of the normal skin microbiota.32
The skin microbiota in patients with AD is altered by endogenous factors, such as FLG mutation, or exogenous stimuli, such as soaps, topical corticosteroids and antibiotics, leading to a modified/non-effective response of the host to allergens, pathogens and tissue damage.32
피부 마이크로바이옴
피부에는
다양한 미생물이 서식하고 있으며,
이들은 수동적으로 서식하는 것이 아니라
숙주 세포와 적극적으로 상호작용하며
선천성 면역 반응에 영향을 미칩니다.63
활성 아토피 피부염 병변에는
박테리아 다양성이 낮고
황색포도상구균이 우세하며,
일단 환자가 통제에 도달하면 세균 환경은 적어도 부분적으로 회복됩니다.
흥미롭게도,
AD가 악화되는 동안
공생 박테리아(표피상구균)의 수가 증가하여
황색포도상구균을 제어하는 보상 메커니즘이 있음을 시사합니다.64
S. epidermidis는
두 가지 AMP(페놀 용해성 모듈린 γ 및 δ)를 생성하는데, 이
는 황색포도상구균,
A군 연쇄상구균,
대장균 등 피부 병원균에 선택적이지만
S. epidermidis와는 싸우지 않습니다.65
또한, 표피상피세포에서 분비되는 LTA는 TLR2 의존적 메커니즘을 통해 조직 손상 시 피부 염증을 억제합니다.66 마지막으로, 표피상피세포에서 분비되는 저분자는 TLR2 신호 전달 경로를 통해 인간 각질 세포의 인간 β-디펜신 발현을 증가시킵니다. 이러한 결과는 피부 미생물이 피부 병원균의 생존을 억제하는 동시에 정상적인 피부 미생물의 회복을 촉진할 수 있다는 가능성을 보여줍니다.32
아토피 피부염 환자의 피부 미생물은 FLG 돌연변이와 같은 내인성 요인이나 비누, 국소 코르티코스테로이드 및 항생제와 같은 외인성 자극에 의해 변화되어 알레르겐, 병원균 및 조직 손상에 대한 숙주의 반응이 변형되거나 비효과적으로 변합니다.32
CONCLUSION
Changes in skin barrier seem to play an undoubtful role in the pathogenesis of AD, connecting the structural changes with the innate and adaptive immune system. AD is a prevalent dermatosis, especially among the pediatric population, but may evolve into a refractory disease, non-responsive to standard anti-inflammatory/immunosuppressive drugs that are currently available. The search of a better understanding of AD pathogenesis will trigger new specific therapeutical targets.
Footnotes
*Study performed atDermatology Department, Faculty of Medicine of Universidade de São Paulo (USP) - São Paulo (SP), Brazil.
Financial Support: FUNADERSP.
Conflict of Interest: None.
REFERENCES
1. Kapoor R, Menon C, Hoffstad O, Bilker W, Leclerc P, Margolis DJ. The prevalence of atopic triad in children with physician-confirmed atopic dermatitis. J Am Acad Dermatol. 2008;58:68–73. [PubMed] [Google Scholar]
2. Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI, Group IPTS Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251–1258. e23. [PubMed] [Google Scholar]
3. Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008;121:1337–1343. [PMC free article] [PubMed] [Google Scholar]
4. Agrawal R, Woodfolk JA. Skin barrier defects in atopic dermatitis. Curr Allergy Asthma Rep. 2014;14:433–433. [PMC free article] [PubMed] [Google Scholar]
5. Addor FA, Aoki V. Skin barrier in atopic dermatitis. An Bras Dermatol. 2010;85:184–194. [PubMed] [Google Scholar]
6. Morita K, Miyachi Y, Furuse M. Tight junctions in epidermis: from barrier to keratinization. Eur J Dermatol. 2011;21:12–17. [PubMed] [Google Scholar]
7. Brown SJ, McLean WH. One remarkable molecule: filaggrin. J Invest Dermatol. 2012;132:751–762. [PMC free article] [PubMed] [Google Scholar]
8. Eckhart L, Lippens S, Tschachler E, Declercq W. Cell death by cornification. Biochim Biophys Acta. 2013;1833:3471–3480. [PubMed] [Google Scholar]
9. Sun R, Celli A, Crumrine D, Hupe M, Adame LC, Pennypacker SD, et al. Lowered humidity produces human epidermal equivalents with enhanced barrier properties. Tissue Eng Part C Methods. 2015;21:15–22. [PMC free article] [PubMed] [Google Scholar]
10. Thyssen JP, Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 2014;134:792–799. [PubMed] [Google Scholar]
11. Wong WJ, Richardson T, Seykora JT, Cotsarelis G, Simon MC. Hypoxia-inducible factors regulate filaggrin expression and epidermal barrier function. J Invest Dermatol. 2015;135:454–461. [PMC free article] [PubMed] [Google Scholar]
12. Fluhr JW, Elias PM, Man MQ, Hupe M, Selden C, Sundberg JP, et al. Is the filaggrinhistidine-urocanic acid pathway essential for stratum corneum acidification. J Invest Dermatol. 2010;130:2141–2144. [PMC free article] [PubMed] [Google Scholar]
13. Vavrova K, Henkes D, Struver K, Sochorova M, Skolova B, Witting MY, et al. Filaggrin deficiency leads to impaired lipid profile and altered acidification pathways in a 3D skin construct. J Invest Dermatol. 2014;134:746–753. [PubMed] [Google Scholar]
14. Miajlovic H, Fallon PG, Irvine AD, Foster TJ. Effect of filaggrin breakdown products on growth of and protein expression by Staphylococcus aureus. J Allergy Clin Immunol. 2010;126:1184–1190. e3. [PMC free article] [PubMed] [Google Scholar]
15. Nomura T, Akiyama M, Sandilands A, Nemoto-Hasebe I, Sakai K, Nagasaki A, et al. Specific filaggrin mutations cause ichthyosis vulgaris and are significantly associated with atopic dermatitis in Japan. J Invest Dermatol. 2008;128:1436–1441. [PubMed] [Google Scholar]
16. Kezic S, O'Regan GM, Yau N, Sandilands A, Chen H, Campbell LE, et al. Levels of filaggrin degradation products are influenced by both filaggrin genotype and atopic dermatitis severity. Allergy. 2011;66:934–940. [PMC free article] [PubMed] [Google Scholar]
17. Brown SJ, Asai Y, Cordell HJ, Campbell LE, Zhao Y, Liao H, et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol. 2011;127:661–667. [PMC free article] [PubMed] [Google Scholar]
An Bras Dermatol. 2016 Jul-Aug; 91(4): 472–478.
doi: 10.1590/abd1806-4841.20164412
PMCID: PMC4999106
PMID: 27579743
Skin barrier in atopic dermatitis: beyond filaggrin*
Mariana Colombini Zaniboni,1 Luciana Paula Samorano,1 Raquel Leão Orfali,1 and Valéria Aoki1
Author information Article notes Copyright and License information PMC Disclaimer
Abstract
Atopic dermatitis is a chronic inflammatory skin disease with a complex pathogenesis, where changes in skin barrier and imbalance of the immune system are relevant factors. The skin forms a mechanic and immune barrier, regulating water loss from the internal to the external environment, and protecting the individual from external aggressions, such as microorganisms, ultraviolet radiation and physical trauma. Main components of the skin barrier are located in the outer layers of the epidermis (such as filaggrin), the proteins that form the tight junction (TJ) and components of the innate immune system. Recent data involving skin barrier reveal new information regarding its structure and its role in the mechanic-immunological defense; atopic dermatitis (AD) is an example of a disease related to dysfunctions associated with this complex.
Keywords: Antimicrobial cationic peptides, Claudins, Dermatitis, atopic, Immunity, innate
INTRODUCTION
Atopic dermatitis (AD) is a highly prevalent dermatosis in the population, especially in children. It has a chronic, inflammatory and pruriginous nature and progresses with periods of exacerbation. Its increasing prevalence in recent decad ranges from 10% to 20% in children and reaches 3% in adults. 1,2 AD can be associated with other manifestations of atopic disease such as asthma and rhinitis, which occur more frequently in patients with recalcitrant AD.1
For many years, the altered immune response in AD has been considered the main mechanism for inflammation and changes in skin permeability (inside-ouside theory). However, the outside-inside theory was then conceived, and the skin barrier defects in AD proved to exert a key role in the pathogenesis of AD.3,4
This review aims to focus on dysfunction of proteins of the skin barrier (filaggrin and claudins 1 and 4) and of components of the innate immune system (pattern recognition receptors, secretory elements, predominant cells of the innate immune system and skin microbiota) in AD patients, which contribute to the constant AD phenotype of xerosis, inflammation and susceptibility to infections.
SKIN BARRIER
Protection and defense are the main functions of the skin. Regulation of the transepidermal water loss (TEWL), defense against the action of external physico-chemical agents and aggression of microorganisms are part of the skin barrier. The stratum corneum (SC) is the main component of such barrier, and is based on the "brick and mortar" structure. Its filmogenic feature is due to the association of SC with surface lipids.5
Filaggrin and proteins of the tight junctions (TJs) have been the most studied components of the skin barrier. Filaggrin, after hydrolyzed, contributes to the formation of relevant components for pH maintenance, moisture and skin protection against microbial agents. TJ protein with active expression, on the other hand, are important to control the selective permeability of the epidermis to build the barrier against the external environment, therefore promoting recognition of the cell "territory".6
Skin barrier proteins with functional relevance
Filaggrin
Filaggrin is a protein originated from pro-filaggrin, produced by keratinocytes. It is the main component of keratohyalin granules, visualized by light microscopy within the granular layer. Conversion of pro-filaggrin into filaggrin, both intracellular proteins, occurs through dephosphorylation and proteolysis by serine proteases, releasing multiple active monomers of filaggrin.7 With the decrease of the water gradient in the outer layers of the epidermis, filaggrin hydrolysis occurs in hygroscopic amino acids.8,9 Factors such as age, ultraviolet B radiation, relative humidity and hypoxia affect this process.10,11
Hygroscopic amino acids, especially arginine, glutamine and histidine are detected within the intercellular space.10 They generate the natural moisturizing factors (NMF), responsible for the maintenance of SC and pH hydration for the production of urocanic acid (UCA) in its cis and trans forms, as well as 5- pyrrolidone carboxylic acid (PCA).10,12,13 Furthermore, these two byproducts filaggrin have inhibitory effects on the Staphylococcus aureus (S. aureus) growth.14
Changes in barrier proteins, such as decreased expression of filaggrin in the skin, and mutations with loss of function in filaggrin gene (FLG), such as those found in ichthyosis vulgaris, have been described in AD.10,15 These mutations lead to increased risk of early onset of the disease, respiratory atopy, allergies, elevated IgE serum levels and persistence of AD in adulthood.16 Moreover, there is a significant relationship between AD with FLG mutation and peanut allergy mediated by IgE, indicating an increased skin permeability and consequent enhanced exposure to allergens.17
Interleukins (IL) 4 and 13, detected in AD lesions, also lead to decreased expression of filaggrin in keratinocytes.18 The family of IL-1 has relevant pro-inflammatory features, such as IL-1α, suggesting that the onset of inflammation may occur due to changes in the skin barrier. Morevoer, there are reports of decrease of NMF in the SC of individuals with AD and mutations in the FLG gene, with increase of IL-1 family cytokines in non-inflammed skin.19
Therefore, patients with AD and deficiency in FLG expression have decreased SC hydration, increased TEWL, and higher pH than non-atopic individuals, with an augmented risk of developing allergies, asthma and rhinitis.20 Changes in the skin barrier due to filaggrin deficiency may also lead to inflammation, and reduced protein expression in keratinocytes (Chart 1).
Chart 1
Key topics on filaggrin
ATOPIC DERMATITIS AND FILAGGRIN:
| • Filaggrin gene (FLG) mutation |
| • Decreased filaggrin expression: |
| • higher risk of early onset of the disease |
| • persistence of atopic dermatitis in adulthood |
| • increased risk of allergies by percutaneous sensitization |
| • association with high serum levels of IgE and other manifestations of atopy |
Tight junctions
TJ are formed by a complex of transmembrane and intracellular proteins found in simple and stratified mammalian epithelia. In normal skin, they are detected in the granular layer, and its expression rapidly increases after injury. They are essential for cell differentiation and keratinization of epidermal cells.6 In skin diseases with altered keratinization, such as psoriasis and ichthyosis vulgaris, they are present even in the deeper layers of the epidermis.21
TJ play an important role in epidermal selective permeability, controlling intercellular flow of substances such as hormones, cytokines and electrolytes, functioning as "gates". This permeability depends directly on the size and ionic specificity of the molecule. In addition to the intercellular permeability function, these structures also act as markers of the cell "territory".22
The intracellular portion of TJ binds to cytoskeletal plasma proteins, while the extracellular portion forms a "loop" in the intercellular space, connecting with the adjacent cell loop. TJ are formed by occludin, claudin, zonula occludens 1 (ZO1) and 2 (ZO2), junctional adhesion molecule-1 (JAM1) and the multi-PDZ-1 protein (MUPP1).23
In 2002, Tsukita and Furuse showed that claudin 1 deficiency in mice led to high TEWL and liver abnormalities, culminating with death.22 These animals showed no structural abnormalitie, but significant loss of function of the skin barrier. A similar clinical condition of claudin 1 deficiency was described in human neonates (ichthyosis-sclerosis-cholangitis syndrome).24
TJ proteins also play an important role in the invasion of some viruses (e.g.: herpes simplex) and bacteria. Some of these organisms use claudin 1 as receptors; others modulate the structure of TJ, inserting effectors, activating signals or even directly connecting to them, resulting in their partial break.25,26 Its expression rapidly increases via activation of toll-like receptor 2 (TLR2).27
The lesional skin of atopic patients contains significant decreased claudin 1 expression, but no claudin 4 reduction, when compared to the skin of non-atopic individuals (Figure 1).28-30 Reduced claudin 1 appears to be related to increased risk of infection by herpes virus type 1 (HSV1) in individuals with AD.25 There is also an inverse relation between the expression of claudin 1 and the presence of the immune response markers Th2, suggesting that this protein affects the immune response to potential environmental allergens (Chart 2).28

Expression of filaggrin (FLG), claudin 1 (CLDN1) and claudin 4 (CLDN4) in skin fragments of adults with atopic dermatitis (AD) stained by immunohistochemistry. (A) Skin fragments of healthy controls: FLG, CLDN1 and CLDN4. (B) Skin fragments of patients with AD, showing reduced expression of FLG, CLDN1 and CLDN4. (C) Expression of FLG, CLDN1 and CLDN 4 (area percentage) in the control group without AD (n=33) compared with patients with AD (n=25). (D) Correlation between disease severity (EASI) and the expression of the proteins in the skin barrier. The line represents the arithmetic mean of the expression of proteins in the skin barrier (percentage area). ** p≤0.01 and *** p≤0.001
Adapted from Batista, et al. 2015.29
Chart 2
Key topics on tight junctions
ATOPIC DERMATITIS AND TIGHT JUNCTIONS:
| • Decreased expression on the skin without injury |
| • Favors infection by herpes-virus type 1 and other viral skin infections |
| • Increased paracellular permeability |
| • Increased risk of allergy to large molecules |
| • Proteins with active expression: |
| • Increase with activation via Toll-like receptor 2 |
| • Decrease with increased Th2 citokynes |
Innate immune system
The innate immune system represents the initial and non-specific response of the human body to external aggressions. This response does not derive or result from target-oriented immune memory, but has an essential role in protecting the individual against potential pathogens. An intact skin barrier is needed, with proper maintenance of its cycle, pH and microbiota. Other componenents of such defense system includes secretory elements, cell receptors, such as pattern recognition receptors (PRR), immune cells and the skin microbiota (Figure 2).

Main components of innate immune system in epidermis and their role in atopic dermatitis (AD). Defects in Toll-like receptor 2 contribute to increased colonization and infection by S. aureus. Decreased AD expression of AMP (catelicidin-LL37 and β-defensin) also favors skin infections. Reduced plasmacytoid dendritic cells in skin injured areas by AD, facilitating certain viral skin infections. Reduced NK cells in AD. S. epidermidis increase the expression of human β-defensin by human keratinocytes through TLR2 signaling pathway. IDEC are increased in AD skin lesion. NOD1 changes are associated with elevated IgE levels in AD individuals. Changes in expression of NLRP1 gene were associated to AD severity

Staphylococcus aureus (S. aureus),

Staphylococcus epidermidis (S. epidermidis),

other bacteria,

staphylococcal enterotox in,

virus,

Toll-like receptors (TLR),

nucleotide-binding oligomerization domain-containing protein (NOD)

NOD-like receptor protein (NLRP)

β-defensina 1,

HBD-2,3 e LL37,

inflammatory dendritic epidermal cells (IDEC),

plasmacytoid DC (pDC).
PATTERN RECOGNITION RECEPTORS (PRR)
The arsenal of PRR comprises members of the toll-like receptors (TLR), nucleotide-binding oligomerization domain-containing protein (NOD-like receptors or NLR), retinoic acid-inducible gene, C-type lectin receptors (CLR) and PGLYRPs (peptidoglycan recognition proteins).31,32
TLR family
TLRs are well-known transmembrane proteins that play as innate receptors. In humans, TLR1-10 have been described and they have the ability to recognize pathogen-associated molecular patterns (PAMPs). TLR1, 2, 4-6,10 are in charge of such recognition on the cell surface, whereas TLR3, 7-9 are found in the endosomes.33 They also recognize endogenous ligands in response to tissue damage, contributing to the maintenance of skin barrier.34,35 TLRs are usually expressed both by innate immune cells, such as DC, NK and macrophages, as well as adaptive immune cells, including T and B cells. Activation of TLR triggers the release of proinflammatory cytokines, therefore modulating the immune response against pathogens. 33
TLR2 is the receptor that recognizes a broad spectrum of PAMPs, including lipopeptides from Gram-positive bacteria, among others.33 There are reports on genetic variants of TLRs that are associated with AD; however, there is emphasis on TLR2, which is capable of recognizing products of the cell wall of S. aureus. AD individuals are more colonized and infected by S. aureus than non-atopic groups, suggesting that mutations of TLR2 may facilitate such susceptibility.36
NLR family
The NLR (NOD like receptors) family has three distinct subfamilies: the NODs (nucleotide-binding oligomerization domain-containing protein), NLRPs (NOD-like receptor protein) and IPAF (ice protease-activating factor).37
NOD receptors are intracellular receptors that respond to a diversity of microbial products.38 NOD1 (also known as CARD4 - caspase activation and recruitment domain 4), selectively respond to Gram-negative bacteria, and NOD2 recognizes a fragment common to all bacteria. NOD1 changes are associated with elevated IgE levels in AD individuals, and are important indicative factors of atopy susceptibility.39 NOD2 mutations that might result in inappropriate immunomodulation, are not only associated with autoimmune diseases but also with AD.40
NRLPs respond to a large variety of ligands, such as DAMPs (damage-associated molecular patterns), ATP and urate crystals, and exogenous agents, such as asbestos and silica. These receptors form a multiprotein complex, named inflammasome, which leads to the production of IL-1β and IL-18 by activation of caspase 1.37,41 There are four main subclasses of inflammasomes: NRLP3, NLRP1, IPAF (also known as NLRC4-NLR Family, CARD Domain Containing 4) and AIM2 (absent in melanoma 2).37
Changes in expression of NLRP1 gene were associated to AD severity.42 Impaired NLRP3 expression may partially explain how skin colonization and infection with S. aureus can contribute to chronic skin inflammation in AD.43 Increased epidermal expression of IL-1β cytokine has been observed in AD patients presenting FLG mutations.19 It was demonstrated enhanced levels of IL-18 both in sera and culture supernatants under staphylococcal enterotoxin A stimuli in AD patients.44
CLR family
C-Type Lectin Receptors (CLRs) contain C-type lectin-domains, therefore recognizing sugars present in microorganisms. KACL (keratinocyte-associated C-type lectin), expressed by human keratinocytes, is highlighted in this group. It triggers cytolytic activity of Natural killer (NK) cells and cytokine secretion; despite changes in the expression and function of this receptor have not been described in AD, atopic patients exhibit defective cytotoxicity of NK cells.45
Antimicrobial peptides (AMP)
AMPs play an important role in the skin innate immunity acting as endogenous antibiotics. Cathelicidin (LL37) and β-defensin family are the main AMPs, but other keratinocyte products are also recognized for their anti-microbial functions, such as ribonuclease (RNase), S100 family, dermcidin and regenerating islet-derived (REG3α).46
While human β-defensin 1 (HDB1) is expressed by normal human keratinocytes, dermal inflammation induces expression of HBD2, HBD3 and LL37. AD skin lesions have is significantly lower levels of AMPs than psoriatic lesions. Reduced expression and secretion of AMPs may contribute to increased susceptibility to skin infections by viruses, bacteria and fungi in AD patients (Chart 3).32
Chart 3
Key topics on the innate immune system (part 1)
Atopic dermatits and innate immune system
| • Changes in pathogen recognition receptors |
| • Defects in Toll-like receptor 2 contribute to increased |
| colonization and infection by S. aureus |
| • Anti-mycobians peptides (AMPs) |
| • Main AMPs: catelicidin (LL37) and β-defensin |
| • Decreased AD expression. Favors skin infections |
Dendritic cells (DC)
DC belong to the family of antigen-presenting cells, and are known as sentinels of the immune system, recognizing and presenting antigens, leading to T cell activation.47,48 They lack other markers of leukocyte lineages (CD3, 14, 16, 19, 20, and 56), and express high levels of MHC class II (HLA-DR) molecules.49 A DC lineage-specific marker has not yet been identified, and the subsets of DC in humans and mice are therefore currently defined by lineage − MHC II+ cells, in combination with various cell surface markers.50
There are two major human DC subsets: CD11c+ myeloid DC (mDC) and CD123+ plasmacytoid DC (pDC); mDC are efficient in the uptake, processing, and presentation of foreign antigens, and under Toll-like receptor (TLR) stimulation, induce secretion of tumor necrosis factor α (TNF-α) and proinflammatory cytokines, such as IL-12. Conversely, pDC are less effective in these processes and mainly known for their function in antiviral immunity.50 The pDC are a critical source for the antiviral type I IFNs (IFNα and IFNβ), and a reduction of these cells in AD skin, facilitate viral skin infections such as eczema herpeticum.38,51
In AD, a single population of inflammatory DC is well described, which belongs to mDC group. They were initially named inflammatory dendritic epidermal cells (IDEC) based on flow cytometry analysis of cells from epidermal suspensions 52-54. IDEC were defined by the following: HLA-DR+LIN-CD11c+CD1a+ and co-express CD206, CD36, FceRI, IgE, CD1b/c, CD11b, among others.55 Yet, IDECs can be modulated by calcineurin inhibitors and topical corticosteroids.51,56
Natural killer cells (NK)
NK cells are capable of destroying cells infected by microorganisms and tumor cells, without previous activation by reconizing the lack of MHC-I espression on the surface of such cells. They release perforins and protease granzime, promoting target cell lysis, and produce a large variety of cytokines, such as TNF-α, IFN-γ, GMCSF, IL-5 and IL-8.51,57 In AD, there is a reduced number of both and in situ and circulating NK.51 In the affected AD tissue, NK cells are in close contact with dendritic cells, indicating that NK cells in direct contact with activated monocytes are ideal targets for apoptosis; this would lead to reduced Th1 cytokine production, and enhanced Th2 immune response, favoring microbial infection.58 Cytokines derived from the keratinocyte, such as TSLP (thymic stromal lymphopoietin), activate NK cells and induce Th2-prone response.59
Regulatory T lymphocytes (Treg)
In patients with AD, circulating regulatory T cells (Treg) (CD4+CD25+FoxP3+ phenotype) are detected in greater numbers and with unchanged immunosuppressive activity.60 These Tregs seem to lose their immunosuppressive activity after stimulation with superantigens, suggesting an increase of effector T cell activation in such individuals.61 Furthermore, the innate immune system produces cytokines inducers of T cells differentiation into Th2, Th17 and Th22.60,61
Other cells of the innate immune system
The innate lymphoid cells (ILCs) group comprises NK cells and ILCs non-NK cells (ILC1, ILC2 and ILC3). They are morphologically very similar to lymphocytes, but lack expression of conventional markers (non-T and non-B cells). They depend on the common γ chain of IL-2 receptor for their development, and on ID2 transcription factor.61 ILC2 has been found in gastrointestinal, skin and lung tissue in humans. Epithelial cytokines IL-25, IL-3 and TSLP, as well as leukotriene D4, activate ILC2 under specific conditions. Studies in animal models of asthma and AD suggest a role of ILC2 in inflammation (Chart 4).62
Chart 4
Key topics on the innate immune system (part 2)
Atopic dermatitis and innate immune system (2):
| • Dendritic cells (DCs) |
| • Reduced plasmacytoid dendritic cells in skin injured areas by AD, facilitating certain viral skin infections |
| • IDECs can be modulated by calcineurin inhibitors and topical corticosteroids |
| • Natural killer cells (NK) |
| • Reduced NK cells in AD |
| • TSLP (thymic stromal lymphopoietin) activates NK cells and induces Th2 cytokines secretion |
| • Regulatory T cells (Tregs) |
| • Increased circulating Treg |
| • Tregs lose their immunosuppressive activity with superantigens of S. aureus |
| • Non-NK innate lymphoid cells (ILC) |
| • Inflammation-promoting role of ILC-2 in animal |
| models of asthma and AD |
Skin Microbiome
There is a wide group of microorganisms that colonize the skin; rather than passive inhabitants, they actively interact with host cells and influence the innate immune response.63 There is poor bacterial diversity in active lesions of AD, with predominance of S. aureus; once the patient reaches control, the bacterial milieu is then at least partially recovered. Interestingly, the number of commensal bacteria (Staphylococcus epidermidis) increases during exacerbations of AD, suggesting a compensatory mechanism to control S. aureus.64 S. epidermidis produces two AMP (phenol-soluble modulins γ and δ), which are selective for skin pathogens, such as S. aureus, group A Streptococcus, and Escherichia coli, but do not combat S. epidermidis.65 Furthermore, LTA released by S. epidermidis inhibits skin inflammation during tissue damage, through a TLR2-dependent mechanism.66 Finally, small molecules secreted by S. epidermidis increase the expression of human β-defensin by human keratinocytes through TLR2 signaling pathway. These findings evidence a potential inhibition of the skin microflora on survival of cutaneous pathogens, while promoting recovery of the normal skin microbiota.32
The skin microbiota in patients with AD is altered by endogenous factors, such as FLG mutation, or exogenous stimuli, such as soaps, topical corticosteroids and antibiotics, leading to a modified/non-effective response of the host to allergens, pathogens and tissue damage.32
CONCLUSION
Changes in skin barrier seem to play an undoubtful role in the pathogenesis of AD, connecting the structural changes with the innate and adaptive immune system. AD is a prevalent dermatosis, especially among the pediatric population, but may evolve into a refractory disease, non-responsive to standard anti-inflammatory/immunosuppressive drugs that are currently available. The search of a better understanding of AD pathogenesis will trigger new specific therapeutical targets.
Footnotes
*Study performed atDermatology Department, Faculty of Medicine of Universidade de São Paulo (USP) - São Paulo (SP), Brazil.
Financial Support: FUNADERSP.
Conflict of Interest: None.
REFERENCES
1. Kapoor R, Menon C, Hoffstad O, Bilker W, Leclerc P, Margolis DJ. The prevalence of atopic triad in children with physician-confirmed atopic dermatitis. J Am Acad Dermatol. 2008;58:68–73. [PubMed] [Google Scholar]
2. Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI, Group IPTS Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251–1258. e23. [PubMed] [Google Scholar]
3. Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008;121:1337–1343. [PMC free article] [PubMed] [Google Scholar]
4. Agrawal R, Woodfolk JA. Skin barrier defects in atopic dermatitis. Curr Allergy Asthma Rep. 2014;14:433–433. [PMC free article] [PubMed] [Google Scholar]
5. Addor FA, Aoki V. Skin barrier in atopic dermatitis. An Bras Dermatol. 2010;85:184–194. [PubMed] [Google Scholar]
6. Morita K, Miyachi Y, Furuse M. Tight junctions in epidermis: from barrier to keratinization. Eur J Dermatol. 2011;21:12–17. [PubMed] [Google Scholar]
7. Brown SJ, McLean WH. One remarkable molecule: filaggrin. J Invest Dermatol. 2012;132:751–762. [PMC free article] [PubMed] [Google Scholar]
8. Eckhart L, Lippens S, Tschachler E, Declercq W. Cell death by cornification. Biochim Biophys Acta. 2013;1833:3471–3480. [PubMed] [Google Scholar]
9. Sun R, Celli A, Crumrine D, Hupe M, Adame LC, Pennypacker SD, et al. Lowered humidity produces human epidermal equivalents with enhanced barrier properties. Tissue Eng Part C Methods. 2015;21:15–22. [PMC free article] [PubMed] [Google Scholar]
10. Thyssen JP, Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 2014;134:792–799. [PubMed] [Google Scholar]
11. Wong WJ, Richardson T, Seykora JT, Cotsarelis G, Simon MC. Hypoxia-inducible factors regulate filaggrin expression and epidermal barrier function. J Invest Dermatol. 2015;135:454–461. [PMC free article] [PubMed] [Google Scholar]
12. Fluhr JW, Elias PM, Man MQ, Hupe M, Selden C, Sundberg JP, et al. Is the filaggrinhistidine-urocanic acid pathway essential for stratum corneum acidification. J Invest Dermatol. 2010;130:2141–2144. [PMC free article] [PubMed] [Google Scholar]
13. Vavrova K, Henkes D, Struver K, Sochorova M, Skolova B, Witting MY, et al. Filaggrin deficiency leads to impaired lipid profile and altered acidification pathways in a 3D skin construct. J Invest Dermatol. 2014;134:746–753. [PubMed] [Google Scholar]
14. Miajlovic H, Fallon PG, Irvine AD, Foster TJ. Effect of filaggrin breakdown products on growth of and protein expression by Staphylococcus aureus. J Allergy Clin Immunol. 2010;126:1184–1190. e3. [PMC free article] [PubMed] [Google Scholar]
15. Nomura T, Akiyama M, Sandilands A, Nemoto-Hasebe I, Sakai K, Nagasaki A, et al. Specific filaggrin mutations cause ichthyosis vulgaris and are significantly associated with atopic dermatitis in Japan. J Invest Dermatol. 2008;128:1436–1441. [PubMed] [Google Scholar]
16. Kezic S, O'Regan GM, Yau N, Sandilands A, Chen H, Campbell LE, et al. Levels of filaggrin degradation products are influenced by both filaggrin genotype and atopic dermatitis severity. Allergy. 2011;66:934–940. [PMC free article] [PubMed] [Google Scholar]
17. Brown SJ, Asai Y, Cordell HJ, Campbell LE, Zhao Y, Liao H, et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol. 2011;127:661–667. [PMC free article] [PubMed] [Google Scholar]