BEYOND REASON
레스베라트롤은 암세포의 포도당수용체를 차단하여 암세포 굶기기에 효과적인 포도껍질, 씨 추출물임. 당연히 부작용은 없음.
혈중 포도당이 높아져 있을때 레스베라트롤은 암세포의 포도당수용체를 닫아 암세포를 굶길 수 있음.
식전 3번복용하면 좋을 듯

Review
Glut 1 in Cancer Cells and the Inhibitory Action of Resveratrol as A Potential Therapeutic Strategy
Angara Zambrano 1,2 , Matías Molt 1, Elena Uribe 2 and Mónica Salas 1,*
1 Instituto de Bioquimica y Microbiologia, Universidad Austral de Chile, Valdivia 0000000, Chile
2 Departamento de Bioquímica y Biología Molecular, Facultad de Ciencias, Universidad de Concepción,
Concepción 4070386, Chile
* Correspondence: monicasalas@uach.cl; Tel.: +56-63-229-3869
Received: 7 May 2019; Accepted: 1 July 2019; Published: 9 July 2019
Abstract: An important hallmark in cancer cells is the increase in glucose uptake. GLUT1 is an
important target in cancer treatment because cancer cells upregulate GLUT1, a membrane protein
that facilitates the basal uptake of glucose in most cell types, to ensure the flux of sugar into metabolic
pathways. The dysregulation of GLUT1 is associated with numerous disorders, including cancer and
metabolic diseases. There are natural products emerging as a source for inhibitors of glucose uptake,
and resveratrol is a molecule of natural origin with many properties that acts as antioxidant and
antiproliferative in malignant cells. In the present review, we discuss how GLUT1 is involved in the
general scheme of cancer cell metabolism, the mechanism of glucose transport, and the importance of
GLUT1 structure to understand the inhibition process. Then, we review the current state-of-the-art of
resveratrol and other natural products as GLUT1 inhibitors, focusing on those directed at treating
dierent types of cancer. Targeting GLUT1 activity is a promising strategy for the development of
drugs aimed at treating neoplastic growth.
Keywords: GLUT1; glucose uptake inhibition; cancer therapy; cancer metabolism; resveratrol
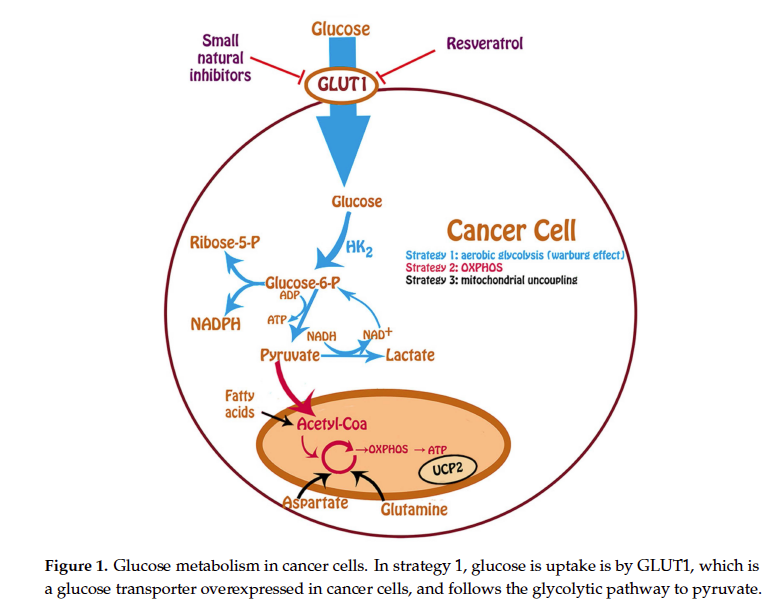
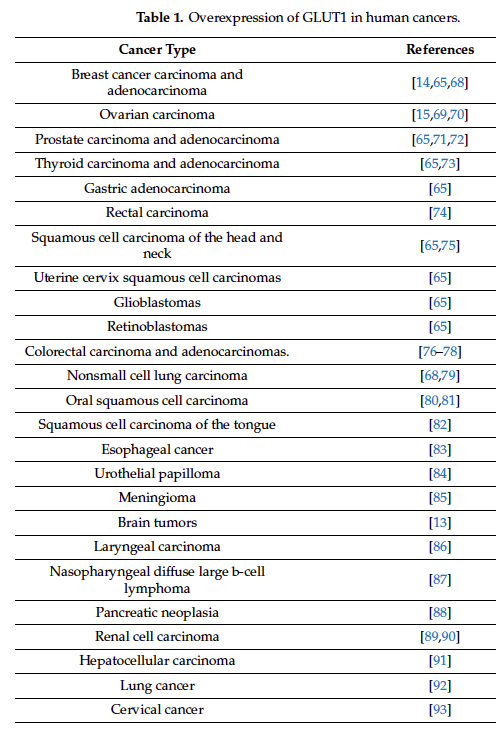
Inhibition of Glucose Uptake by Resveratrol
Resveratrol (3,5,40-trihydroxystilbene or RSV) is a polyphenolic natural product that attracted great interest mainly due to its anticarcinogenic, anti-inflammatory, and cardioprotective properties [111–113].
RSV has structural similarities with tyrosine kinases that are known inhibitors of GLUT1 [114,115]. For this reason, we performed experiments in order to investigate the relationship between RSV and this transporter. By using kinetic assays, we observed for the first time that RSV inhibit the glucose uptake in human leukemic cell lines U-937 and HL-60, by a direct interaction with the internal face of GLUT1, in a noncompetitive mode [116].
With regard to RSV and glucose uptake, studies in dierent human ovarian cancer cells have shown that treatments with RSV were able to inhibit glucose uptake, lactate production, Akt, and mTOR
signaling, and cell viability depending on the dose and time used [117–119]. There are a great number of studies relating RSV and glucose uptake in cancer cells and in pathological conditions such as
insulin resistance that we summarize in Table 2.
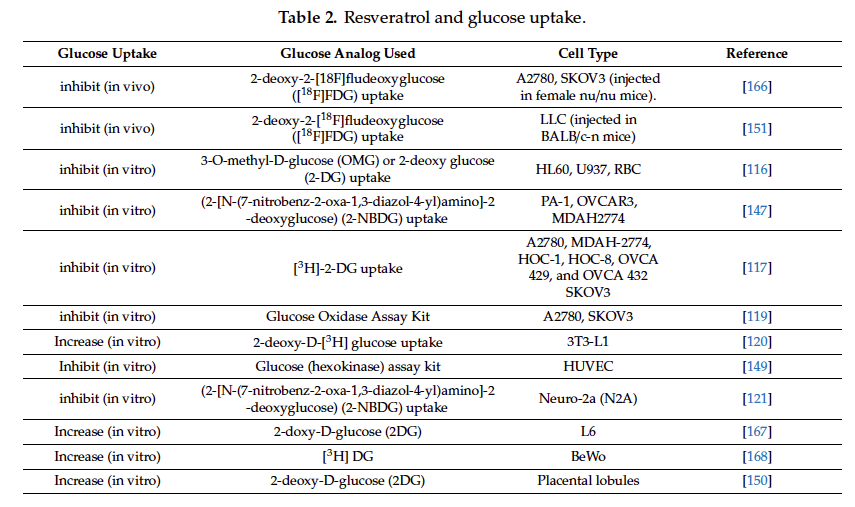
Most of the experiments were done in vitro, but also in vivo using labeled glucose analogs. In cancer cells it is observed that resveratrol inhibited the uptake of glucose, favoring the anticancer action; but in pathological conditions such as insulin resistance or diabetes, resveratrol increased glucose uptake and insulin sensitivity favoring the anti diabetic effect [120]. Other studies in neuronal cells showing that RSV had an inhibitory effect of the glucose uptake, favoring the neuronal regulation of glucose and insulin sensitivity [121]. Among other mechanisms of action RSV also targets a great number of intracellular molecules implicated in cell cycle control and apoptosis induction [122–126].
RSV is an attractive candidate for cancer therapy because of its unique capacity to affect the mTOR/AMPK pathway at different levels. By inhibiting mTOR and ribosomal protein S6 kinase, and by activating AMPK, RSV exerts a potent short-term effect on metabolism. The type of cell death observed in cancer cells treated with RSV has been reported as apoptosis or autophagy [118,119]. Van Ginkel et al. [127] concluded that elevated levels of RSV lead
to tumor regression and widespread tumor cell death. The underlying mechanism involves direct
activation of the intrinsic and extrinsic apoptotic pathway. Thus, in normal adipocytes, it has been
observed that RSV induces apoptosis at concentrations greater than 20 M, while in insulin-resistant
adipocytes; RSV stimulates glucose transport via SIRT1-AMPK-Akt. These results suggest that RSV
can behave dierently according to the dose used and the cell type and the metabolic state [120].
Recently, Dai et al. [128] showed that RSV inhibits the growth and proliferation of MGC-803 cells
of gastric cancer in a dose- and time-dependent way by downregulating the expression of genes
related with Wnt pathway as -catenin, c-myc, and cyclin D1, proteins related with GLUTs gene
regulation. On the other hand, Kleszcz, et al. [129] did not observe inhibition of c-myc gene expression
by resveratrol in FaDu hypopharyngeal carcinoma cells, but the doses used were significantly lower.
With respect to autophagy, a number of studies show that RSV induces autophagy and cell death in
cancer cells when they are nutrient-deprived and that RSV could act by inducing a starvation-like
signaling response [117]. Indeed, activation of the JNK pathway by RSV leads to the induction of genes that participate in both the initial and late steps of autophagy in CML cells [130 ,131 ]. RSV could also
impact mitochondrial membrane potential, the respiration chain, and ATP synthesis [132 ].
RSV also stimulates the molecular pathway dependent on sirtuins, histone deacetylases,
that regulates the activity of transcription factors in many tissues related to energy metabolism [133 ].
RSV induces the expression of silent information regulator-6 (SIRT6) in hypopharyngeal carcinoma
FaDu cell line [129 ]. SIRT6 influences the expression of several glycolytic genes such as GLUT1, aldolase, pyruvate dehydrogenase kinase 1 (PDK1), and phosphofructokinase 1 (PFK1) [134 ]. Furthermore, RSV is known as an activator of SIRT1 and has been related to diabetes progress as a target for its treatment [135 ,136 ], inflammation, and neuroprotection [137 ]. There are data suggesting that RSV has hypoglycemic properties in diabetic rats and restore glycolytic enzyme activities [138 ,139 ].
With respect to the repertoire of glycolytic isoenzymes involved in the action of RSV, Iqbal et al.
observed that RSV down-regulate pyruvate kinase 2 (PKM2) expression by inhibiting mTOR signaling
and suppressed cancer metabolism, characterized by a decreased glucose uptake, lower lactate
production (aerobic glycolysis), and reduced anabolism (macromolecule synthesis) in various cancer
cell lines [140 ]. Recently, it was observed that RSV, by targeting PKM2 and ERK1/ 2, destabilizes BCL-2 protein level finally leading to apoptosis in human melanoma cells [141 ]. In addition, the re-expression of the embryonic isoenzyme M2 of pyruvate kinase in cancerous cells has been related to a stronger glycolytic phenotype and a proliferative advantage in hypoxic conditions [142 ]. HK2 seems to
be associated with mitochondria, linking ATP production in this organelle to cytosolic glucose
phosphorylation. The release of mitochondrial HK2 could explain the increase in cytosolic hexokinase
activity implicated in the onset of apoptosis [143 ]. In cardiomyocytes, during anoxia/ deoxygenation
injury, RSV exerts a protective e ect by promoting the linkage of voltage-dependent anion channel 1
(VDAC1) to HK2 [144 ]. HK2 links up with VDAC1 forming a polymeric channel that finally stimulates cell survival [145 ].
Recently, RSV showed a decrease in mRNA and protein levels of GLUT1, HK2, PFK1, and PKM2
which finally caused inhibition of aerobic glycolysis in a study of VEGF-angiogenesis in human
umbilical vein endothelial cells, putting forth the role of RSV in the regulation of pathological
angiogenesis [146 ].
Specifically, RSV exerts effects on the GLUT1 transporter at different levels, such as:
(a) A direct inhibitory action on the protein, which has been demonstrated by kinetic assays on cancer cells (116) and interrupting the traffic to the plasma membrane [147 ].
(b) An inhibitory effect on mRNA expression for GLUT [145 ,148 –150 ]
(c) Regulating many transcription factors that in turn regulate the expression of GLUT1 such as HIF-1alpha and c-Myc [129 ,151 ]
(d) Regulating GLUT1 expression through various signaling pathways such as: AMPK [147 ], Wnt [127 ,128 ], Jnk kinases [129 ,130 ], sirtuins and histone deacetylases [132 ].
(e) Also regulating miRNA expression of GLUT1.
RSV also is able to regulate glucose uptake, metabolism and signaling pathway in cancer cells
through regulation of specific microRNAs (miRNAs). Resveratrol a ects the miRNA machinery in
positive and negative manners; it is suggested that this regulatory activity is likely to be advantageous for cancer treatment and prevention. There are many miRNAs that are dysregulated in cancers [152 –157 ].
In resveratrol-treated prostate cancer cells there are significant upregulations of 28 miRNAs and
downregulations of 23 miRNAs. Among these, two miRNA clusters, such as miR-17-92 and miR-106ab, are known oncomirs. Subsequent analyses showed significant downregulation of these oncomirs in
resveratrol-treated prostate cancer cells [158 ]. In the case of breast cancer, miR-663 and miR-744 have been found to negatively regulate eEF1A2, resveratrol induces a 4.5-fold upregulation of miR-663 and a two-fold increase in miR-744 [152 ], resveratrol also controls breast cancer cell proliferation by
inducing tumor-suppressive miRNAs (miR-34a, miR-424, and miR-503) via the p53 pathway and
then by suppressing heterogeneous nuclear ribonucleoprotein A1 (HNRNPA1), which is associated
with tumor progression [159 ]. There are many interesting studies about the molecular mechanisms associating resveratrol and miRNA regulation in cancer. For example, resveratrol targets the oncogenic
expression of miR-21, thus blocking the PI3K-Akt signaling [160 ], and it also reduces MMP2 via
upregulation of miR-328 in osteosarcoma cells [161 ].
Several miRNAs have an important impact on metabolism in cancer cells. Some miRNAs
such as miR-150 were consistently decreased in cell lines and osteosarcoma tissues as compared
to osteoblast cells and normal bone; the ectopic overexpression of miR-150 inhibits osteosarcoma
cell proliferation and suppresses glucose uptake. On the other hand, loss of function of miR-150
enhanced osteosarcoma cell proliferation and increased glucose uptake and lactate secretion [107 ].
In colon cancer cells, the miRNA-143 overexpression inhibits glucose uptake and glucose transporter
1 (GLUT1) expression [162 ]. Many miRNAs are associated to regulated GLUTs expression, for
example; miR-21a-5p, miR-29a-3p, miR-29c-3p, miR-93-5p, miR-106b-5p, miR-133a-3p, miR-133b-3p,
miR-222-3p, and miR-223-3p have been reported to directly and/ or indirectly regulate the GLUT4
expression; and their expressions are altered principally in the diabetes condition [163 ].
Recent evidences demonstrate that miRNAs play important roles in certain e ects of resveratrol
on cell metabolism. Pyruvate kinase M2 (PKM2) has been found to be overexpressed in di erent
cancers [164 ]; it has been shown that resveratrol represses PKM2 by increasing the expression of
miR-326. Also, resveratrol improves mitochondrial function. Specifically, the miR-27b was significantly
induced in a dose-dependent way in skeletal muscle and C2C12 myoblast treated with resveratrol,
and miR-27b overexpression improves mitochondrial function in a Sirt1-dependent manner [165 ].
Genistein


Abstract
Background:
Genistein is a natural isoflavone with many health benefits, including antitumour effects. Increased hypoxia-inducible factor 1 α (HIF-1α) levels and glycolysis in tumour cells are associated with an increased risk of mortality, cancer progression, and resistance to therapy. However, the effect of genistein on HIF-1α and glycolysis in hepatocellular carcinoma (HCC) is still unclear.
Methods:
Cell viability, apoptosis rate, lactate production, and glucose uptake were measured in HCC cell lines with genistein incubation. Lentivirus-expressed glucose transporter 1 (GLUT1) or/and hexokinase 2 (HK2) and siRNA of HIF-1α were used to test the direct target of genistein. Subcutaneous xenograft mouse models were used to measure in vivoefficacy of genistein and its combination with sorafenib.
Results:
Genistein inhibited aerobic glycolysis and induced mitochondrial apoptosis in HCC cells. Neither inhibitors nor overexpression of HK2 or GLUTs enhance or alleviate this effect. Although stabiliser of HIF-1αreversed the effect of genistein, genistein no longer has effects on HIF-1α siRNA knockdown HCC cells. In addition, genistein enhanced the antitumour effect of sorafenib in sorafenib-resistant HCC cells and HCC-bearing mice.
Conclusions:
Genistein sensitised aerobic glycolytic HCC cells to apoptosis by directly downregulating HIF-1α, therefore inactivating GLUT1 and HK2 to suppress aerobic glycolysis. The inhibitory effect of genistein on tumour cell growth and glycolysis may help identify effective treatments for HCC patients at advanced stages.
MORIN
- 1Agro Processing and Technology Division, National Institute for Interdisciplinary Science and Technology (CSIR), Thiruvananthapuram, India
- 2Academy of Scientific and Innovative Research, New Delhi, India
The study under investigation focuses on in vitro antiproliferative efficacy of the flavonoid morin and the mechanisms by which it inhibits the growth of colon cancer using SW480 colon cancer cells with emphasis on Warburg effect. It was found that the cell proliferation was significantly inhibited by morin in a dose and time dependent manner. Morin induced apoptosis that was correlated with increased levels of reactive oxygen species formation and loss of mitochondrial membrane potential of the cells. In addition, an increase in cleaved PARP, cleaved caspase 3, cleaved caspase 8, cleaved caspase 9 and Bax as well as a decrease in Bcl 2 was observed, indicating morin is inducing both intrinsic as well as extrinsic pathway of apoptosis. This was further confirmed by using downstream caspase 3 inhibitor which indicated that caspase 3 inhibition reduces morin induced cell death. Moreover, the impact of morin on over all energy status when determined in terms of total cellular ATP level showed a decline with low level of glucose uptake and Glut1 expression. The results indicate that morin exerts antiproliferative activity by inducing apoptosis and by reducing Warburg effect in the evaluated cell lines and provide preliminary evidence for its anticancer activity.
람네틴
Abstract
The present study aimed to investigate whether rhamnetin induced apoptosis in human breast cancer cells and the underlying molecular mechanism of this anti cancer effect. The treatment of MCF-7 cells with rhamnetin was able to significantly inhibit cell proliferation and induce caspase-3/9 activity in a dose- and time-dependent manner, compared with untreated cells. In addition, treatment with rhamnetin was able to significantly promote the expression of p53 protein and microRNA (miR-)34a compared with untreated cells. The treatment with rhamnetin also suppressed the expression of Notch1 protein in MCF-7 cells compared with untreated cells. Subsequently, miR-24a expression was promoted in rhamnetin-treated MCF-7 cells using a miR-34a plasmid. The overexpression of miR-34a was able to significantly inhibit cell viability and induce caspase-3/9 activity in MCF-7 cells following treatment with rhamnetin. Furthermore, the overexpression of miR-34a was able to significantly promote the expression of p53 protein and miR-34a, and suppress the expression of Notch1 protein in rhamnetin-treated MCF-7 cells. Therefore, the results of the present study demonstrated that rhamnetin induced apoptosis in human breast cancer cells via the miR-34a/Notch-1 signaling pathway.
Keywords: rhamnetin, breast cancer, microRNA 34a, Notch-1
이소함네틴 플라보노이드
Isorhamnetin flavonoid synergistically enhances the anticancer activity and apoptosis induction by cis-platin and carboplatin in non-small cell lung carcinoma (NSCLC)
Bao-Yi Zhang, Yan-Ming Wang, [...], and Shao-Rong Han
Additional article information
Abstract
The development of novel antitumor drugs for the treatment of non-small cell lung carcinoma NSCLC is imperative in order to improve the efficacy of lung cancer therapy and prognosis. In the current study, we demonstrated the antitumor activity of isorhamnetin and its combinations with cisplatin and carboplatin against A-549 lung cancer cells. In order to assess the anticancer enhancing effect of isorhamnetin on cisplatin and carboplatin, A-549 cells were treated with isorhamnetin, cisplatin, carboplatin and their combinations and cell viability, cell apoptosis, cell cycle arrest as well as loss of mitochondrial membrane potential were evaluated by MTT assay, flow cytometry, confocal microscopy and fluorescence microscopy. The effect of the drugs on cancer cell migration, microtubule depolymerization as well activation of caspases was also studied. The results revealed that, as compared to single drug treatment, the combination of isorhamnetin with cisplatin and carboplatin resulted in greater effect in inhibiting cancer cell growth and inducing apoptosis. Combination of isorhamnetin with cisplatin and carboplatin resulted in more potent apoptosis induction as revealed by fluorescence microscopy using AO/PI double staining. Isorhamnetin and its combinations also triggered microtubule distortion and depolymerization. The combination of isorhamnetin with cisplatin and carboplatin increased the number of cells in G2/M phase dramatically as compared to single drug treatment. Moreover, isorhamnetin and its combinations with known anticancer drugs induced disruption of the mitochondrial membrane potential as well as activation of caspases 3, 9 and poly-(ADP-ribose) polymerase in A-549 cells. Isorhamnetin as well as its combinations with cisplatin and carboplatin resulted in inhibition of cancer cell migration significantly. Results of the current study suggest that isorhamnetin combinations with cisplatin and carboplatin might be a potential clinical chemotherapeutic approach for NSCLC.
Keywords: Non-small cell lung carcinoma, isorhamnetin, apoptosis, cell migration, tubulin, chemotherapy
비오카닌 에이
Perspectives Regarding the Role of Biochanin A in Humans

Chen Yu
1,

Peng Zhang
1,2,

Lixin Lou
1 and
 Yang Wang1,2*
Yang Wang1,2*- 1Department of Infectious Diseases, First Hospital of Jilin University, Changchun, China
- 2Department of Pediatrics, University of Oklahoma Health Sciences Center, Oklahoma City, OK, United States
Biochanin A (BCA) is an isoflavone mainly found in red clover with poor solubility and oral absorption that is known to have various effects, including anti-inflammatory, estrogen-like, and glucose and lipid metabolism modulatory activity, as well as cancer preventive, neuroprotective, and drug interaction effects. BCA is already commercially available and is among the main ingredients in many types of supplements used to alleviate postmenopausal symptoms in women. The activity of BCA has not been adequately evaluated in humans. However, the results of many in vitro and in vivo studies investigating the potential health benefits of BCA are available, and the complex mechanisms by which BCA modulates transcription, apoptosis, metabolism, and immune responses have been revealed. Many efforts have been exerted to improve the poor bioavailability of BCA, and very promising results have been reported. This review focuses on the major effects of BCA and its possible molecular targets, potential uses, and limitations in health maintenance and treatment.

테오필린
테오필린(theophylline, 1,3-디메틸크산틴(1,3-dimethylxanthine))은 만성 폐쇄성 폐질환(COPD), 천식 등 호흡기 질환의 치료에 사용되는 잔틴 약물로서, 다양한 브랜드명으로 판매되고 있다. 잔틴계에 속하는 이 물질은 구조와 약리적 특성이 테오브로민, 카페인과 유사성을 가지며 자연에서 쉽게 발견되며 차(차나무), 코코아(카카오)에 존재한다. 적은 양의 테오필린은 간의 카페인 대사 처리 산물 가운데 하나이다.[1]
테오필린은 차잎에서 최초로 추출되었으며 1888년 독일의 생물학자 알브레히트 코셀에 의해 화학적으로 식별되었다.[2][3] 7년 뒤, 1,3-dimethyluric acid으로 시작되는 화학합성이 에밀 피셔, Lorenz Ach에 의해 기술되었다.[4] 테오필린 합성의 대안 방식인 퓨린은 1900년 독일의 다른 과학자 Wilhelm Traube에 의해 도입되었다.[5] 테오필린의 최초의 임상적 활용은 1902년 이뇨제로서 사용되기 시작했다.[6] 천식 치료로 처음 보고되기까지 20년의 시간이 더 걸렸다.[7] 이 약물은 Theostat 20, Theostat 80라는 이름으로 최대 1970년대까지 시럽으로 처방되었으며 1980년대 초 Quibron이라는 이름의 알약 형태로 판매되었다.
Theophylline exhibits anti-cancer activity viasuppressing SRSF3 in cervical and breast cancer cell lines
Yung-Lung Chang, Yu-Juei Hsu, [...], and Shih-Ming Huang
Additional article information
Abstract
Caffeine, theophylline, and theobromine are the most well-known members of methylxanthines. Caffeine-induced serine/arginine-rich splicing factor 2, SRSF2, and SRSF3 are required for the alternative splicing of a subset of cancer-associated genes. However, it remains to be investigated whether and how theophylline and theobromine as well as caffeine exert their antitumor effects through mediating the alternative splicing process. Here, we reveal that theophylline down-regulated SRSF3 expression and switched p53 from alpha into a beta isoform as caffeine did in HeLa and MCF-7 cells via the reverse-transcriptase polymerase chain reaction and Western blot analysis. Further functional studies show that theophylline induced cellular apoptosis, senescence, and decreased colony formation. Interestingly, theophylline had a suppressive effect on cellular proliferation, whereas caffeine enhanced cellular proliferation rates via the 5-bromo-2-deoxyuridine analysis. Theophylline and caffeine had no effect on MCF-10A cells, which is a normal breast cell line. Our results provide an insight that theophylline as well as caffeine could be repurposed as antitumor leading compounds via the downregulation of splicing factor SRSF3 and its target genes.
phloretin

Abstract
Apple is a rich source of bioactive phytochemicals that help improve health by preventing and/or curing many disease processes, including cancer. One of the apple polyphenols is phloretin [2′,4′,6′-Trihydroxy-3-(4-hydroxyphenyl)-propiophenone], which has been widely investigated for its antioxidant, anti-inflammatory and anti-cancer activities in a wide array of preclinical studies. The efficacy of phloretin in suppressing xenograft tumor growth in athymic nude mice implanted with a variety of human cancer cells, and the ability of the compound to interfere with cancer cells signaling, have made it a promising candidate for anti-cancer drug development. Mechanistically, phloretin has been reported to arrest the growth of tumor cells by blocking cyclins and cyclin-dependent kinases and induce apoptosis by activating mitochondria-mediated cell death. The blockade of the glycolytic pathway via downregulation of GLUT2 mRNA and proteins, and the inhibition of tumor cells migration, also corroborates the anti-cancer effects of phloretin. This review sheds light on the molecular targets of phloretin as a potential anti-cancer and anti-inflammatory natural agent.
Keywords: phloretin, cell proliferation, inflammation, apoptosis, glucose uptake, migration
Nordihydroguaiaretic acid inhibits growth of cervical cancer SiHa cells by up-regulating p21
- Authors:
- Peng Gao
- Fei Zhai
- Lei Guan
- Jie Zheng
View Affiliations
- Published online on: November 10, 2010 https://doi.org/10.3892/ol.2010.205
- Pages: 123-128
Abstract
Nordihydroguaiaretic acid (NDGA) and its derivatives possess anti-cancer effects on various types of cancer via the induction of apoptosis or cell cycle arrest. This study proved that NDGA inhibited cervical cancer SiHa cell growth and induced cell cycle arrest at the G1 phase, which may be a consequence of cell cycle kinase inhibitor p21 induction. NDGA promoted acetylation of histone H3 in total and p21 gene-associated chromatin. This effect is gene selective, since NDGA has no impact on the p27 gene. NDGA also inhibited HPV-16 E6 gene transcription, which in turn resulted in the restoration of p53 protein levels. The silencing mediator for retinoid and thyroid hormone receptors (SMRT) is a key component of the HDAC3-HDAC4-N-CoR/SMRT complex. We found that NDGA significantly inhibited the transcription of SMRT, which, together with p53, may aid in the detection of the increase of histone H3 acetylation within the p21 gene. Our results suggest that NDGA induces p21 transcription by selectively elevating histone H3 acetylation associated with p21 gene and p53 protein levels via the inhibition of HPV-16 E6 expression.
고시폴
- 면화씨 추출물

Gossypol induces cell death by activating apoptosis and autophagy in HT-29 cells
- Authors:
- Ming‑Dong Lu
- Li‑Yi Li
- Pi‑Hong Li
- Tao You
- Fei‑Hai Wang
- Wei‑Jian Sun
- Zhi‑Qiang Zheng
View Affiliations
- Published online on: June 19, 2017 https://doi.org/10.3892/mmr.2017.6804
- Pages: 2128-2132
Abstract
Gossypol is a polyphenolic, yellowish compound derived from cottonseed extract. The present study examined the effects of gossypol on the apoptosis and autophagy of HT‑29 cells. A Cell Counting Kit‑8 assay, Annexin V‑FITC, JC‑1 staining and western blotting were used to identify the viability of cells, stages of apoptosis and the expression levels of the signaling proteins. Gossypol promoted apoptosis and induced the loss of mitochondrial membrane potential. Further investigation of the apoptotic mechanism revealed that gossypol increased the ratio of B‑cell lymphoma 2 (Bcl-2)-associated X protein/Bcl‑2 protein levels and upregulated the expression of caspase‑3. Gossypol also enhanced the activity of microtubule‑associated protein light chain 3 LC3‑II and Beclin‑1 and downregulated LC3‑I, in a dose‑dependent manner. Together, these finding suggested that gossypol may be a novel and potential antitumor agent.
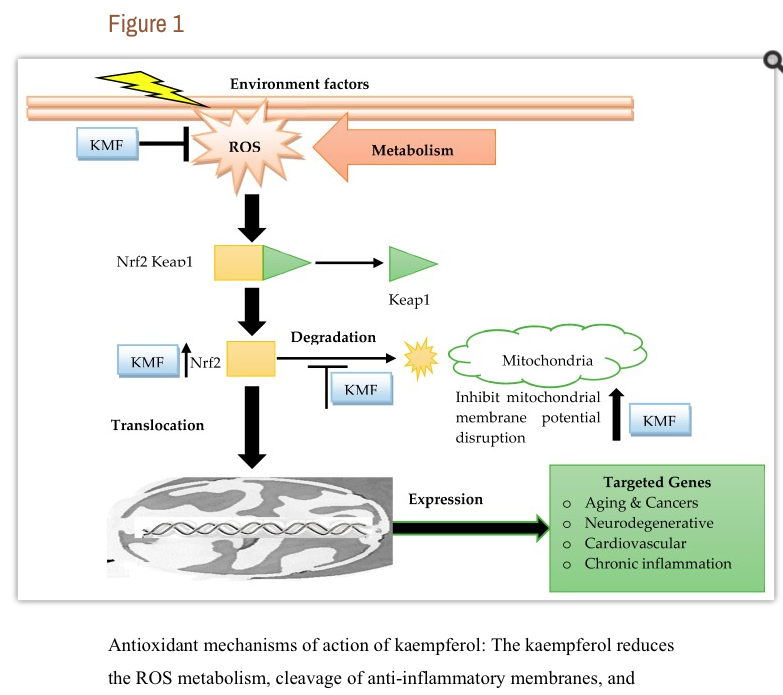
캠퍼롤
Abstract
A marked decrease in human cancers, including breast cancer, bone cancer, and cervical cancer, has been linked to the consumption of vegetable and fruit, and the corresponding chemoprotective effect has been associated with the presence of several active molecules, such as kaempferol. Kaempferol is a major flavonoid aglycone found in many natural products, such as beans, bee pollen, broccoli, cabbage, capers, cauliflower, chia seeds, chives, cumin, moringa leaves, endive, fennel, and garlic. Kaempferol displays several pharmacological properties, among them antimicrobial, anti-inflammatory, antioxidant, antitumor, cardioprotective, neuroprotective, and antidiabetic activities, and is being applied in cancer chemotherapy. Specifically, kaempferol-rich food has been linked to a decrease in the risk of developing some types of cancers, including skin, liver, and colon. The mechanisms of action include apoptosis, cell cycle arrest at the G2/M phase, downregulation of epithelial-mesenchymal transition (EMT)-related markers, and phosphoinositide 3-kinase/protein kinase B signaling pathways. In this sense, this article reviews data from experimental studies that investigated the links between kaempferol and kaempferol-rich food intake and cancer prevention. Even though growing evidence supports the use of kaempferol for cancer prevention, further preclinical and clinical investigations using kaempferol or kaempferol-rich foods are of pivotal importance before any public health recommendation or formulation using kaempferol.
Keywords: kaempferol, pharmacokinetics, pharmacodynamics, antioxidant, anticancer, chemoprevention, apoptosis, cell cycle arrest, metastasis, reactive oxygen species
Abstract
Kaempferol is a polyphenol antioxidant found in fruits and vegetables. Many studies have described the beneficial effects of dietary kaempferol in reducing the risk of chronic diseases, especially cancer. Epidemiological studies have shown an inverse relationship between kaempferol intake and cancer. Kaempferol may help by augmenting the body’s antioxidant defense against free radicals, which promote the development of cancer. At the molecular level, kaempferol has been reported to modulate a number of key elements in cellular signal transduction pathways linked to apoptosis, angiogenesis, inflammation, and metastasis. Significantly, kaempferol inhibits cancer cell growth and angiognesis and induces cancer cell apoptosis, but on the other hand, kaempferol appears to preserve normal cell viability, in some cases exerting a protective effect. The aim of this review is to synthesize information concerning the extraction of kaempferol, as well as to provide insights into the molecular basis of its potential chemo-preventative activities, with an emphasis on its ability to control intracellular signaling cascades that regulate the aforementioned processes. Chemoprevention using nanotechnology to improve the bioavailability of kaempferol is also discussed.
Keywords: Dietary flavonoid, Kaempferol, Angiogenesis, Apoptosis, Signal transduction, Metastasis, Nanotechnology
커큐민
Abstract
Cancer is the second leading cause of death in the world and one of the major public health problems. Despite the great advances in cancer therapy, the incidence and mortality rates of cancer remain high. Therefore, the quest for more efficient and less toxic cancer treatment strategies is still at the forefront of current research. Curcumin, the active ingredient of the Curcuma longaplant, has received great attention over the past two decades as an antioxidant, anti-inflammatory, and anticancer agent. In this review, a summary of the medicinal chemistry and pharmacology of curcumin and its derivatives in regard to anticancer activity, their main mechanisms of action, and cellular targets has been provided based on the literature data from the experimental and clinical evaluation of curcumin in cancer cell lines, animal models, and human subjects. In addition, the recent advances in the drug delivery systems for curcumin delivery to cancer cells have been highlighted.
Keywords: curcumin, anticancer, structure activity relationship, cellular pathway, mechanism of action, delivery system









 Thomas Sithara
Thomas Sithara K. B. Arun1,
K. B. Arun1,  H. P. Syama
H. P. Syama T. R. Reshmitha
T. R. Reshmitha P. Nisha
P. Nisha Yang Wang
Yang Wang








