Abstract
Histamine intolerance results from a disequilibrium of accumulated histamine and the capacity for histamine degradation. Histamine is a biogenic amine that occurs to various degrees in many foods. In healthy persons, dietary histamine can be rapidly detoxified by amine oxidases, whereas persons with low amine oxidase activity are at risk of histamine toxicity. Diamine oxidase (DAO) is the main enzyme for the metabolism of ingested histamine. It has been proposed that DAO, when functioning as a secretory protein, may be responsible for scavenging extracellular histamine after mediator release. Conversely, histamine N-methyltransferase, the other important enzyme inactivating histamine, is a cytosolic protein that can convert histamine only in the intracellular space of cells. An impaired histamine degradation based on reduced DAO activity and the resulting histamine excess may cause numerous symptoms mimicking an allergic reaction. The ingestion of histamine-rich food or of alcohol or drugs that release histamine or block DAO may provoke diarrhea, headache, rhinoconjunctival symptoms, asthma, hypotension, arrhythmia, urticaria, pruritus, flushing, and other conditions in patients with histamine intolerance. Symptoms can be reduced by a histamine-free diet or be eliminated by antihistamines. However, because of the multifaceted nature of the symptoms, the existence of histamine intolerance has been underestimated, and further studies based on double-blind, placebo-controlled provocations are needed. In patients in whom the abovementioned symptoms are triggered by the corresponding substances and who have a negative diagnosis of allergy or internal disorders, histamine intolerance should be considered as an underlying pathomechanism.
INTRODUCTION
Histamine intolerance results from a disequilibrium of accumulated histamine and the capacity for histamine degradation. The main enzyme for metabolism of ingested histamine is diamine oxidase (DAO) (1–5). An impaired histamine degradation based on a reduced DAO activity and the resulting excess of histamine may cause numerous symptoms mimicking an allergic reaction. Ingestion of histamine-rich food (6), alcohol (7–9), or drugs (10–13) that release histamine or block DAO may provoke diarrhea, headache (14), congestion of the nose, asthmatoid wheezing (6, 8, 15), hypotension, arrhythmia, urticaria (16, 17), pruritus, flushing, and other conditions in these patients. Approximately 1% of the population has histamine intolerance, and 80% of those patients are middle-aged (18). Because of the multifaceted symptoms, the existence of histamine intolerance is frequently underestimated, or its symptoms are misinterpreted. Clinical symptoms and their provocation by certain foods and beverages appear similar in different diseases, such as food allergy and intolerance of sulfites, histamine, or other biogenic amines (eg, tyramine). Therefore, the differentiation of the causal agent in adverse reactions to food, alcohol, and drugs is a difficult challenge. There is poor evidence of adverse reactions to these agents based on double-blind, placebo-controlled (DBPC) provocations (19). However, a better understanding of the pathophysiology, clinical picture, trigger factors, and diagnostic tools may help to clarify the confusing debate surrounding histamine intolerance.
HISTAMINE AND HISTAMINE METABOLISM
Histamine (2-[4-imidazolyl]ethylamine) was discovered in 1910 by Dale and Laidlaw (20), and it was identified as a mediator of anaphylactic reactions in 1932 (21). Histamine belongs to the biogenic amines and is synthesized by the pyridoxal phosphate (vitamin B-6)–containing L-histidine decarboxylase (HDC) from the amino acid histidine. It is synthesized by mast cells, basophils, platelets, histaminergic neurons, and enterochromaffine cells, where it is stored intracellularly in vesicles and released on stimulation. Histamine is a potent mediator of numerous biologic reactions. Besides the well-known triggering of degranulation of mast cells by crosslinking of the FcεRI receptor by specific allergens, several other nonimmunologic stimuli, such as neuropeptides, complement factors (ie, C3a and C5a), cytokines, hyperosmolarity, lipoproteins, adenosine, superoxidases (22), hypoxia, chemical and physical factors (eg, extreme temperatures, traumas) (23), or alcohol and certain food and drugs, may activate mast cells.
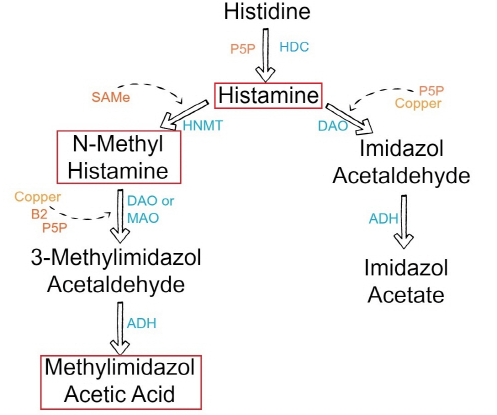
Histamine exerts its effects by binding to its 4 receptors [histamine 1 receptor (H1R), H2R, H3R, and and H4R] on target cells in various tissues (Figure 1, Table 1). It causes smooth muscle cell contraction, vasodilatation, increased vascular permeability and mucus secretion, tachycardia, alterations of blood pressure, and arrhythmias, and it stimulates gastric acid secretion and nociceptive nerve fibers. In addition, histamine has been known to play various roles in neurotransmission, immunomodulation, hematopoiesis, wound healing, day-night rhythm, and the regulation of histamine- and polyamine-induced cell proliferation and angiogenesis in tumor models (24, 25) and intestinal ischemia (26). Histamine can be metabolized in 2 ways: by oxidative deamination by DAO (former name: histaminase) or by ring methylation by histamine-N-methyltransferase (HNMT) (27) (Figure 2, Table 2). Whether histamine is catabolized by DAO or HNMT is supposed to depend on the localization of histamine. The DAO protein is stored in plasma membrane–associated vesicular structures in epithelial cells and is secreted into the circulation on stimulation (28, 29). Therefore, it has been proposed that DAO may be responsible for scavenging extracellular histamine (eg, after ingestion of histamine-rich food) after mediator release. Conversely, HNMT, the second most important enzyme inactivating histamine, is a cytosolic protein (30), which can convert histamine only in the intracellular space of cells (31, 32). Thus, the enzymes do not seem to compete for the substrate, although they have a similar affinity for histamine and they are expressed in some overlapping tissues. HNMT has a slightly higher affinity for histamine [Michaelis-Menten constant (kM): 6–13 μmol/L] than does DAO (kM: 20 μmol/L). In mammals, DAO expression is restricted to specific tissues; the highest activities are shown for small bowel and colon ascendens (4, 5, 33) and for placenta and kidney (28, 31). Lower DAO activity has been discussed as a potential indicator of intestinal mucosa damage in inflammatory and neoplastic diseases (17, 24, 34) and in persons undergoing chemotherapy (35). HNMT is widely expressed in human tissues; the greatest expression is in kidney and liver, followed by spleen, colon, prostate, ovary, spinal cord cells, bronchi, and trachea (36). HNMT is regarded as the key enzyme for histamine degradation in the bronchial epithelium (37).
Summary of histamine-mediated symptoms. Adapted with permission from Maintz L et al. Dtsch Artzebl 2006;103:A3477-83.
Summary of the histamine metabolism. The biogenic amine histamine is synthesized by decarboxylation of the amino acid histidine catalyzed by L-histidine decarboxylase (HDC) (1). Histamine can be metabolized by extracellular oxidative deamination of the primary amino group by diamine oxidase (DAO) (2) or intracellular methylation of the imidazole ring by histamine-N-methyltransferase (HNMT) (3). Therefore, insufficient enzyme activity caused by enzyme deficiency or inhibition may lead to accumulation of histamine. Both enzymes can be inhibited by their respective reaction products in a negative feedbackloop (4). N-Methylhistamine is oxidatively deaminated to N-methyl-imidazole acetaldehyde by monoamine oxidase B (MAO B) (5) or by DAO (6). Because the methylation pathway takes place in the cytosolic compartment of cells, MAO B (5) has been suggested to catalyze this reaction in vivo (35).
Histamine effects according to plasma histamine concentration (ng/mL)
| Histamine | Clinical effect |
|---|---|
| 0–1 | Reference |
| 1–2 | ↑ Gastric acid secretion ↑ Heart rate |
| 3–5 | Tachycardia, headache, flush, urticaria, pruritus |
| 6–8 | ↓ Arterial pressure |
| 7–12 | Bronchospasm |
| ≈100 | Cardiac arrest |
Characteristics of the histamine-degrading enzymes diamine oxidase (DAO) and histamine N-methyl-transferase (HNMT)1
| DAO | HNMT | |
|---|---|---|
| Gene | ||
| Gene map locus | Chromosome 7q35 | Chromosome 2q22 |
| Gene | 10 kbp, 5 exons, 4 introns | 35 kbp, 6 exons |
| Associated with SNPs | Inflammatory and neoplastic gastrointestinal diseases such as food allergy, gluten-sensitive enteropathy, Crohn disease, ulcerative colitis, and colon adenoma | Asthma |
| Protein | Soluble homodimeric glycoprotein of MR 200 000 with subunits of 70–125 kDa; 750 amino acid residues | Soluble, cytosolic protein of MR 33 000 with subunits of 29–34 kDa; 292 amino acid residues |
| Enzyme | ||
| Group | Copper-containing amine oxidases | Methyltransferases |
| Active form | Homodimer with the active-site cofactor 2,4,5-trihydroxyphenylalanine quinone (Topa quinone) | Monomer with a 2-domain structure |
| Enzyme kinetics (km) | Histamine, 20 μmol/L | Histamine, 6–13 μmol/L |
| Putrescine, 350 μmol/L | S-adenosyl-L-methionine, 6–10 μmmol/L | |
| Spermidine, 3 mmol/L | ||
| Optimum pH | 7.2 | 7.5–9.0 |
| Inhibititors | Copper-chelating agents, eg cyanide Carbonylgroup reagents, eg, aminoguanidine, semibarbacide | Reaction products: N-methylhistamine, S-adenosyl-L-homocysteine |
| Sulphydryl groups: p-chloromercuriobenzoate | ||
| Major expression | Intestine, kidney, placenta | Highest: kidney and liver; considerable: spleen, colon, prostate, ovary, spinal cord cells, trachea, and bronchi; to a smaller amount, nearly ubiquitous expression |
| Storage | Plasma membrane–associated vesicular structures in epithelial cells, secretion into the circulation upon stimulation | Cytosolic compartment of the cells |
| Function | Extracellular scavenger of histamine and other diamines by oxidative deamination of the primary amino group of histamine | Intracellular histamine inactivation by methylation of the imidazole ring |
SNPs, single-nucleotide polymorphisms; kbp, kilobase pair; MR, molecular weight; kDa, kiloDalton; kM, Michaelis-Menten constant.
ETIOPATHOGENESIS OF HISTAMINE INTOLERANCE
Different mechanisms have been proposed as causing histamine intolerance (38). Histamine intolerance can develop through both increased availability of histamine and impaired histamine degradation. Underlying conditions for increased availability may be an endogenous histamine overproduction caused by allergies, mastocytosis, bacterias, gastrointestinal bleeding, or increased exogenous ingestion of histidine or histamine by food or alcohol. Other biogenic amines, such as putrescine, may also be involved in displacing histamine from its mucosal mucine linkage, which results in an increase of free absorbable histamine in circulation. However, the main cause of histamine intolerance is an impaired enzymatic histamine degradation caused by genetic or acquired impairment of the enzymatic function of DAO or HNMT. Gastrointestinal diseases with altered enterocytes also may cause decreased production of DAO (17, 33, 39). Yet another cause can be competitive inhibition of histamine degradation of DAO by other biogenic amines, alcohol (7–9), or drugs (10, 12, 40). Acquired histamine intolerance may be transient and therefore reversible after the elimination of causes, such as by discontinuing DAO-blocking drugs. DAO inhibits the transepithelial permeation of exogenous histamine (41, 42), and impaired DAO activity results in increased enteral histamine uptake with consequent increased plasma histamine concentrations (10, 41) and corresponding symptoms. Increased amounts of histamine metabolites may also inhibit HNMT, the second enzyme metabolizing histamine (6, 43).
THE GENETIC BACKGROUND OF HISTAMINE INTOLERANCE
Recently, a potential genetic background of a reduced histamine metabolism has also been investigated. The human DAO gene spans ≈10 kbp and is located on chromosome 7q35 (27) Various single-nucleotide polymorphisms (SNPs) in the DAO gene have been shown to be associated with inflammatory and neoplastic gastrointestinal diseases, such as food allergy (44), gluten-sensitive enteropathy, Crohn disease, ulcerative colitis, and colon adenoma (45–47). No significant difference in the distribution of the investigated HNMT alleles could be shown between patients with gastrointestinal diseases and control subjects (45, 47), but a functional relevant polymorphism of the HNMT gene (chromosome 2q22) has been described for white asthma patients (48). Conversely, this association could not be observed in Japanese (49), German pediatric (50), and East Indian (51) populations. Thus, histamine intolerance seems to be acquired mostly through the impairment of DAO activity caused by gastrointestinal diseases or through the inhibition of DAO, but the high interindividual variations in the expression of DAO in the gut and the association of SNPs in the DAO gene with gastrointestinal diseases provide evidence for a genetic predisposition in a subgroup of patients with histamine intolerance (27).
CLINICAL PICTURE
Basal plasma histamine concentrations of 0.3 to 1.0 ng/mL are considered normal (52). Exceeding the individual histamine tolerance gives rise to concentration-dependent histamine-mediated symptoms (15, 53, 54) (Table 1). Even healthy persons may develop severe headache or flushing due to ingestion of massive amounts of histamine as is known from studies of scromboid poisoning (55). It has been shown that inhibition of DAO followed by oral histamine administration may induce severe and even life-threatening reactions, such as hypotension, bronchospasm, or shock (10, 43). Recurrent anaphylactic reactions have been reported in patients with hyperhistaminemia (56). In histamine-sensitive patients with reduced DAO activity, symptoms occur even after the ingestion of the small amounts of histamine that are well tolerated by healthy persons. Symptoms can be manifest via the abovementioned actions of histamine in multiple organs, such as the gastrointestinum, lung, skin, cardiovascular system, and brain, according to the expression of histamine receptors. Typical symptoms of histamine intolerance include gastrointestinal disorders, sneezing, rhinorrhea and congestion of the nose, headache (14, 57), dysmenorrhea, hypotonia, arrhythmias (58, 59), urticaria (16, 60), pruritus, flushing, and asthma (7, 8).
Histamine and headache
Headache can be induced dose-dependently by histamine in healthy persons as well as in patients with migraine (53, 61). Histamine-induced headache is a vascular headache caused mainly by nitrate monoxide (62). Histamine releases endothelial nitrate monoxide upon stimulation of H1R, which is also expressed in the large intracranial arteries (63). In migraine patients, plasma histamine concentrations have been shown to be elevated both during headache attacks and during symptom-free periods. An increase in the number of brain mast cells is associated with pathologic conditions such as migraine, cluster headache, and multiple sclerosis (64). Many migraine patients have histamine intolerance evidenced by reduced DAO activity, triggering of headache by food rich in histamine (eg, long-ripened cheese or wine), and the alleviation of headache (ie, disappearance of symptoms) under a histamine-free diet (57, 65) and therapy with antihistamines (66).
Histamine and gastrointestinum
Besides headache, gastrointestinal ailments including diffuse stomach ache, colic, flatulence, and diarrhea are leading symptoms of histamine intolerance. Elevated histamine concentrations and diminished DAO activities have been shown for various inflammatory and neoplastic diseases such as Crohn disease (17), ulcerative colitis (67), allergic enteropathy (39), food allergy (33, 68, 69), and colorectal neoplasmas (24). In the colonic mucosa of patients with food allergy, a concomitant reduced HNMT (70) and an impaired total histamine degradation capacity (THDC) (69) have been found (33), so that the enzymes cannot compensate each other. Therefore, an impaired histamine metabolism has been suggested to play a role in the pathogenesis of these diseases.
Histamine and airways
During or immediately after the ingestion of histamine-rich food or alcohol, rhinorrea or nasal obstruction may occur in patients with histamine intolerance; in extreme cases, asthma attacks also may occur. Reduced HNMT activity has been shown for patients with food allergy (70) and asthma bronchiale (71).
Histamine and food
Histamine and other biogenic amines are present to various degrees in many foods, and their presence increases with maturation (1, 72). The formation of biogenic amines in food requires the availability of free amino acids, the presence of decarboxylase-positive microorganisms, and conditions allowing bacterial growth and decarboxylase activity. Free amino acids either occur as such in foods or may be liberated by proteolysis during processing or storage (73). Numerous bacterias and some yeast display high HDC activity and thus have the capacity to form histamine. Histidine is generated from autolytic or bacterial processes (74). Therefore, high concentrations of histamine are found mainly in products of microbial fermentation, such as aged cheese (75), sauerkraut, wine (76), and processed meat (77, 78) (Table 3) or in microbially spoiled food. Thus, histamine, tyramine, putrescine, and cadaverine serve as indicators of hygienic food quality (73). Tyramine and putrescine also may lead to intolerance reactions in combination with histamine. Possible explanations may be the inhibition of DAO by other amines (43) or the promotion of histamine liberation from the mucosa by putrescine (34).