참고) neuron, epithelial cell, leukocyte, fibroblast는 highly polarized cell --> asymmetric cell division

Neurobiol Dis. Author manuscript; available in PMC 2020 Feb 1.
Published in final edited form as:
Neurobiol Dis. 2019 Feb; 122: 35–40.
Published online 2018 Jul 5. doi: 10.1016/j.nbd.2018.07.005
PMCID: PMC6366665
NIHMSID: NIHMS1009564
PMID: 29981842
Autophagy and Mitophagy in ALS
Chantell S. Evans1 and Erika L. F. Holzbaur1,*
Author information Copyright and License information PMC Disclaimer
The publisher's final edited version of this article is available at Neurobiol Dis
Abstract
Amyotrophic lateral sclerosis (ALS) is a debilitating and incurable disease involving the loss of motor neurons and subsequent muscle atrophy. Genetic studies have implicated deficits in autophagy and/or mitophagy in the onset of the disease. Here we review recent progress in our understanding of the pathways for autophagy and mitophagy in neurons, and how these pathways may be affected by mutations in genes including DCTN1, OPTN, TBK1, VCP, and C9ORF72. We also discuss the implications of modulating autophagy in ALS, highlighting both the potential of the approach and the concerns raised by targeting this pathway as a therapeutic strategy in neurodegenerative disease.
근위축성 측삭 경화증(ALS)은 운동 신경세포의 손실과 그에 따른 근육 위축을 수반하는 쇠약하고 난치성 질환입니다. 유전학적 연구에 따르면 자가포식 및/또는 미토파지의 결핍이 이 질환의 발병과 관련이 있는 것으로 밝혀졌습니다. 여기에서는 뉴런의 자가포식 및 미토파지 경로에 대한 이해의 최근 진전 사항과 이러한 경로가 DCTN1, OPTN, TBK1, VCP, C9ORF72를 포함한 유전자의 돌연변이에 의해 어떻게 영향을 받을 수 있는지에 대해 검토합니다.
또한 루게릭병에서 자가포식 조절이 갖는 의미에 대해 논의하고, 신경 퇴행성 질환의 치료 전략으로서 이 경로를 표적으로 삼는 접근법의 잠재력과 제기되는 우려를 모두 강조합니다.
INTRODUCTION
Initially described in the late 19th century by Charcot and Joffroy, our scientific understanding of the devastating neurodegenerative disease Amyotrophic lateral sclerosis (ALS) has progressed significantly, particularly through the identification of causative mutations for familial forms of the disease. Genetic studies have implicated key pathways involved in pathogenesis, including autophagy and mitophagy. However, this knowledge has not yet led to the development of effective therapeutic strategies, suggesting that there are still key gaps in our understanding of the underlying mechanisms. This review summarizes progress, investigating neuronal autophagy and mitophagy, and how that knowledge contributes to our understanding of the pathobiology of ALS.
19세기 후반 샤르코와 조프로이에 의해 처음 기술된 이후, 치명적인 신경 퇴행성 질환인 근위축성 측삭 경화증(ALS)에 대한 과학적 이해는 특히 가족성 질환의 원인 돌연변이 확인을 통해 상당한 진전을 이루었습니다. 유전자 연구는 자가포식 및 미토파지를 포함하여 발병에 관여하는 주요 경로를 밝혀냈습니다. 그러나 이러한 지식이 아직 효과적인 치료 전략의 개발로 이어지지는 않았으며, 이는 근본적인 메커니즘에 대한 이해에 여전히 중요한 격차가 있음을 시사합니다. 이 리뷰에서는 신경세포의 자가포식과 미토파지를 조사하고 이러한 지식이 루게릭병의 병태생리를 이해하는 데 어떻게 기여하는지에 대한 진전 사항을 요약합니다.
WHAT IS ALS?
ALS is a debilitating neuromuscular disease characterized by the progressive degeneration of motor neurons in the spinal cord and brain. Degeneration of these motor neurons leads to neuromuscular denervation, atrophy of voluntary skeletal muscles, and ultimately paralysis and death. The worldwide incidence of ALS is estimated at ~5/100,000 (Chio et al., 2013). Onset is usually age-dependent, although rare genetic forms may have a juvenile onset. Global estimates indicate that incidence peaks between the ages of 50 and 75 years (Chia et al., 2018). Disease progression is usually rapid, leading to death within 3–5 years of initial onset. There is currently no cure for this fatal disease, and available therapeutic options show only limited effectiveness.
Approximately 10% of ALS cases are familial in origin, with the remainder sporadic and of unknown cause. Possible causative factors for sporadic ALS include environmental factors such as infectious disease, chronic neuroinflammation, injury or trauma, and toxin exposure. However, the major shared risk factor across both sporadic and familial forms is aging.
루게릭병은 척수와 뇌의 운동 뉴런이 점진적으로 퇴화되는 것을 특징으로 하는 쇠약성 신경근육 질환입니다. 이러한 운동 신경세포의 퇴행은 신경근 탈신경화, 자발적 골격근 위축, 궁극적으로 마비와 사망으로 이어집니다. 루게릭병의 전 세계 발병률은 약 5/100,000명으로 추정됩니다(Chio et al., 2013). 발병은 일반적으로 연령에 따라 다르지만 드문 유전적 형태는 청소년기에 발병할 수 있습니다. 전 세계적으로 50세에서 75세 사이에 발병률이 최고조에 이르는 것으로 추정됩니다(Chia et al., 2018). 일반적으로 질병 진행이 빨라 최초 발병 후 3~5년 이내에 사망에 이르게 됩니다. 현재 이 치명적인 질병을 치료할 수 있는 방법은 없으며, 현재 사용 가능한 치료 옵션은 제한적인 효과만을 보여줍니다.
루게릭병의 약 10%는 가족력이 있으며, 나머지는 산발적으로 발생하고 원인을 알 수 없는 경우가 많습니다. 산발성 루게릭병의 원인으로는 감염성 질환, 만성 신경염증, 부상 또는 외상, 독소 노출과 같은 환경적 요인이 있습니다. 그러나 산발성 및 가족성 루게릭병 모두에서 공통적으로 나타나는 주요 위험 요인은 노화입니다.
Progress on genetic causes of ALS has been very rapid in recent years, with ~30 different genes now implicated (Chia et al., 2018). Surprisingly, these genes do not fit easily into a single cellular pathway, although some themes do emerge. For example, a number of ALS-associated genes encode RNA-binding proteins, including TDP43 and FUS, suggesting that alterations in ribostasis may contribute to pathogenesis. Alterations in proteostasis, mitochondrial function, cytoskeletal integrity and intracellular trafficking have also been implicated (Taylor et al., 2016).
루게릭병의 유전적 원인에 대한 연구는 최근 몇 년 동안 매우 빠르게 진전되어 현재 약 30개의 유전자가 관련되어 있습니다(Chia et al., 2018). 놀랍게도 이러한 유전자들은 일부 테마가 나타나기는 하지만 단일 세포 경로에 쉽게 들어맞지 않습니다. 예를 들어, 다수의 루게릭병 관련 유전자는 TDP43과 FUS를 포함한 RNA 결합 단백질을 코딩하는데, 이는 골격성 변화가 발병에 기여할 수 있음을 시사합니다. 단백질 항상성, 미토콘드리아 기능, 세포 골격 완전성 및 세포 내 이동의 변화도 관련되어 있습니다(Taylor et al., 2016).
Strikingly, several of the ALS-associated genes identified to date have been functionally implicated in autophagy and/or mitophagy, specifically the clearance of protein aggregates and/or damaged mitochondria. ALS genes known to function in autophagy include OPTN, TBK1, and SQSTM1. More broadly, proteins encoded by the genes C9ORF72, VCP, CHMP2B, VAPB, ALS2, and DCTN1 have all been implicated in vesicular trafficking and may affect autophagy either directly or indirectly. A central role for autophagy in ALS is well supported by the pathology of the disease, which commonly includes the accumulation of protein aggregates and swollen or dystrophic mitochondria in motor neurons of affected patients (Taylor et al., 2016).
놀랍게도 현재까지 확인된 루게릭병 관련 유전자 중 일부는 오토파지 및/또는 미토파지, 특히 단백질 응집체 및/또는 손상된 미토콘드리아의 제거에 기능적으로 관련되어 있습니다. 오토파지에 작용하는 것으로 알려진 루게릭병 유전자로는 OPTN, TBK1, SQSTM1이 있습니다. 더 광범위하게는 C9ORF72, VCP, CHMP2B, VAPB, ALS2, DCTN1 유전자에 의해 코딩된 단백질이 모두 소포 수송에 관여하며 오토파지에 직간접적으로 영향을 미칠 수 있습니다. 루게릭병에서 자가포식의 중심적인 역할은 루게릭병 환자의 운동 신경세포에 단백질 응집체와 부종 또는 영양 장애가 있는 미토콘드리아가 축적되는 질병의 병리학에 의해 잘 뒷받침됩니다(Taylor et al., 2016).
Here we review the two best characterized pathways for autophagy in neurons, axonal autophagy and mitophagy, and discuss the available evidence linking dysfunction in these pathways to the onset and progression of ALS. We also highlight a few of the many questions that remain, such as how mutations that are ubiquitously expressed may lead to the specific loss of motor neurons, and how cell-autonomous and cell-non-autonomous mechanisms may contribute (Box 1). We also discuss the possibilities and limitations of therapeutic interventions affecting autophagy in either neurons or their support cells.
여기에서는 뉴런의 자가포식에 대한 가장 잘 특성화된 두 가지 경로인 축삭 자가포식과 미토파지를 검토하고, 이러한 경로의 기능 장애와 루게릭병의 발병 및 진행을 연결하는 이용 가능한 증거에 대해 논의합니다. 또한 유비쿼터스적으로 발현되는 돌연변이가 어떻게 운동 뉴런의 특정 소실로 이어질 수 있는지, 세포 자율 및 세포 비자율 메커니즘이 어떻게 기여할 수 있는지 등 아직 남아 있는 많은 질문 중 몇 가지를 강조합니다(상자 1). 또한 뉴런이나 그 지원 세포의 자가포식에 영향을 미치는 치료적 개입의 가능성과 한계에 대해서도 논의합니다.
BOX 1:
Outstanding questions regarding the role of autophagy in ALS
운동 뉴런에 오토파지가 필요한가요? (2017)의 데이터는 신경근 접합부의 구조와 기능에 대한 Atg7 녹다운의 영향을 조사했으며 비교적 경미한 결손을보고 했습니까? 장기적인 녹다운 시에는 더 심각한 결손이 나타날까요?
자가포식 및/또는 미토파지에 필요한 유전자의 돌연변이가 세포 자율적 또는 세포 비자율적 경로를 통해 질병을 유발합니까? 예를 들어, 운동 뉴런에서 돌연변이 OPTN 또는 TBK1의 발현이 질병을 유발하기에 충분한가요, 아니면 돌연변이 SOD1에서 발견 된 것처럼 신경교 기능 장애도 기여합니까 (Taylor et al., 2016)?
모든 신경세포 미토콘드리아는 동일한 품질 관리 메커니즘의 적용을 받나요? Sung 등(2016)의 데이터에 따르면 미토파지는 생체 내에서 드물고 체세포에만 국한되어 있다고 합니다. 미토파지가 공간적으로 제한되어 있는지 또는 뉴런 전체에서 발생하는지를 확인하기 위해서는 아직 연구가 더 필요합니다. 또한 뉴런에서 다른 미토콘드리아 품질 관리 메커니즘의 기여는 아직 불분명합니다.
미토파지 결핍과 신경 염증 사이의 메커니즘적 연관성은 무엇일까요? PINK1과 파킨의 하류에서 미토파지 경로 내에서 기능하는 것으로 확인된 유전자에는 이전에 염증 경로에 관여하는 것으로 알려진 유전자 인 OPTN과 TBK1이 포함됩니다.
자가포식/미토파지 경로의 결함은 산발성 루게릭병의 발병에 어느 정도 기여하나요? 유전자 연구에 따르면 루게릭병의 발병에는 단백질 정체, 골격 정체, 미토콘드리아 건강 및 신경 염증을 포함한 여러 경로가 관여하는 것으로 나타났습니다. 이러한 경로가 산발성 루게릭병에 어느 정도 기여하는지는 아직 밝혀지지 않았습니다.
자가포식을 강화하는 치료법이 운동 신경세포 건강에 도움이 될까요, 아니면 해가 될까요? 여러 모델 시스템의 데이터에 따르면 자가포식 활성화는 질병의 발병과 진행에 차별적인 영향을 미칠 수 있다고 합니다.
AUTOPHAGY IN NEURONS
Autophagy is a critical pathway to maintain homeostasis and to respond to cellular stress. The molecular components of the autophagy pathway have been well characterized over several decades of research in model systems, such as yeast. Studies in mammalian cells indicate that the overall pathway is highly conserved. More recently, however, there has been a growing appreciation that autophagy is specifically tuned in highly differentiated cells, with distinct, tissue-specific differences apparent in regulation of autophagy, such as cellular responses to stress. For example, Mizushima et al. (2004) used a transgenic mouse model expressing the autophagosome marker GFP-LC3B to demonstrate that upon starvation autophagy is significantly upregulated in tissues such as liver, but not in brain (Mizushima et al., 2004).
오토파지는 항상성을 유지하고 세포 스트레스에 대응하는 데 중요한 경로입니다. 자가포식 경로의 분자 구성 요소는 효모와 같은 모델 시스템에서 수십 년에 걸친 연구를 통해 잘 특성화되었습니다. 포유류 세포를 대상으로 한 연구에 따르면 전반적인 경로가 매우 잘 보존되어 있습니다. 그러나 최근에는 스트레스에 대한 세포 반응과 같이 오토파지의 조절에 뚜렷한 조직별 차이가 나타나면서 오토파지가 고도로 분화된 세포에서 특별히 조정된다는 인식이 확산되고 있습니다. 예를 들어, 미즈시마 등(2004)은 오토파지솜 마커 GFP-LC3B를 발현하는 형질전환 마우스 모델을 사용하여 기아 시 간과 같은 조직에서는 오토파지가 유의하게 상향 조절되지만 뇌에서는 그렇지 않다는 사실을 입증했습니다(Mizushima et al., 2004).
Differentiated and highly polarized cells, such as neurons, exhibit spatially segregated pathways for autophagy and mitophagy (Maday and Holzbaur, 2014; Sung et al., 2016). Direct imaging of autophagy in neurons expressing GFP-labeled Atg8 (LC3B in mammals) has demonstrated a robust, constitutive pathway for autophagosome formation in the distal axon both in vitro and in vivo (Maday and Holzbaur, 2014; Maday et al., 2012; Neisch et al., 2017). Autophagosomes form in a stepwise manner in the growth cone of neurons in culture and at synaptic sites such as the neuromuscular junction in vivo (Neisch et al., 2017; Stavoe et al., 2016). Once formed, distal autophagosomes are rapidly transported toward the soma via the microtubule-based motor cytoplasmic dynein and its activator dynactin (Figure 1; (Maday et al., 2012; Neisch et al., 2017)). Of note, a G59S mutation in the dynactin subunit p150Glued, encoded by the DCTN1 gene, has been identified as the cause of the late-onset and slowly progressive motor neuron disease HMN7B (Puls et al., 2003). Thus, mutations in DCTN1 can be considered as a very rare cause of ALS (Chia et al., 2018).
뉴런과 같이 분화되고 고도로 분극화된 세포는 오토파지와 미토파지를 위한 공간적으로 분리된 경로를 나타냅니다(Maday and Holzbaur, 2014; Sung et al., 2016). GFP 표지 Atg8(포유류의 LC3B)을 발현하는 뉴런에서 오토파지를 직접 이미징하면 시험관 및 생체 내 원위 축삭에서 오토파지솜 형성을 위한 견고하고 구성적인 경로가 입증되었습니다(Maday and Holzbaur, 2014; Maday et al., 2012; Neisch et al., 2017). 오토파지좀은 배양 중인 신경세포의 성장 원뿔과 생체 내 신경근 접합부와 같은 시냅스 부위에서 단계적으로 형성됩니다(Neisch et al., 2017; Stavoe et al., 2016). 일단 형성된 원위 오토파지솜은 미세소관 기반의 운동성 세포질 다이닌과 그 활성화제인 다이낙틴을 통해 체질 쪽으로 빠르게 운반됩니다(그림 1; (Maday et al., 2012; Neisch et al., 2017)). 주목할 만한 점은 DCTN1 유전자에 의해 암호화되는 다이낙틴 서브유닛 p150Glued의 G59S 돌연변이가 늦게 발병하고 서서히 진행되는 운동 신경 질환인 HMN7B의 원인으로 밝혀졌다는 것입니다(Puls et al., 2003). 따라서 DCTN1의 돌연변이는 루게릭병의 매우 드문 원인으로 간주될 수 있습니다(Chia et al., 2018).
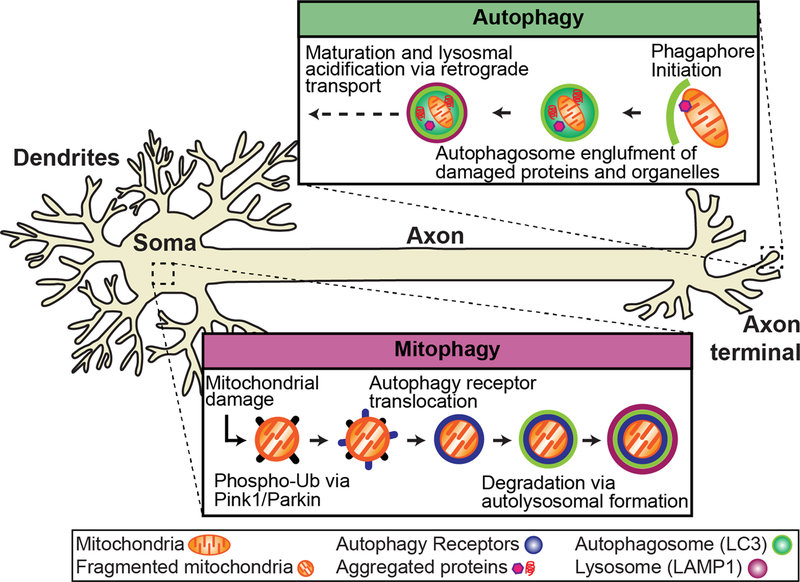
Autophagy and mitophagy in the neuron.
Autophagy and mitophagy are constitutive mechanisms important to in the maintenance of neuronal homeostasis that have been implicated in ALS. Autophagy occurs at the distal tip of the axon, where autophagosomes engulf damaged/aged mitochondria and protein aggregates. Through retrograde transport, the autophagosome matures and fuses with lysosomes to form a mature autolysosome that is degraded in the soma. Evidence suggests that mitophagy is spatially distinct from autophagy and occurs primarily in the soma. Damaged mitochondria are marked for degradation via phospho-ubiquitination of outer mitochondrial membrane proteins via PINK1/Parkin. Autophagy receptors (i.e. OPTN) are recruited to mitochondria and initiate the translocation of the autophagy machinery to degrade damaged mitochondria via lysosomal fusion.
자가포식과 미토파지는 루게릭병에 관여하는 신경세포 항상성 유지에 중요한 구성 메커니즘입니다. 오토파지는 축삭의 말단에서 발생하며, 여기서 오토파지는 손상되거나 노화된 미토콘드리아와 단백질 응집체를 삼켜버립니다. 오토파지는 역행 수송을 통해 성숙하고 리소좀과 융합하여 체내에서 분해되는 성숙한 오토리소좀을 형성합니다. 미토파지는 오토파지와 공간적으로 구별되며 주로 체내에서 발생한다는 증거가 있습니다. 손상된 미토콘드리아는 PINK1/Parkin을 통해 외부 미토콘드리아 막 단백질의 인산화 유비퀴틴화를 통해 분해되는 것으로 표시됩니다. 오토파지 수용체(예: OPTN)는 미토콘드리아로 모집되어 리소좀 융합을 통해 손상된 미토콘드리아를 분해하기 위한 오토파지 기계의 전좌를 시작합니다.
Axonal autophagosomes form constitutively (Maday and Holzbaur, 2014; Maday and Holzbaur, 2016; Maday et al., 2012; Neisch et al., 2017; Stavoe et al., 2016), and cargo-loading appears to be nxxxxonselective (Maday et al., 2012). Engulfed cargos found within axonal autophagosomes include mitochondrial fragments and aggregated proteins (Figure 1), and may include synaptic vesicles under some circumstances (Maday et al., 2012; Okerlund et al., 2017; Wong and Holzbaur, 2014b). Both in vitro and in vivo, autophagosome formation is most prominent in the distal axon (Maday and Holzbaur, 2014; Maday and Holzbaur, 2016; Neisch et al., 2017; Soukup et al., 2016; Stavoe et al., 2016; Vanhauwaert et al., 2017).
축삭 자가포식체는 구성적으로 형성되며(Maday and Holzbaur, 2014; Maday and Holzbaur, 2016; Maday 등, 2012; Neisch 등, 2017; Stavoe 등, 2016), 화물 적재는 비선택적인 것으로 보입니다(Maday 등, 2012). 축삭 자가포식체 내에서 발견되는 포획화물에는 미토콘드리아 조각과 응집된 단백질이 포함되며(그림 1), 일부 상황에서는 시냅스 소포가 포함될 수 있습니다(Maday et al., 2012; Okerlund et al., 2017; Wong and Holzbaur, 2014b). 시험관 및 생체 내 모두에서 오토파지솜 형성은 원위 축삭돌기에서 가장 두드러집니다(Maday and Holzbaur, 2014; Maday and Holzbaur, 2016; Neisch et al., 2017; Soukup et al., 2016; Stavoe et al., 2016; Vanhauwaert et al., 2017).
During transport toward the soma, autophagosomes fuse with late endosomes and lysosomes to form a more mature, acidified organelle known as an autolysosome. In contrast to newly formed autophagosomes which are primarily found in the distal axon, autolysosomes are found along the length of the axon and accumulated within the soma (Maday and Holzbaur, 2016). The autolysosome contains degradative proteases such as cathepsins and efficiently degrades internalized cargos so that the components can be released and recycled. As effective autophagy depends on fusion of nascent autophagosomes with functional lysosomes to form a proteolytically active compartment, conditions that affect lysosomal integrity, such as mutations in the ALS-linked gene VCP (Papadopoulos et al., 2017) or extracellular deposits of β-amyloid (Gowrishankar et al., 2015) affect both lysosomal and autophagosomal processing in neurons.
soma로 이동하는 동안 오토파지좀은 후기 엔도좀 및 리소좀과 융합하여 오토리소좀으로 알려진 보다 성숙하고 산성화된 세포 소기관을 형성합니다. 주로 원위 축삭에서 발견되는 새로 형성된 오토파지솜과 달리, 오토리소좀은 축삭의 길이를 따라 발견되며 체내에 축적됩니다(Maday and Holzbaur, 2016). 오토리소좀은 켑신과 같은 분해성 프로테아제를 포함하고 있으며, 내재화된 화물을 효율적으로 분해하여 성분이 방출되고 재활용될 수 있도록 합니다. 효과적인 자가포식은 초기 자가포식체와 기능성 리소좀이 융합하여 단백질 분해 활성 구획을 형성하는 데 달려 있기 때문에 루게릭병 관련 유전자 VCP의 돌연변이(파파도풀로스 등, 2017) 또는 β-아밀로이드의 세포 외 침착(고우리샨카 등, 2015)과 같이 리소좀 완전성에 영향을 미치는 조건은 뉴런의 리소좀 및 자가포식체 처리 모두에 영향을 미칩니다.
ALS-LINKED GENES IMPLICATED IN AUTOPHAGY
In addition to DCTN1, which encodes an essential component of the microtubule-based motor complex that moves autophagosomes from the axon toward the soma, the protein products of multiple ALS-linked genes have been implicated in the dynamics or regulation of autophagy. These include p62/SQSTM1 (Gal et al., 2009), a ubiquitin-binding protein that associates with both protein aggregates (for example, see (Rudnick et al., 2017)) and damaged mitochondria (Wong and Holzbaur, 2014a). Optineurin/OPTN is another ubiquitin-binding protein that is also implicated in the autophagic clearance of aggregated proteins (Korac et al., 2013) and depolarized or damaged mitochondria (Wong and Holzbaur, 2014a). As noted below, both p62/SQSTM1 and OPTN are recruited to ubiquitinated substrates, and induce their sequestration by an autophagosome via their LC3-binding motifs. TBK1 was identified as an ALS-linked gene by exome sequencing, and is a kinase known to phosphorylate both p62 and OPTN (Cirulli et al., 2015). While cellular assays have clearly established roles for p62, OPTN, and TBK1 in the autophagic clearance of protein aggregates as well as mitophagy (discussed below), these three proteins have also been implicated in innate immunity pathways, and thus may cause disease by mechanisms unrelated to autophagy/mitophagy. Further work is required to establish the critical pathogenic mechanism or mechanisms involved.
축삭에서 체세포로 오토파지를 이동시키는 미세소관 기반 모터 복합체의 필수 구성 요소를 암호화하는 DCTN1 외에도 여러 루게릭병 관련 유전자의 단백질 산물이 오토파지의 역학 또는 조절에 관여하는 것으로 밝혀졌습니다. 여기에는 단백질 응집체와 결합하는 유비퀴틴 결합 단백질인 p62/SQSTM1(Gal et al., 2009) 및 손상된 미토콘드리아(Wong and Holzbaur, 2014a 참조)가 포함됩니다. 옵티뉴린/OPTN은 또 다른 유비퀴틴 결합 단백질로서 응집된 단백질의 자가포식 제거(Korac et al., 2013) 및 탈분극 또는 손상된 미토콘드리아(Wong and Holzbaur, 2014a)에도 관련되어 있습니다. 아래에 언급된 바와 같이, p62/SQSTM1과 OPTN은 모두 유비퀴틴화된 기질에 모집되며, LC3 결합 모티프를 통해 오토파지솜에 의한 격리를 유도합니다. TBK1은 엑솜 시퀀싱을 통해 루게릭병 관련 유전자로 확인되었으며, p62와 OPTN을 모두 인산화하는 것으로 알려진 키나아제입니다(Cirulli et al., 2015). 세포 분석을 통해 단백질 응집체의 자가포식 제거와 미토파지(아래에서 설명)에서 p62, OPTN, TBK1의 역할이 명확히 밝혀졌지만, 이 세 단백질은 선천성 면역 경로에도 관여하므로 자가포식/미토파지와 무관한 메커니즘에 의해 질병을 유발할 수 있습니다. 중요한 병원성 메커니즘을 규명하기 위해서는 추가적인 연구가 필요합니다.
Other ALS-associated genes have also been linked to autophagy, although their specific roles remain to be determined. These include VCP, implicated in the autophagy of stress granules (Buchan et al., 2013) and the maintenance of lysosomal integrity (Papadopoulos et al., 2017), and VAPB, an ER-tethering protein (Dong et al., 2016). Expansion of hexanucleotide repeats within the noncoding sequence of the C9ORF72 gene are the most frequent cause of familial ALS (DeJesus-Hernandez et al., 2011; Renton et al., 2011). Recent data implicate the protein product of the C9ORF72 gene in the regulation of ULK1, and thus in the initiation of autophagy; however, C9ORF72 has also been proposed to function in the regulation of lysosomal fusion or function (Amick et al., 2016; Jung et al., 2017; Yang et al., 2016). However, it remains unclear if decreased C9ORF72 expression contributes to the onset of neurodegeneration, as mouse knockouts do not show a neuron-specific phenotype (O’Rourke et al., 2016); other mechanisms such as RAN translation or the formation of RNA foci may instead drive pathogenesis (Ash et al., 2013; Mori et al., 2013; Zu et al., 2011; Zu et al., 2013)
다른 루게릭병 관련 유전자도 오토파지와 관련이 있지만, 구체적인 역할은 아직 밝혀지지 않았습니다. 여기에는 스트레스 과립의 자가포식(Buchan 외, 2013) 및 리소좀 완전성 유지(Papadopoulos 외, 2017)에 관여하는 VCP와 ER-테더링 단백질(Dong 외, 2016)인 VAPB가 포함됩니다. C9ORF72 유전자의 비코딩 서열 내 헥사뉴클레오티드 반복의 확장은 가족성 루게릭병의 가장 흔한 원인입니다(DeJesus-Hernandez 외., 2011; Renton 외., 2011). 최근 데이터에 따르면 C9ORF72 유전자의 단백질 산물은 ULK1의 조절과 자가포식의 시작에 관여하지만, C9ORF72는 리소좀 융합 또는 기능의 조절에도 작용하는 것으로 제안되었습니다(Amick et al., 2016; Jung et al., 2017; Yang et al., 2016). 그러나 마우스 녹아웃은 신경세포 특이적 표현형을 나타내지 않기 때문에 C9ORF72 발현 감소가 신경 퇴행의 발병에 기여하는지는 아직 불분명하며, 대신 RAN 번역이나 RNA 초점 형성과 같은 다른 메커니즘이 발병을 주도할 수 있습니다(Ash et al., 2013; Mori et al., 2013; Zu et al., 2011; Zu et al., 2013).
AUTOPHAGIC CLEARANCE OF PROTEIN AGGREGATES IN ALS
In vitro studies of neurons expressing the ALS-linked mutation SOD1G93A demonstrated the uptake of mutant SOD1 into axonal autophagosomes (Maday et al., 2012). Surprisingly, over-expression of the mutant protein did not result in upregulation of autophagosome formation or flux, suggesting that the axonal pathway for autophagosome biogenesis has a limited ability to respond to proteotoxic stress. Similar studies in neurons expressing mutant Huntingtin support this conclusion, as Huntingtin aggregates were readily taken up by axonal autophagosomes, but no increase in biogenesis or flux was induced by the expression of this aggregation-prone protein (Wong and Holzbaur, 2014b). This study demonstrated a detrimental effect of mutant Huntingtin on the ability of autophagosomes to degrade their cargo, suggesting that while autophagosome biogenesis is not effectively upregulated, turnover may be inhibited leading to the accumulation of autophagosomes within neurons exposed to proteotoxic stress.
루게릭병 관련 돌연변이 SOD1G93A를 발현하는 뉴런에 대한 시험관 내 연구에서 돌연변이 SOD1이 축삭 오토파지솜으로 흡수되는 것이 입증되었습니다(Maday et al., 2012). 놀랍게도 돌연변이 단백질의 과발현은 오토파지솜 형성이나 플럭스의 상향 조절을 초래하지 않았으며, 이는 오토파지솜 생성을 위한 축삭 경로가 단백질 독성 스트레스에 반응하는 능력이 제한적이라는 것을 시사합니다. 돌연변이 헌팅틴을 발현하는 뉴런을 대상으로 한 유사한 연구에서도 헌팅틴 응집체가 축삭 오토파지좀에 의해 쉽게 흡수되었지만 이 응집성 단백질의 발현에 의해 생체 생성이나 플럭스의 증가가 유도되지 않았기 때문에 이러한 결론을 뒷받침합니다(Wong and Holzbaur, 2014b). 이 연구는 돌연변이 헌팅틴이 오토파지솜의 화물 분해 능력에 해로운 영향을 미치는 것을 보여 주었으며, 이는 오토파지솜 생성이 효과적으로 상향 조절되지는 않지만 회전율이 억제되어 단백질 독성 스트레스에 노출된 뉴런 내에 오토파지솜이 축적될 수 있음을 시사합니다.
Rudnick et al. (2017) examined autophagy in motor neurons in a transgenic mouse model expressing SOD1G93A, and identified the formation of large ubiquitinated protein aggregates positive for p62, the product of the ALS-associated gene SQSTM1. Both large autophagosomes positive for Atg8 homologs, ~3 μm in diameter and termed round-bodies, and skein-like inclusions were observed. The SOD1G93A transgenic mouse has a stereotypical time course of disease onset, progression and death, allowing the investigators to compare autophagosome accumulation to disease progression. Prior to frank onset of disease, round bodies were relatively abundant while the skein-like inclusions became more prevalent with disease progression. Potentially, the accumulation of the skein-like inclusions may reflect a deficit in the ability of the motor neuron to effectively clear protein aggregates via autophagy, due to either an aging-dependent decline in autophagy or the high burden of proteotoxic stress. While the formation of p62-positive aggregates was most prominent in motor neurons early in disease progression, similar structures were observed in interneurons at later time points, indicating that while the observed pathology induced by over-expression of mutant SOD1G93A preferentially affected motor neurons, it was not motor neuron-specific. Together, these studies suggest that overexpression of aggregation-prone proteins readily overwhelms the ability of the neuron to effectively clear aggregates by autophagy.
(2017)은 SOD1G93A를 발현하는 형질전환 마우스 모델에서 운동 신경세포의 자가포식을 조사한 결과, 루게릭병 관련 유전자 SQSTM1의 산물인 p62에 양성인 대형 유비퀴틴화 단백질 응집체가 형성되는 것을 확인했습니다. 직경이 약 3 μm이고 둥근 몸체라고 불리는 Atg8 상동체에 양성인 대형 오토파지솜과 타래와 같은 내포물이 모두 관찰되었습니다. SOD1G93A 형질 전환 마우스는 질병 발병, 진행 및 사망의 전형적인 시간 경과를 가지고 있어 연구자들은 오토파지솜 축적과 질병 진행을 비교할 수 있습니다. 질병이 본격적으로 발병하기 전에는 원형체가 상대적으로 많았고, 질병이 진행됨에 따라 실 모양의 내포물이 더 많이 나타났습니다. 잠재적으로, 타래형 내포물의 축적은 노화에 따른 자가포식의 감소 또는 단백질 독성 스트레스의 높은 부담으로 인해 운동 뉴런이 자가포식을 통해 단백질 응집체를 효과적으로 제거하는 능력의 결핍을 반영할 수 있습니다. p62 양성 응집체의 형성은 질병 진행 초기에 운동 뉴런에서 가장 두드러졌지만, 이후 시점의 인터뉴런에서도 유사한 구조가 관찰되었으며, 이는 돌연변이 SOD1G93A의 과발현에 의해 유도된 병리가 운동 뉴런에 우선적으로 영향을 미치지만 운동 뉴런에 특이적이지 않다는 것을 나타냅니다. 이 연구들을 종합하면 응집되기 쉬운 단백질의 과발현은 자가포식에 의해 응집물을 효과적으로 제거하는 뉴런의 능력을 쉽게 압도한다는 것을 시사합니다.
MITOPHAGY IN NEURONS
Damaged or aged mitochondria are selectively sequestered and eliminated through the autophagic process of mitophagy (Tal et al., 2007). This pathway is important for neuronal homeostasis and is most commonly linked to Parkinson’s disease, due to the discovery of two proteins (PINK1 and Parkin) linked to the familial form of the disease (Kitada et al., 1998; Valente et al., 2004; Youle and Narendra, 2011). However, many neurodegenerative diseases overlap on the subcellular level with defects in neuronal mitophagy. In the case of ALS, the accumulation of damaged or dysfunctional mitochondria are thought to be a contributing factor to the disease. Despite our knowledge of this pathway and disease links between PINK-1/Parkin and OPTN/TBK1 to Parkinson’s disease and ALS, respectively, it remains unclear as to the significance of this overlap (Cirulli et al., 2015; Freischmidt et al., 2015; Kitada et al., 1998; Maruyama and Kawakami, 2013; Valente et al., 2004).
The mitophagy pathway has been worked out in detail in immortalized cell lines, identifying a highly regulated process that involves the stepwise recruitment of multiple proteins (Lazarou et al., 2015; Moore and Holzbaur, 2016; Wong and Holzbaur, 2014a). Upon damage, PINK1 is stabilized on the mitochondrial surface which leads to the recruitment of an E3 ubiquitin ligase, Parkin (for reviews of PINK1/Parkin mitophagy see (Nguyen et al., 2016; Pickrell and Youle, 2015; Youle and Narendra, 2011)). Phospho-ubiquitination of outer mitochondrial membrane proteins initiates a feed-forward cascade that marks mitochondria for degradation (Kane et al., 2014; Kazlauskaite et al., 2014; Matsuda et al., 2010; Narendra et al., 2008; Narendra et al., 2010). Specificity for the autophagosome engulfment of damaged mitochondria relies on OPTN, an autophagy receptor, and its kinase TBK1 (Lazarou et al., 2015; Moore and Holzbaur, 2016; Wong and Holzbaur, 2014a). Translocation of OPTN to damaged mitochondria leads to LC3B recruitment and degradation via lysosomal fusion (Figure 1; (Moore and Holzbaur, 2016; Rogov et al., 2014; Wong and Holzbaur, 2014a)). It should be noted that p62 is also recruited to damage mitochondria, but is independent of OPTN and does not lead to recruitment of LC3B (Wong and Holzbaur, 2014a).
In immortalized cell lines, OPTN translocation was shown to initiate within tens of minutes of mitochondrial damage via a mitochondrial KillerRed (mt-KR) construct, where light-induced activation of mt-KR causes local damage to mitochondria vis ROS production (Bulina et al., 2006), or CCCP treatment, an inhibitor of oxidative phosphorylation; robust OPTN recruitment and stabilization was observed in ~40 minutes (Moore and Holzbaur, 2016; Wong and Holzbaur, 2014a). The use of an ALS-associated E478G ubiquitin binding-deficient mutant of OPTN significantly decreased translocation to damaged mitochondria, as the mutant failed to stably associate with the mitochondrial surface (Lazarou et al., 2015; Wong and Holzbaur, 2014a). Moreover, the use of an ALS-linked TBK1 mutant significantly reduced OPTN and LC3B recruitment to damaged mitochondria; this was also observed by silencing TBK1 or using a potent TBK1 inhibitor (Lazarou et al., 2015; Moore and Holzbaur, 2016). Therefore, loss of function of OPTN or TBK1 results in impaired mitophagy and accumulation of damaged mitochondria. Thus, inefficient turnover of mitochondria via ALS-associated mutants could be a contributing factor leading to mitochondrial dysfunction and accumulation, a prevalent feature in the motor neurons of ALS patients.
Surprisingly, substantially less is known about the regulation of mitophagy in neurons. As neurons are highly polarized and require long-range transport, it remains controversial whether distal mitochondria are subject to the same quality control mechanisms as those near the cell body. Since prompt removal of damaged mitochondria is critical for cell viability and the energy demands of neuronal mitochondria are substantial, it raises the question of how/where Pink1/Parkin-mediated mitophagy occur in neurons? Using mt-KR or global damage via antimycin a treatment, Ashrafi et. al (2014) showed that damage to axonal mitochondria triggered translocation of the mitophagy machinery within tens of minutes. In neurons lacking PINK1 or Parkin, recruitment was not visualized. It should be noted that despite the implications of PINK1 and Parkin in neurodegenerative disease, knockout mice fail to develop significant neurological defects (Whitworth and Pallanck, 2017). The authors concluded that while autophagosome biogenesis is a constitutive pathway that occurs distally in neurons, autophagosome induced formation around damaged mitochondria is a distinct pathway that can occur anywhere along the axon (Ashrafi et al., 2014).
This idea was recently called into question by studies in Drosophila which suggested that the soma is the primary focus of PINK1/Parkin-mediated mitophagy in neurons. Devireddy and colleagues showed that while loss of PINK1 reduced mitochondrial membrane potential, it failed to lead to an accumulation of mitochondria or alter mitochondrial length in axons. However, irregular mitochondrial morphology was observed in the soma (Devireddy et al., 2015). Consistent with this notion, Parkin-deficient flies had normal motility and morphology in the axons of motor neurons, but an abnormal mitochondrial network in the cell body (Sung et al., 2016). The authors argued that in motor neurons in vivo mitophagy is a rare event due to an additional quality control step that allows only healthy mitochondria to exit the soma and enter the axon.
Much of the work thus far to study neuronal mitophagy has focused on a PINK1/Parkin-mediated process. The use of chemical uncouplers has shown that mitophagy in neurons is remarkably slow compared to the time-course that was observed in immortalized cell lines. Additionally, neuronal mitochondria are much more resistant to mitophagy initiation than organelles in cell lines, which could be due to the fact that they are terminally differentiated. Thus, it is plausible that neurons have developed additional quality control mechanisms, prior to mitophagy, as preventative measures to decrease the number of stressed or aged organelles likely to enter the mitophagy pathway (Sugiura et al., 2014). For example, recent work from Lin et al. (2017) has provided new evidence to suggest that stressed neuronal mitochondria are removed from the axon via mitochondria-derived cargos and that this pathway is independent of Parkin, Drp1, and autophagy. The authors observed an alteration in mitochondrial membrane potential when synatphilin (SNPH), a mitochondrial anchoring protein, was overexpressed suggesting that axonal transport of mitochondria is required to maintain integrity and health. Additionally, stressed mitochondria release SNPH containing vesicles as a mechanism to shift from a stationary position to actively transport damaged mitochondria from the axon for degradation (Lin et al., 2017). This finding is in line with a recent report showing mature mitochondria are tethered near presynaptic terminals in cortical neurons (Lewis et al., 2016). Together these results suggest that aged mitochondria are more susceptible to damage due to their immobilization.
It is plausible that these discrepancies in mitophagy location are due to the types of treatments (i.e. global vs. local damage), as well as the severity of the chemical uncouplers (i.e. CCCP or antimycin a) used to induce mitochondrial damage. Additionally, the rates of mitophagy could be heavily influenced by in vitro versus in vivo model systems (Sung et al., 2016). While Pink1/Parkin-mediated mitophagy in immortalized cell lines appeared to be straightforward, it has become apparent that this mechanism in neurons is complex and work is still needed to fully address where and how this process occurs. For example, the interplay of mitophagy and neuroinflammation is now receiving attention, as neuroinflammation is a hallmark of ALS and increasing evidence suggests that mitophagy and neuroinflammation are linked (Komine and Yamanaka, 2015; Oakes et al., 2017).
AUTOPHAGY: FRIEND OR FOE FOR THE TREATMENT OF ALS?
Autophagy and mitophagy are considered to be essential homeostatic pathways in neurons, as genetic ablation of key autophagy genes is sufficient to induce the degeneration of neurons (Hara et al., 2006; Komatsu et al., 2006). Further, the identification of mutations in proteins thought to function within the autophagy/mitophagy pathways (SQSTM1, C9ORF72, PINK1, Parkin, OPTN, TBK1) in both Parkinson’s disease and ALS further highlights the potential importance of these cellular mechanisms.
In cellular models of ALS, there have been reports that pathology can be rescued by the induction of autophagy. For example, Marrone et al. (2018) developed iPSC lines expressing wild type or mutant FUS-eGFP, and observed the recruitment of both proteins to stress granules upon oxidative stress in both iPSCs and an induced mixed population of neuronal cells containing motor neurons. Dynamics of stress granule formation were altered in mutant FUS-eGFP iPSCs and could be rescued by treating the cells with mTOR inhibitors that can induce autophagy (Marrone et al., 2018). Of note, the drug-induced induction of autophagy in iPSC-derived neurons was much less than that observed in the parent iPSC lines, consistent with observations that mTOR inhibition does not effectively upregulate autophagy in neurons (Maday and Holzbaur, 2016).
Surprisingly, however, Rudnick et al. (2017) reported that the inhibition of autophagy induced by a targeted knockdown of Atg7 is not sufficient to induce motor neuron cell death in mice up to 150 days old, although abnormal synaptic structure and function were observed in Atg7 conditional knockout (cKO) mice. Crossing Atg7-cKO mice to mice expressing mutant SOD1 led to some counter-intuitive findings. The deficiency in autophagy induced by depletion of Atg7 led to an earlier disease onset by some but not all measures, as onset of hind limb tremor was observed 22 days earlier but there was no difference in disease-associated weight loss. However, depletion of Atg7 was found to extend lifespan by ~22%, although motor neuron survival was not affected as compared to mice expressing mutant SOD1 in the presence of wildtype levels of Atg7 (Rudnick et al., 2017).
In a parallel study, rilmenidine was administered to induce autophagy in the SOD1G93A mouse. While autophagy was upregulated, both motor neuron degeneration and symptom progression were deleteriously affected (Perera et al., 2017). Non-cell autonomous effects of autophagy modulation must also be considered (Fabbrizio et al., 2017). For example, Staats et al. (2013) found that rapamycin treatment of SOD1G93A mice did not increase survival. However, rapamycin has an immunosuppressive effect on lymphocytes. To avoid this complication, SOD1G93A mice were crossed to RAG1−/− mice to generate animals deficient in mature lymphocytes. In this line, rapamycin treatment induced a mild extension of lifespan (6.5 days). Disease onset was not affected, instead the principal effect was on disease progression (Staats et al., 2013).
Together, results to date raise concern that therapeutic approaches aimed generally at enhancing autophagy may not be uniformly beneficial for patients with ALS. More nuanced strategies may be effective, such as enhancing lysosomal function and thus counteracting the accumulation of autophagosomes or autolysosomes without sufficient degradative ability. These strategies must be built on a better understanding of the cell biology of autophagy in neurons, and how the dynamics of autophagy are affected in both familial and sporadic ALS.
CONCLUSIONS
Recent progress is beginning to map out how and when autophagy occurs in neurons, and how neurons use this pathway to counter cellular stressors such as proteotoxic stress or damaged mitochondria. Studies at the cellular level are profiting from the recent advances in the genetics of ALS and other, related neurodegenerative diseases. We are beginning to understand how disease-associated genes can be grouped into consensus pathways, allowing an improved focus on disease-causing mechanisms involved in pathogenesis. While current data clearly implicate defects in autophagy and mitophagy in familial ALS, further research is necessary to understand the molecular basis for the defects, as well as the most effective approaches to intervene (Box 1). Hopefully, the progress made in understanding familial cases will then directly inform our understanding of the predominant, sporadic forms of ALS, and lead to the design of more effective therapeutic approaches in future.
최근 뉴런에서 자가포식이 언제 어떻게 일어나는지, 그리고 뉴런이 단백질 독성 스트레스나 손상된 미토콘드리아와 같은 세포 스트레스 요인에 대응하기 위해 이 경로를 어떻게 사용하는지 파악하는 데 진전이 이루어지고 있습니다. 세포 수준에서의 연구는 루게릭병 및 기타 관련 신경 퇴행성 질환의 유전학이 최근 발전함에 따라 많은 이점을 얻고 있습니다. 우리는 질병 관련 유전자를 합의된 경로로 그룹화할 수 있는 방법을 이해하기 시작했으며, 이를 통해 발병에 관여하는 질병 유발 메커니즘에 더 집중할 수 있게 되었습니다. 현재 데이터는 가족성 루게릭병에서 자가포식 및 미토파지의 결함을 분명히 시사하지만, 이러한 결함의 분자적 근거와 가장 효과적인 개입 방법을 이해하기 위해서는 추가 연구가 필요합니다(상자 1). 가족성 루게릭병에 대한 이해의 진전이 산발적으로 발생하는 루게릭병에 대한 이해에 직접적인 정보를 제공하고, 향후 보다 효과적인 치료 접근법을 설계하는 데 도움이 되기를 바랍니다.
HIGHLIGHTS
ACKNOWLEDGMENTS
The authors thank Phuong Nguyen for her careful reading of the manuscript, and gratefully acknowledge funding from the HHMI Hanna H. Gray Fellowship to CSE and NIH R37 NS060698 to ELFH.
REFERENCES
- Amick J, Roczniak-Ferguson A, and Ferguson SM. 2016. C9orf72 binds SMCR8, localizes to lysosomes, and regulates mTORC1 signaling. Mol Biol Cell 27:3040–3051. [PMC free article] [PubMed] [Google Scholar]
- Ash PE, Bieniek KF, Gendron TF, Caulfield T, Lin WL, Dejesus-Hernandez M, van Blitterswijk MM, Jansen-West K, Paul JW 3rd, Rademakers R, Boylan KB, Dickson DW, and Petrucelli L. 2013. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron 77:639–646. [PMC free article] [PubMed] [Google Scholar]
- Ashrafi G, Schlehe JS, LaVoie MJ, and Schwarz TL. 2014. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. The Journal of cell biology 206:655–670. [PMC free article] [PubMed] [Google Scholar]
- Buchan JR, Kolaitis RM, Taylor JP, and Parker R. 2013. Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell 153:1461–1474. [PMC free article] [PubMed] [Google Scholar]
- Bulina ME, Chudakov DM, Britanova OV, Yanushevich YG, Staroverov DB, Chepurnykh TV, Merzlyak EM, Shkrob MA, Lukyanov S, and Lukyanov KA. 2006. A genetically encoded photosensitizer. Nature biotechnology 24:95–99. [PubMed] [Google Scholar]
- Chia R, Chio A, and Traynor BJ. 2018. Novel genes associated with amyotrophic lateral sclerosis: diagnostic and clinical implications. Lancet Neurol 17:94–102. [PMC free article] [PubMed] [Google Scholar]
- Chio A, Logroscino G, Traynor BJ, Collins J, Simeone JC, Goldstein LA, and White LA. 2013. Global epidemiology of amyotrophic lateral sclerosis: a systematic review of the published literature. Neuroepidemiology 41:118–130. [PMC free article] [PubMed] [Google Scholar]
- Cirulli ET, Lasseigne BN, Petrovski S, Sapp PC, Dion PA, Leblond CS, Couthouis J, Lu YF, Wang Q, Krueger BJ, Ren Z, Keebler J, Han Y, Levy SE, Boone BE, Wimbish JR, Waite LL, Jones AL, Carulli JP, Day-Williams AG, Staropoli JF, Xin WW, Chesi A, Raphael AR, McKenna-Yasek D, Cady J, Vianney de Jong JM, Kenna KP, Smith BN, Topp S, Miller J, Gkazi A, Consortium FS, Al-Chalabi A, van den Berg LH, Veldink J, Silani V, Ticozzi N, Shaw CE, Baloh RH, Appel S, Simpson E, Lagier-Tourenne C, Pulst SM, Gibson S, Trojanowski JQ, Elman L, McCluskey L, Grossman M, Shneider NA, Chung WK, Ravits JM, Glass JD, Sims KB, Van Deerlin VM, Maniatis T, Hayes SD, Ordureau A, Swarup S, Landers J, Baas F, Allen AS, Bedlack RS, Harper JW, Gitler AD, Rouleau GA, Brown R, Harms MB, Cooper GM, Harris T, Myers RM, and Goldstein DB. 2015. Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science 347:1436–1441. [PMC free article] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GY, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, and Rademakers R. 2011. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72:245–256. [PMC free article] [PubMed] [Google Scholar]
- Devireddy S, Liu A, Lampe T, and Hollenbeck PJ. 2015. The Organization of Mitochondrial Quality Control and Life Cycle in the Nervous System In Vivo in the Absence of PINK1. The Journal of neuroscience : the official journal of the Society for Neuroscience 35:9391–9401. [PMC free article] [PubMed] [Google Scholar]
- Dong R, Saheki Y, Swarup S, Lucast L, Harper JW, and De Camilli P. 2016. Endosome-ER Contacts Control Actin Nucleation and Retromer Function through VAP-Dependent Regulation of PI4P. Cell 166:408–423. [PMC free article] [PubMed] [Google Scholar]
- Fabbrizio P, Amadio S, Apolloni S, and Volonte C. 2017. P2X7 Receptor Activation Modulates Autophagy in SOD1-G93A Mouse Microglia. Front Cell Neurosci 11:249. [PMC free article] [PubMed] [Google Scholar]
- Freischmidt A, Wieland T, Richter B, Ruf W, Schaeffer V, Muller K, Marroquin N, Nordin F, Hubers A, Weydt P, Pinto S, Press R, Millecamps S, Molko N, Bernard E, Desnuelle C, Soriani MH, Dorst J, Graf E, Nordstrom U, Feiler MS, Putz S, Boeckers TM, Meyer T, Winkler AS, Winkelman J, de Carvalho M, Thal DR, Otto M, Brannstrom T, Volk AE, Kursula P, Danzer KM, Lichtner P, Dikic I, Meitinger T, Ludolph AC, Strom TM, Andersen PM, and Weishaupt JH. 2015. Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nature neuroscience 18:631–636. [PubMed] [Google Scholar]
- Gal J, Strom AL, Kwinter DM, Kilty R, Zhang J, Shi P, Fu W, Wooten MW, and Zhu H. 2009. Sequestosome 1/p62 links familial ALS mutant SOD1 to LC3 via an ubiquitin-independent mechanism. J Neurochem 111:1062–1073. [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar S, Yuan P, Wu Y, Schrag M, Paradise S, Grutzendler J, De Camilli P, and Ferguson SM. 2015. Massive accumulation of luminal protease-deficient axonal lysosomes at Alzheimer’s disease amyloid plaques. Proceedings of the National Academy of Sciences of the United States of America 112:E3699–3708. [PMC free article] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, and Mizushima N. 2006. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441:885–889. [PubMed] [Google Scholar]
- Jung J, Nayak A, Schaeffer V, Starzetz T, Kirsch AK, Muller S, Dikic I, Mittelbronn M, and Behrends C. 2017. Multiplex image-based autophagy RNAi screening identifies SMCR8 as ULK1 kinase activity and gene expression regulator. Elife 6. [PMC free article] [PubMed] [Google Scholar]
- Kane LA, Lazarou M, Fogel AI, Li Y, Yamano K, Sarraf SA, Banerjee S, and Youle RJ. 2014. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. The Journal of cell biology 205:143–153. [PMC free article] [PubMed] [Google Scholar]
- Kazlauskaite A, Kondapalli C, Gourlay R, Campbell DG, Ritorto MS, Hofmann K, Alessi DR, Knebel A, Trost M, and Muqit MM. 2014. Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65. Biochem J 460:127–139. [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, and Shimizu N. 1998. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392:605–608. [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, and Tanaka K. 2006. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441:880–884. [PubMed] [Google Scholar]
- Komine O, and Yamanaka K. 2015. Neuroinflammation in motor neuron disease. Nagoya J Med Sci 77:537–549. [PMC free article] [PubMed] [Google Scholar]
- Korac J, Schaeffer V, Kovacevic I, Clement AM, Jungblut B, Behl C, Terzic J, and Dikic I. 2013. Ubiquitin-independent function of optineurin in autophagic clearance of protein aggregates. J Cell Sci 126:580–592. [PMC free article] [PubMed] [Google Scholar]
- Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, and Youle RJ. 2015. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524:309–314. [PMC free article] [PubMed] [Google Scholar]
- Lewis TL Jr., Turi GF, Kwon SK, Losonczy A, and Polleux F. 2016. Progressive Decrease of Mitochondrial Motility during Maturation of Cortical Axons In Vitro and In Vivo. Current biology : CB 26:2602–2608. [PMC free article] [PubMed] [Google Scholar]
- Lin MY, Cheng XT, Tammineni P, Xie Y, Zhou B, Cai Q, and Sheng ZH. 2017. Releasing Syntaphilin Removes Stressed Mitochondria from Axons Independent of Mitophagy under Pathophysiological Conditions. Neuron 94:595–610 e596. [PMC free article] [PubMed] [Google Scholar]
- Maday S, and Holzbaur EL. 2014. Autophagosome biogenesis in primary neurons follows an ordered and spatially regulated pathway. Dev Cell 30:71–85. [PMC free article] [PubMed] [Google Scholar]
- Maday S, and Holzbaur EL. 2016. Compartment-Specific Regulation of Autophagy in Primary Neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 36:5933–5945. [PMC free article] [PubMed] [Google Scholar]
- Maday S, Wallace KE, and Holzbaur EL. 2012. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. The Journal of cell biology 196:407–417. [PMC free article] [PubMed] [Google Scholar]
- Marrone L, Poser I, Casci I, Japtok J, Reinhardt P, Janosch A, Andree C, Lee HO, Moebius C, Koerner E, Reinhardt L, Cicardi ME, Hackmann K, Klink B, Poletti A, Alberti S, Bickle M, Hermann A, Pandey UB, Hyman AA, and Sterneckert JL. 2018. Isogenic FUS-eGFP iPSC Reporter Lines Enable Quantification of FUS Stress Granule Pathology that Is Rescued by Drugs Inducing Autophagy. Stem Cell Reports 10:375–389. [PMC free article] [PubMed] [Google Scholar]
- Maruyama H, and Kawakami H. 2013. Optineurin and amyotrophic lateral sclerosis. Geriatrics & gerontology international 13:528–532. [PubMed] [Google Scholar]
- Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, Kimura M, Komatsu M, Hattori N, and Tanaka K. 2010. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. The Journal of cell biology 189:211–221. [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yamamoto A, Matsui M, Yoshimori T, and Ohsumi Y. 2004. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 15:1101–1111. [PMC free article] [PubMed] [Google Scholar]
- Moore AS, and Holzbaur EL. 2016. Dynamic recruitment and activation of ALS-associated TBK1 with its target optineurin are required for efficient mitophagy. Proceedings of the National Academy of Sciences of the United States of America 113:E3349–3358. [PMC free article] [PubMed] [Google Scholar]
- Mori K, Weng SM, Arzberger T, May S, Rentzsch K, Kremmer E, Schmid B, Kretzschmar HA, Cruts M, Van Broeckhoven C, Haass C, and Edbauer D. 2013. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science 339:1335–1338. [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, and Youle RJ. 2008. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. The Journal of cell biology 183:795–803. [PMC free article] [PubMed] [Google Scholar]
- Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, and Youle RJ. 2010. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 8:e1000298. [PMC free article] [PubMed] [Google Scholar]
- Neisch AL, Neufeld TP, and Hays TS. 2017. A STRIPAK complex mediates axonal transport of autophagosomes and dense core vesicles through PP2A regulation. The Journal of cell biology 216:441–461. [PMC free article] [PubMed] [Google Scholar]
- Nguyen TN, Padman BS, and Lazarou M. 2016. Deciphering the Molecular Signals of PINK1/Parkin Mitophagy. Trends Cell Biol 26:733–744. [PubMed] [Google Scholar]
- O’Rourke JG, Bogdanik L, Yanez A, Lall D, Wolf AJ, Muhammad AK, Ho R, Carmona S, Vit JP, Zarrow J, Kim KJ, Bell S, Harms MB, Miller TM, Dangler CA, Underhill DM, Goodridge HS, Lutz CM, and Baloh RH. 2016. C9orf72 is required for proper macrophage and microglial function in mice. Science 351:1324–1329. [PMC free article] [PubMed] [Google Scholar]
- Oakes JA, Davies MC, and Collins MO. 2017. TBK1: a new player in ALS linking autophagy and neuroinflammation. Mol Brain 10:5. [PMC free article] [PubMed] [Google Scholar]
- Okerlund ND, Schneider K, Leal-Ortiz S, Montenegro-Venegas C, Kim SA, Garner LC, Waites CL, Gundelfinger ED, Reimer RJ, and Garner CC. 2017. Bassoon Controls Presynaptic Autophagy through Atg5. Neuron 93:897–913 e897. [PubMed] [Google Scholar]
- Papadopoulos C, Kirchner P, Bug M, Grum D, Koerver L, Schulze N, Poehler R, Dressler A, Fengler S, Arhzaouy K, Lux V, Ehrmann M, Weihl CC, and Meyer H. 2017. VCP/p97 cooperates with YOD1, UBXD1 and PLAA to drive clearance of ruptured lysosomes by autophagy. The EMBO journal 36:135–150. [PMC free article] [PubMed] [Google Scholar]
- Perera ND, Sheean RK, Lau CL, Shin YS, Beart PM, Horne MK, and Turner BJ. 2017. Rilmenidine promotes MTOR-independent autophagy in the mutant SOD1 mouse model of amyotrophic lateral sclerosis without slowing disease progression. Autophagy:1–18. [PMC free article] [PubMed]
- Pickrell AM, and Youle RJ. 2015. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 85:257–273. [PMC free article] [PubMed] [Google Scholar]
- Puls I, Jonnakuty C, LaMonte BH, Holzbaur EL, Tokito M, Mann E, Floeter MK, Bidus K, Drayna D, Oh SJ, Brown RH Jr., Ludlow CL, and Fischbeck KH. 2003. Mutant dynactin in motor neuron disease. Nat Genet 33:455–456. [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita VM, Kaivorinne AL, Holtta-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chio A, Restagno G, Borghero G, Sabatelli M, Consortium I, Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, and Traynor BJ. 2011. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72:257–268. [PMC free article] [PubMed] [Google Scholar]
- Rogov V, Dotsch V, Johansen T, and Kirkin V. 2014. Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Molecular cell 53:167–178. [PubMed] [Google Scholar]
- Rudnick ND, Griffey CJ, Guarnieri P, Gerbino V, Wang X, Piersaint JA, Tapia JC, Rich MM, and Maniatis T. 2017. Distinct roles for motor neuron autophagy early and late in the SOD1(G93A) mouse model of ALS. Proceedings of the National Academy of Sciences of the United States of America 114:E8294–E8303. [PMC free article] [PubMed] [Google Scholar]
- Soukup SF, Kuenen S, Vanhauwaert R, Manetsberger J, Hernandez-Diaz S, Swerts J, Schoovaerts N, Vilain S, Gounko NV, Vints K, Geens A, De Strooper B, and Verstreken P. 2016. A LRRK2-Dependent EndophilinA Phosphoswitch Is Critical for Macroautophagy at Presynaptic Terminals. Neuron 92:829–844. [PubMed] [Google Scholar]
- Staats KA, Hernandez S, Schonefeldt S, Bento-Abreu A, Dooley J, Van Damme P, Liston A, Robberecht W, and Van Den Bosch L. 2013. Rapamycin increases survival in ALS mice lacking mature lymphocytes. Molecular neurodegeneration 8:31. [PMC free article] [PubMed] [Google Scholar]
- Stavoe AK, Hill SE, Hall DH, and Colon-Ramos DA. 2016. KIF1A/UNC-104 Transports ATG-9 to Regulate Neurodevelopment and Autophagy at Synapses. Dev Cell 38:171–185. [PMC free article] [PubMed] [Google Scholar]
- Sugiura A, McLelland GL, Fon EA, and McBride HM. 2014. A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. The EMBO journal 33:2142–2156. [PMC free article] [PubMed] [Google Scholar]
- Sung H, Tandarich LC, Nguyen K, and Hollenbeck PJ. 2016. Compartmentalized Regulation of Parkin-Mediated Mitochondrial Quality Control in the Drosophila Nervous System In Vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience 36:7375–7391. [PMC free article] [PubMed] [Google Scholar]
- Tal R, Winter G, Ecker N, Klionsky DJ, and Abeliovich H. 2007. Aup1p, a yeast mitochondrial protein phosphatase homolog, is required for efficient stationary phase mitophagy and cell survival. The Journal of biological chemistry 282:5617–5624. [PubMed] [Google Scholar]
- Taylor JP, Brown RH Jr., and Cleveland DW. 2016. Decoding ALS: from genes to mechanism. Nature 539:197–206. [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, and Wood NW. 2004. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 304:1158–1160. [PubMed] [Google Scholar]
- Vanhauwaert R, Kuenen S, Masius R, Bademosi A, Manetsberger J, Schoovaerts N, Bounti L, Gontcharenko S, Swerts J, Vilain S, Picillo M, Barone P, Munshi ST, de Vrij FM, Kushner SA, Gounko NV, Mandemakers W, Bonifati V, Meunier FA, Soukup SF, and Verstreken P. 2017. The SAC1 domain in synaptojanin is required for autophagosome maturation at presynaptic terminals. The EMBO journal 36:1392–1411. [PMC free article] [PubMed] [Google Scholar]
- Whitworth AJ, and Pallanck LJ. 2017. PINK1/Parkin mitophagy and neurodegeneration-what do we really know in vivo? Curr Opin Genet Dev 44:47–53. [PubMed] [Google Scholar]
- Wong YC, and Holzbaur EL. 2014a. Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proceedings of the National Academy of Sciences of the United States of America 111:E4439–4448. [PMC free article] [PubMed] [Google Scholar]
- Wong YC, and Holzbaur EL. 2014b. The regulation of autophagosome dynamics by huntingtin and HAP1 is disrupted by expression of mutant huntingtin, leading to defective cargo degradation. The Journal of neuroscience : the official journal of the Society for Neuroscience 34:1293–1305. [PMC free article] [PubMed] [Google Scholar]
- Yang M, Liang C, Swaminathan K, Herrlinger S, Lai F, Shiekhattar R, and Chen JF. 2016. A C9ORF72/SMCR8-containing complex regulates ULK1 and plays a dual role in autophagy. Sci Adv 2:e1601167. [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, and Narendra DP. 2011. Mechanisms of mitophagy. Nature reviews. Molecular cell biology 12:9–14. [PMC free article] [PubMed] [Google Scholar]
- Zu T, Gibbens B, Doty NS, Gomes-Pereira M, Huguet A, Stone MD, Margolis J, Peterson M, Markowski TW, Ingram MA, Nan Z, Forster C, Low WC, Schoser B, Somia NV, Clark HB, Schmechel S, Bitterman PB, Gourdon G, Swanson MS, Moseley M, and Ranum LP. 2011. Non-ATG-initiated translation directed by microsatellite expansions. Proceedings of the National Academy of Sciences of the United States of America 108:260–265. [PMC free article] [PubMed] [Google Scholar]
- Zu T, Liu Y, Banez-Coronel M, Reid T, Pletnikova O, Lewis J, Miller TM, Harms MB, Falchook AE, Subramony SH, Ostrow LW, Rothstein JD, Troncoso JC, and Ranum LP. 2013. RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proceedings of the National Academy of Sciences of the United States of America 110:E4968–4977. [PMC free article] [PubMed] [Google Scholar]