
Brain Behav Immun. Author manuscript; available in PMC 2011 May 1.
Published in final edited form as:
Brain Behav Immun. 2010 May; 24(4): 525–528.
Published online 2009 Nov 5. doi: 10.1016/j.bbi.2009.10.015
PMCID: PMC2856781
NIHMSID: NIHMS157681
PMID: 19896530
The immunoregulatory role of dopamine: an update
Chandrani Sarkar,a Biswarup Basu,b Debanjan Chakroborty,a Partha Sarthi Dasgupta,b,* and Sujit Basua,c,*
Author information Copyright and License information PMC Disclaimer
The publisher's final edited version of this article is available at Brain Behav Immun
Abstract
The neurotransmitter dopamine (DA) is an important molecule bridging the nervous and immune systems. DA through autocrine/paracrine manner modulates the functions of immune effector cells by acting through its receptors present in these cells. DA also has unique and opposite effects on T cell functions. Although DA activates naïve or resting T cells, but it inhibits activated T cells. In addition, changes in the expression of DA receptors and their signaling pathways especially in T cells are associated with altered immune functions in disorders like schizophrenia and Parkinson’s disease. These results suggest an immunoregulatory role of DA. Therefore targeting DA receptors and their signaling pathways in these cells by using DA receptor agonists and antagonists may be useful for the treatment of diseases where DA induced altered immunity play a pathogenic role.
신경 전달 물질인 도파민(DA)은
신경계와 면역계를 연결하는 중요한 분자입니다.
DA는
면역 효과 세포에 존재하는 수용체를 통해 작용하여
자율신경/파라크린 방식으로
면역 효과 세포의 기능을 조절합니다.
DA는
또한 T 세포 기능에 독특하고 상반된 영향을 미칩니다.
DA는
순진하거나 휴지기에 있는 T 세포를 활성화하지만,
활성화된 T 세포는 억제합니다.
또한,
특히 T 세포에서 DA 수용체의 발현과
신호 경로의 변화는 정신분열증이나 파킨슨병과 같은 질환에서
면역 기능의 변화와 관련이 있습니다.
이러한 결과는
DA의 면역 조절 역할을 시사합니다.
따라서
DA 수용체 작용제와 길항제를 사용하여
이러한 세포에서 DA 수용체와 그 신호 경로를 표적으로 삼는 것은
DA에 의해 유도된 변화된 면역이 병원성 역할을 하는 질환의 치료에 유용할 수 있습니다.
Keywords: dopamine, immunity, T cells, dendritic cells, macrophages, NK cells, B cells, microglia
1. Introduction
Besides conventional roles of neurotransmitters in neural communication, a large amount of evidence indicates that neurotransmitters mediate cross talk between the nervous and immune systems (Eskandari and Sternberg, 2002). Among these neurotransmitters, the role of DA is particularly interesting because in addition to regulating behavior, movement, endocrine, cardiovascular, renal and gastrointestinal functions (Basu et al., 1995; Chakroborty et al., 2008; Mezey et al., 1999; Missale et al., 1998), DA can also modulate immune functions (Basu and Dasgupta, 2000). DA is synthesized by different immune effector cells and its receptors are present in these cells (Basu et al., 1993; Basu and Dasgupta, 2000; Eldrup et al., 1989; Ferrari et al., 2004; Kirillova et al., 2008; Le Fur et al., 1980; McKenna et al., 2002; Nakano et al., 2008, 2009). Furthermore the sympathetic innervation of lymphoid tissues can also be dopaminergic in nature, particularly during stress (Bencsics et al., 1997; Mignini et al., 2003). As the majority of recent reports indicate unique interactions between dopamine and T cells, the main focus of this mini-review is on DA mediated regulation of T cell function.
신경 통신에서 신경 전달 물질의 전통적인 역할 외에도
신경 전달 물질이
신경계와 면역계 간의 상호 작용을 매개한다는 많은 증거가 있습니다(Eskandari and Sternberg, 2002).
이러한
신경전달물질 중 DA의 역할은
행동, 운동, 내분비, 심혈관, 신장 및 위장 기능을 조절할 뿐만 아니라(Basu 외, 1995; Chakroborty 외, 2008; Mezey 외, 1999; Missale 외, 1998) 면역 기능도 조절할 수 있기 때문에 특히 흥미롭습니다(Basu and Dasgupta, 2000).
DA는
다양한 면역 효과 세포에 의해 합성되며
이러한 세포에는 수용체가 존재합니다(Basuet al., 1993; Basu and Dasgupta, 2000; Eldrup et al., 1989; Ferrari et al., 2004; Kirillova et al., 2008; Le Fur et al., 1980; McKenna et al., 2002; Nakano et al., 2008, 2009).
또한
림프 조직의 교감 신경 분포는
특히 스트레스를 받는 동안 도파민을 분비할 수 있습니다(Bencsics 등, 1997; Mignini 등, 2003).
최근 보고의 대부분은
도파민과 T 세포 간의 독특한 상호작용을 나타내므로,
이 미니 리뷰의 주요 초점은 DA를 매개로 한 T 세포 기능 조절에 있습니다.
2. DA modulate the functions of immune effector cells by acting through its receptors present in these cells
DA is a monoamine catecholamine neurotransmitter, which acts through its D1 and D2 classes of receptors present in the target cells (Missale et al., 1998). The D1 class includes the D1 and D5 subtypes, which on activation increase intracellular cAMP (Missale et al., 1998). In contrast, the D2 class of receptors, which includes D2, D3, and D4 subtypes, inhibits intracellular cAMP on stimulation (Missale et al., 1998).
Several studies now indicate the presence of DA D1 D2, D3, D4 and D5 receptors in normal human leukocytes (Ferrari et al., 2004; Kirillova et al., 2008; McKenna et al., 2002; Nakano et al., 2008, 2009). Among the leukocyte subpopulations, T lymphocytes, monocytes have low, neutrophils, eosinophils have moderate and B, NK cells have high and more consistent expression of DA receptors (McKenna et al., 2002). In addition, DA D1 receptors are present in human dendritic cells (Nakano et al., 2008). Recently DA uptake system has been identified in the lymphocytes (Amenta et al., 2001; Caronti et al., 2001; Mill et al., 2002).
Furthermore, dopaminergic innervation of lymphoid tissues through sympathetic nerves has also been described, thereby suggesting direct DA-mediated neural regulation of immune effector cells (Bencsics et al., 1997; Mignini et al., 2003). Although these cells primarily come in contact with DA in lymph nodes, spleen, bone marrow and circulation (Basu and Dasgupta, 2000), but DA synthesized and released by T and dendritic cells can also act on DA receptors present in the T cells through an autocrine/paracrine loop (Bergquist, et al., 1994; Cosentino, et al., 2007; Nakano et al., 2009). DA regulates several important functions of these cells (Besser et al., 2005; Ghosh et al., 2003; Levite et al., 2001; Saha et al., 2001a, 2001b; Sarkar et al., 2006; Watanabe et al., 2006). Thus understanding the changes in the immune disorders associated with abnormal dopaminergic activities will help to elucidate the immunomodulatory role of this important neurotransmitter (Basu and Dasgupta, 2000).
3. Altered immunity is seen in diseases with abnormal dopamine function
Altered immune functions have been observed in diseases like schizophrenia and Parkinson’s disease with abnormal dopaminergic systems (Ilani et al., 2001; Nagai et al., 1996; Wandinger et al., 1999). A significantly higher expression of DA D3 receptors and increased IFN-γ synthesis by T cells are reported in untreated schizophrenic patients (Boneberg et al., 2006; Ilani et al., 2001). On the contrary, decreased expression of DA D3 receptors and IFNγ synthesis by peripheral lymphocytes are seen in Parkinson’s disease (Nagai et al., 1996; Wandinger et al., 1999). Because DA D3 receptor mediated increase in IFNγ synthesis by T cells has been demonstrated (Ilani et al., 2004), therefore these immune abnormalities are probably due to the changes in expression of DA D3 receptors and its signaling pathways in the T cells of patients with schizophrenia and Parkinson’s disease (Ilani et al., 2004).
Furthermore as dysfunction of the central dopaminergic system is associated with Parkinson’s disease and schizophrenia, it will be therefore important to mention here that animal studies have indicated brain DA mediated regulation of peripheral immune functions (Basu and Dasgupta, 2000). It has also been recently shown that central dopaminergic hypoactivity increases the risk of inflammation during infection or tissue injury (Engler et al. 2009).
4. DA regulates the functions of immune effector cells through autocrine/paracrine loop
CD4+CD25+ regulatory T lymphocytes (Tregs) are specialized T cells, which play a key role in the control of immune homeostasis (Cosentino et al., 2007). Recently, it has been demonstrated that Tregs contain substantial amounts of dopamine (Cosentino et al., 2007), which after being released acts on the DA D1 receptors present in these cells and subsequently suppress IL-10 and TGFβ synthesis by these cells (Cosentino et al., 2007). In addition, the released DA by acting on DA D1 receptors down-regulates Treg-dependent inhibition of effector T-lymphocyte proliferation and this occurs without affecting the production of TNFα or IFNγ (Cosentino et al., 2007).
Similarly, a paracrine regulatory loop of DA has been shown in the interface of dendritic and T cells (Nakano et al., 2009). DA stored in human monocytic-dendritic cells following its release acts on the D1 receptors present in the naïve T cells, increase cyclic AMP and cause differentiation of these cells into Th2 lineage in response to anti-CD3 plus anti-CD28 mAb (Nakano et al., 2009). However, in absence of dopamine release, T cell differentiation shifts towards Th1 lineage (Nakano et al., 2009). DA is released from these cells following antigen-specific dendritic-T cell interaction (Nakano et al., 2009). Furthermore as stimulation of cAMP increase DA concentration in dendritic cells and because DA by acting through its D1 receptors can increase cAMP concentration, therefore it is possible that the released DA auto-regulates its synthesis in these cells via acting through DA D1 receptors present in these cells (Nakano et al., 2009). These findings indicate that endogenous DA subserves an autocrine/paracrine regulatory loop in the cells of the immune system (Cosentino et al., 2007; Nakano et al., 2009).
5. DA activates resting T cells in absence of any additional stimulating agent
Stimulation of DA D2 and D3 receptors in normal resting peripheral human T lymphocytes, activate α4β1 and α5β1 integrins in these cells, thereby promoting adhesion of these cells to the extracellular matrix component, fibronection (Levite et al., 2001). This action of DA may be critical for trafficking and extravasation of T cells across the blood vessels and tissue barriers (Levite et al., 2001). This study is supported by Watanabe et al., 2006 who have shown that DA stimulates adhesion of CD8+ T cells to fibronectin and ICAM through integrins. These authors have further demonstrated that DA induced chemotactic migration of naïve CD8+Tcells is synergistic with chemokines like CCL19, CCL21 and CXCL12 (Watanabe et al., 2006). This action of DA is reported to be mediated through its D3 receptors present in these cells (Watanabe et al., 2006). DA is also reported to induce cytokine secretion by resting T cells (Besser et al., 2005). It has been shown that stimulation of DA D3 and D1/D5 receptors increase the secretion of TNFα and stimulation of DA D2 receptors induce IL-10 secretion without affecting the secretion of IFNγ and IL-4 (Besser et al., 2005) (Fig. 1A).
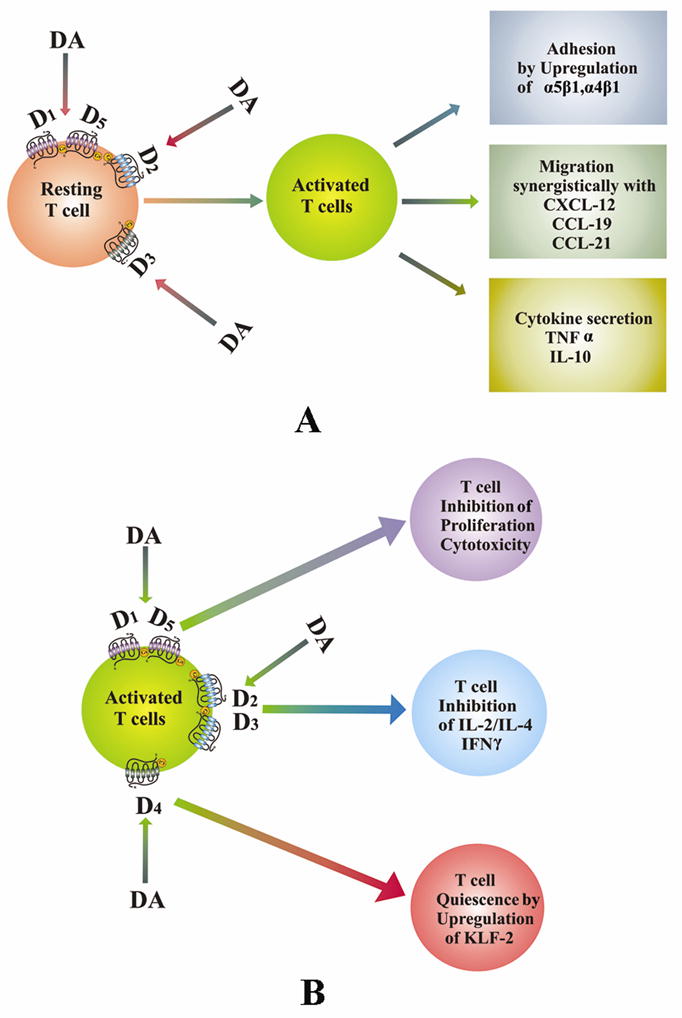
Role of dopamine in T cell functions. (A). DA activates naïve or resting T cells. DA by acting through its receptors stimulates adhesion, migration and cytokine secretion by these cells. (B). DA inhibits activated T cells. DA inhibits the activation of T cells when present during stimulation of T cell receptors and thus inhibits proliferation, cytokine secretion and induces T cell quiescence in these cells by acting through DA receptors. DA, dopamine; D1–D5, dopamine D1–D5 receptors.
6. DA inhibits activation of stimulated T cells
Although DA activated resting T cells, but anti CD3 and IL-2 induced proliferation and cytotoxicity of CD4+ and CD8+ T cells collected from normal human subjects are significantly inhibited when these cells are treated in vitro with high DA concentration observed in the plasma (48.6 pg/ml) of lung cancer patients suffering from uncoping stress (Saha et al., 2001a, 2001b). The molecular mechanism of this action is attributed to DA D1 receptor induced increase in the intracellular cAMP (Saha et al., 2001a, 2001b). Similarly, stimulation of DA D2 and D3 receptors in T cells has been shown to inhibit activated T cell receptor induced cell proliferation, and secretion of IL-2, IFNγ and IL-4 by down regulating the expressions of non receptor tyrosine kinase lck and fyn (Ghosh et al., 2003) (Fig. 1B).
We have also recently shown that stimulation of DA D4 receptors in human T cells during T cell receptor activation is associated with its quiescence (Sarkar et al., 2006). DA induces quiescence of these cells by upregulating the transcription factor, KLF2 via inhibition of ERK1/ERK2 in these cells (Sarkar et al., 2006) (Fig. 1B).
7. DA modulates the functions of NK cells, Splenic cells, Macrophages, B cells and Microglial cells
There are reports which indicate that DA can also modulate the functions of other cells in the immune system (Basu and Dasgupta, 2000). Although Reduced NK cell activities and ovalbumine induced delayed type hypersensitivity responses are reported in animals with hyperdopaminergic systems (Teunis et al., 2004; Kavelaars et al., 2005), but increased LPS-induced cytokine production by macrophages and ovalbumine induced humoral responses have been observed in these animals (Kavelaars et al., 2005).
Moreover, DA treatment has shown to stimulate the proliferation of murine splenocytes by acting through its D2 receptors present in these cells (Carr et al., 2003). A recent report indicates DA mediated inhibition of proliferation in both resting and malignant B lymphocytes (Meredith et al., 2006). Also, DA promotes apoptosis in cycling B cells through oxidative stress. However this action of DA is not demonstrated in resting lymphocytes (Meredith et al., 2006).
Microglial cells are the important immune effector cells in the brain (Chang and Liu, 2000; Färber et al., 2005; Orr et al., 2005; Theodore et al., 2008). After CNS infection, exposure to inflammatory stimuli, or interaction with blood-derived cells, these cells become activated to perform several innate immune functions, including induction of inflammation, cytotoxicity, and regulation of T-cell responses through presentation of antigen. Recent studies have demonstrated the presence of both DA D1 and D2 classes of receptors in the microglial cells (Chang and Liu, 2000; Färber et al., 2005). DA by acting via its D1 receptors regulates the synthesis of microglial nitric oxide, an immune mediator with high antiviral activity (Chang and Liu, 2000; Färber et al., 2005). It has been also shown that DA regulates the migration of these cells in vitro by acting through its receptors present in these cells (Färber et al., 2005).
8. Summary and conclusions
Taken together, the studies outlined above indicate that there is a well defined dopaminergic system in immunity (Basu and Dasgupta, 2000), DA is an important regulator of normal immunity (Basu and Dasgupta, 2000) and changes in the status of DA concentrations and/or receptors, especially in the T cells are responsible for abnormal immune functions seen in patients with schizophrenia and Parkinson’s disease (Ilani et al., 2001, 2004; Nagai et al., 1996; Wandinger et al., 1999). It will be therefore interesting to study if this DA mediated changes in the immune system is also linked to the etiology of these diseases (Ilani et al., 2001, 2004; Nagai et al., 1996; Wandinger et al., 1999).
Because a recent report indicates functional dopaminergic system in the thymus of rats (Mignini et al., 2009), therefore DA may have a role in the maturation and selection of lymphocytes (Mignini et al., 2009). It will therefore be prudent to investigate whether DA has any role in the formation of memory T cells since DA is not only synthesized in T cells, but there is also a functional dopaminergic autocrine regulatory loop in these cells (Bergquist, et al., 1994; Cosentino, et al., 2007). Therefore, elucidation of the detailed mechanisms by which DA activates resting T cells and inhibits stimulated T cells will be necessary to design new and effective therapies in future to modulate the functions of T cells in both health and diseases.
Finally, and most importantly, DA and its agonists or antagonists are being used in the clinics at present for the treatment of other diseases (Katzung, 2004); therefore rapid clinical trials may be undertaken using these inexpensive drugs for the treatment of immune disorders. However these drugs should be used with caution in patients with microbial sepsis as it may suppress the immune functions in these patients (Oberbeck, et al., 2006).
위에 요약된 연구들을 종합하면,
면역에는 도파민 시스템이 잘 정의되어 있고(Basu and Dasgupta, 2000),
DA는 정상 면역의 중요한 조절자이며(Basu and Dasgupta, 2000),
특히 T 세포에서 DA 농도 및/또는 수용체의 상태 변화가 정신분열증과 파킨슨병 환자에서
나타나는 면역 기능 이상에 관여합니다(Ilani 등, 2001, 2004; Nagai 등, 1996; Wandinger et al., 1999).
따라서
DA를 매개로 한 면역 체계의 변화가
이러한 질병의 병인과도 관련이 있는지 연구하는 것은
흥미로울 것입니다(Ilani 등, 2001, 2004; Nagai 등, 1996; Wandinger 등, 1999).
최근 보고에 따르면 쥐의 흉선에서 도파민 시스템이 기능적으로 작동하므로(Mignini et al., 2009), DA가 림프구의 성숙과 선택에 영향을 미칠 수 있습니다(Migninietal., 2009). 따라서 DA는 T 세포에서 합성 될뿐만 아니라 이러한 세포에 기능적 도파민 성 자율 조절 루프가 있기 때문에 DA가 기억 T 세포의 형성에 어떤 역할을하는지 조사하는 것이 신중할 것입니다 (Bergquist 등, 1994; Cosentino 등, 2007).
따라서
향후 건강과 질병 모두에서
T 세포의 기능을 조절하기 위한 새롭고 효과적인 치료법을 설계하려면
DA가 휴면 T 세포를 활성화하고
자극된 T 세포를 억제하는 자세한 메커니즘을 밝혀야 할 것입니다.
마지막으로,
가장 중요한 것은 DA와
그 작용제 또는 길항제가
현재 다른 질병의 치료를 위해 임상에서 사용되고 있다는 것입니다(Katzung, 2004).
따라서
면역 질환 치료를 위해 이러한 저렴한 약물을 사용하여
신속한 임상 시험이 수행될 수 있습니다.
그러나 이러한 약물은
미생물성 패혈증 환자에게는
면역 기능을 억제할 수 있으므로 주의해서 사용해야 합니다(Oberbeck 등, 2006).
Acknowledgments
This work was supported in parts by DRDO (LSRB/24/EPB/2001) Government of India Grant (P.S.D.); Council of Scientific and Industrial Research Government of India Fellowship 9/30(43)/2005-EMR-1 to B.B., National Institutes of Health, USA Grants CA118265 (S. B.), CA 124763 (S. B.), and Department of Defense Grant, USA grant W81XWH-07-1-0051 (S. B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amenta F, Bronzetti E, Cantalamessa F, El-Assouad D, Felici L, Ricci A, Tayebati SK. Identification of dopamine plasma membrane and vesicular transporters in human peripheral blood lymphocytes. J Neuroimmunol. 2001;117:133–142. [PubMed] [Google Scholar]
- Basu S, Dasgupta PS, Chowdhury Roy J. Altered plasma level and uptake of dopamine by platelets in some human malignant tumors. Biog Amines. 1995;11:31–38. [Google Scholar]
- Basu S, Dasgupta PS. Dopamine, a neurotransmitter, influences the immune system. J Neuroimmunol. 2000;102:113–124. [PubMed] [Google Scholar]
- Basu S, Dasgupta PS, Lahiri T, Chowdhury JR. Uptake and biodistribution of dopamine in bone marrow, spleen and lymph nodes of normal and tumor bearing mice. Life Sci. 1993;53:415–424. [PubMed] [Google Scholar]
- Bencsics A, Sershen H, Baranyi M, Hashim A, Lajtha A, Vizi ES. Dopamine, as well as norepinephrine, is a link between noradrenergic nerve terminals and splenocytes. Brain Res. 1997;761:236–243. [PubMed] [Google Scholar]
- Bergquist J, Tarkowski A, Ekman R, Ewing A. Discovery of endogenous catecholamines in lymphocytes and evidence for catecholamine regulation of lymphocyte function via an autocrine loop. Proc Natl Acad Sci U S A. 1994;91:12912–12916. [PMC free article] [PubMed] [Google Scholar]
- Besser MJ, Ganor Y, Levite M. Dopamine by itself activates either D2, D3 or D1/D5 dopaminergic receptors in normal human T-cells and triggers the selective secretion of either IL-10, TNF alpha or both. J Neuroimmunol. 2005;169:161–171. [PubMed] [Google Scholar]
- Boneberg EM, von Seydlitz E, Pröpster K, Watzl H, Rockstroh B, Illges H. D3 dopamine receptor mRNA is elevated in T cells of schizophrenic patients whereas D4 dopamine receptor mRNA is reduced in CD4+-T cells. J Neuroimmunol. 2006;173:180–187. [PubMed] [Google Scholar]
- Caronti B, Antonini G, Calderaro C, Ruggieri S, Palladini G, Pontieri FE, Colosimo C. Dopamine transporter immunoreactivity in peripheral blood lymphocytes in Parkinson’s disease. J Neural Transm. 2001;108:803–807. [PubMed] [Google Scholar]
- Carr L, Tucker A, Fernandez-Botran R. In vivo administration of L-dopa or dopamine decreases the number of splenic IFN gamma-producing cells. J Neuroimmunol. 2003;137:87–93. [PubMed] [Google Scholar]
- Chang JY, Liu LZ. Catecholamines inhibit microglial nitric oxide production. Brain Res Bull. 2000;52:525–530. [PubMed] [Google Scholar]