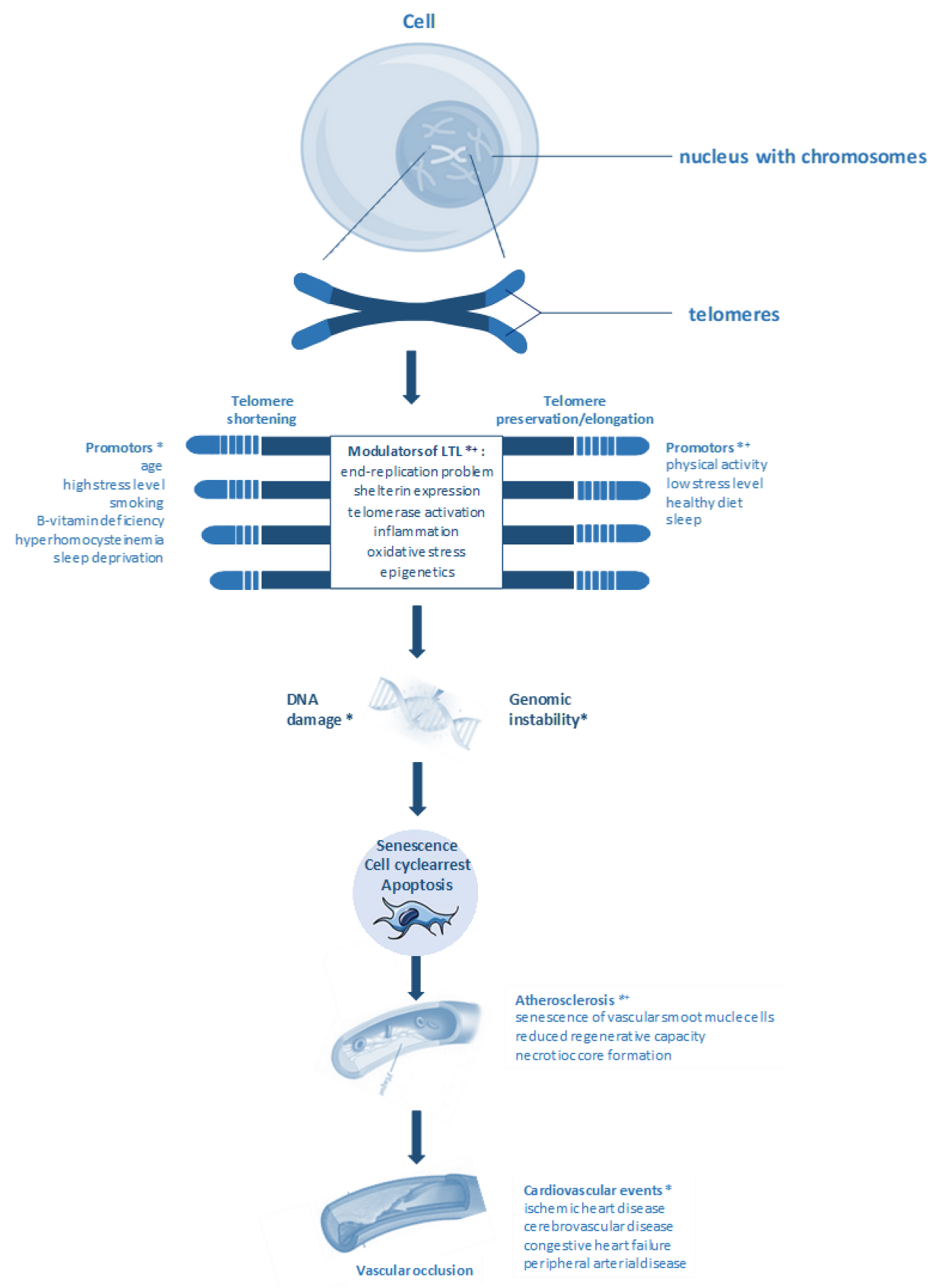
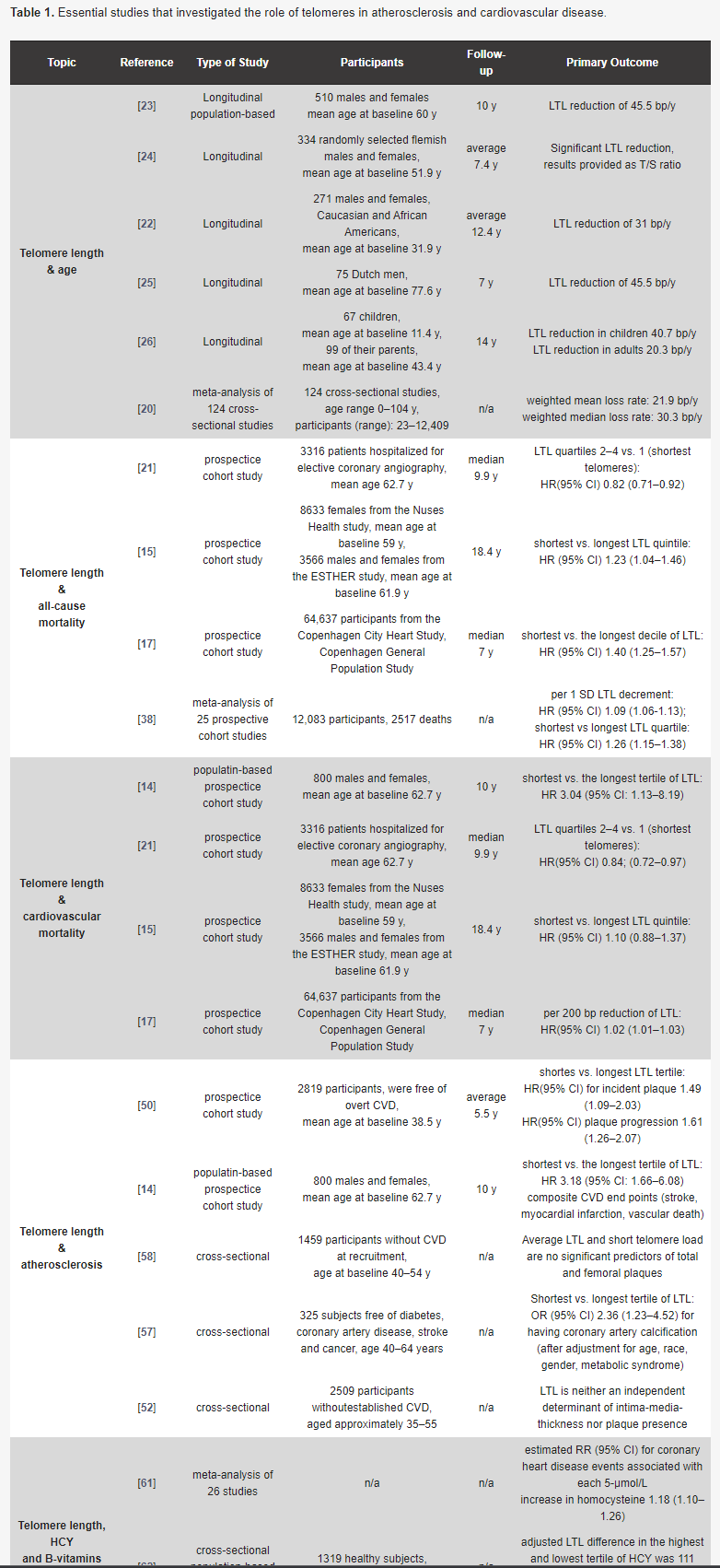
The Importance of Telomere Shortening for Atherosclerosis and Mortality
Author to whom correspondence should be addressed.
Academic Editor: Andy Wessels
J. Cardiovasc. Dev. Dis. 2020, 7(3), 29; https://doi.org/10.3390/jcdd7030029
Received: 15 May 2020 / Revised: 29 July 2020 / Accepted: 30 July 2020 / Published: 6 August 2020
https://cafe.daum.net/panicbird/S5zw/195
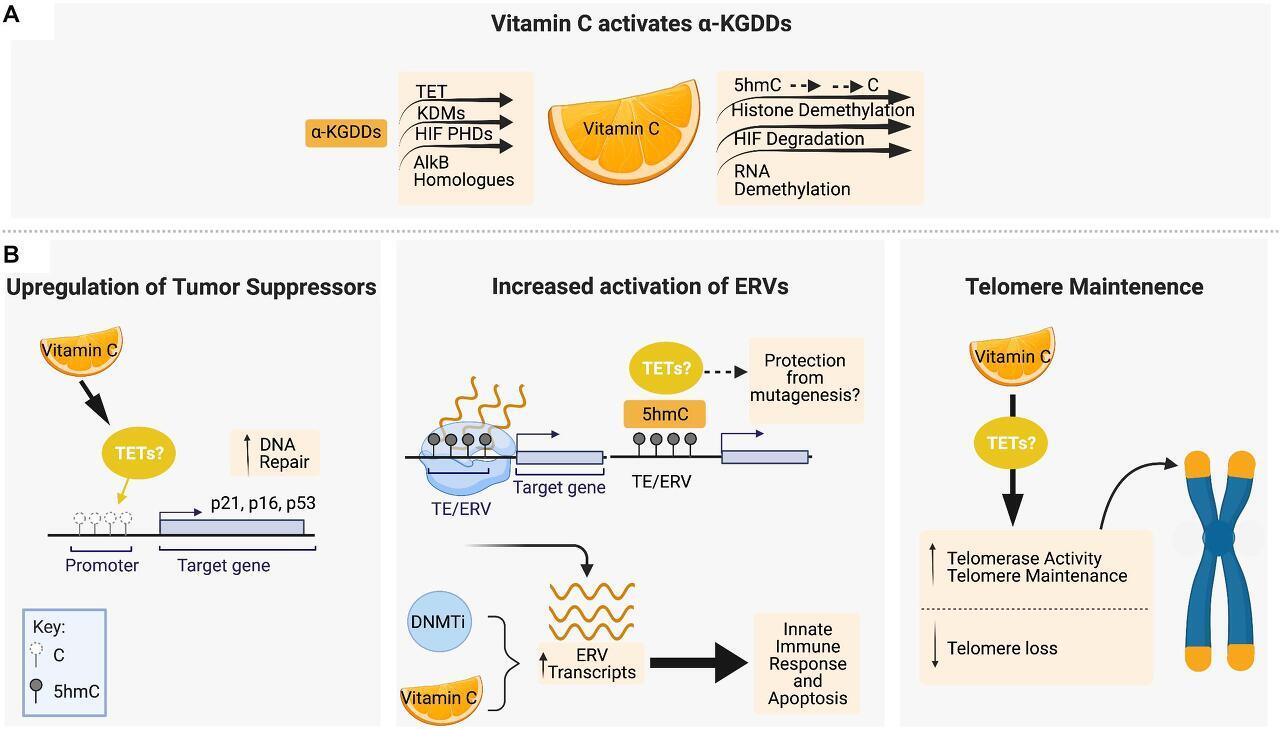
FIGURE 2. Vitamin C maintains genomic stability through interactions with epigenetic regulators, tumor suppressor upregulation and telomere maintenance. (A) Vitamin C serves as a cofactor for members of the α-ketoglutarate-dependent dioxygenases (α-KGDD) family such as TETs, KDMs, HIF PHDs, and ALKBHs, which can work to maintain genomic stability through the demethylation of 5mC residues, histone demethylation, HIF-1α degradation, and RNA demethylation, respectively. (B) Vitamin C supplementation has been shown to promote DNA demethylation in the promotor regions of tumor suppressor loci encoding p16, p21, and p53. Vitamin C promotes DNA demethylation and 5hmC formation at transposable elements (TEs) in the genome, leading to upregulated expression of endogenous retroviral genes (ERVs) in combination with DNA methyltransferase inhibitors (DNMTis). In cancer cells, ERV upregulation initiates an innate immune response and apoptotic cell death which is enhanced by vitamin C. Vitamin C treatment has also been shown to increase telomerase activity and the expression of genes that protect telomere integrity and decrease the rate of telomere loss. Figure created with BioRender.com .
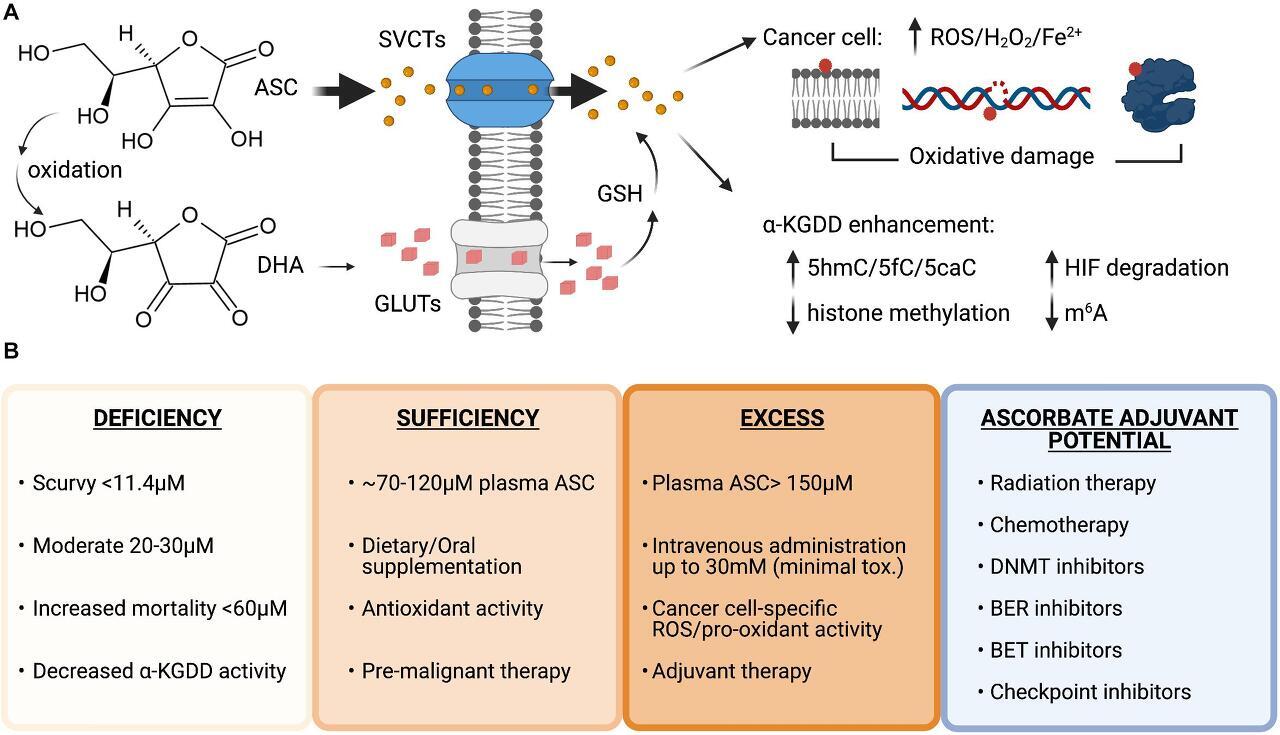
Vitamin C uptake and therapeutic potential as an anti-cancer agent. (A) Structures of ascorbate (ASC) and dehydroascorbate (DHA). ASC primarily enters cells through sodium-dependent vitamin C transporters (SVCTs) and can be transported in its oxidized form as DHA via glucose transporters (GLUTs) then reduced back to ASC by the antioxidant glutathione (GSH) once inside the cell. ASC can enhance the activity of α-ketoglutarate dependent dioxygenases (α-KGDD), and high doses can act as a pro-oxidant, creating increased levels of reactive oxygen species (ROS), H2O2 and increased redox active iron levels (Fe 2+ ) that cause lipid, DNA, and protein oxidation in cancer cells. (B) Plasma ascorbate levels correlate with disease states, overall health, and therapeutic potential. Ascorbate deficiency is associated with increased all-cause mortality and lower α-KGDD activity. Oral supplementation can restore plasma levels to normal and allow vitamin C antioxidant properties to provide benefit as a pre-malignant therapy potentially lowering genomic instability and preventing transformation. High-dose intravenous administration of ASC can be used to generate millimolar (mM) plasma concentrations and generate ROS and pro-oxidant damage to cancer cells. Vitamin C has currently shown efficacy as an adjuvant for many cancer therapies in pre-clinical and clinical trials in combination with standard chemotherapy, DNA hypomethylating agents and targeted inhibitors: Carboplatin and Paclitaxel, Azacitidine, poly-adenosine diphosphate-ribose polymerase (PARP) inhibitors (Olaparib), immune checkpoint inhibitors (anti-PD1 and anti-CTLA4), and bromodomain and extraterminal domain (BET) inhibitors (JQ1). Figure created with BioRender.com .
Review Article | Open Access
Volume 2017 | Article ID 8936156 | https://doi.org/10.1155/2017/8936156
Show citation
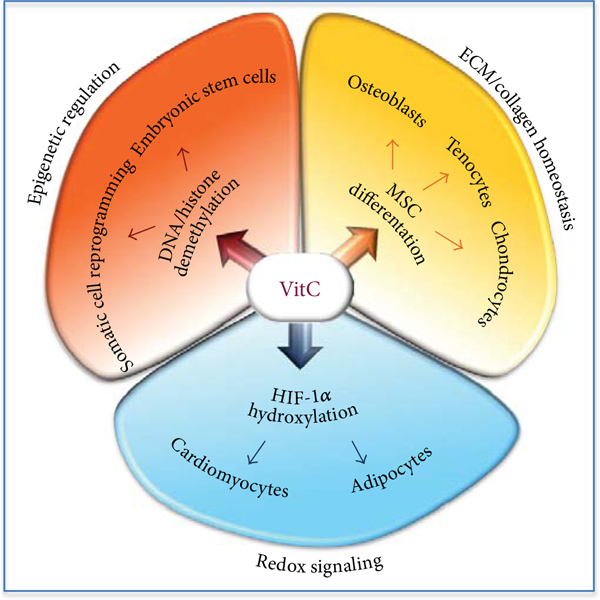
Vitamin C in Stem Cell Biology: Impact on Extracellular Matrix Homeostasis and Epigenetics
다음검색