Eur J Nutr. 2018; 57(1): 1–24.
Published online 2017 Apr 9. doi: 10.1007/s00394-017-1445-8
PMCID: PMC5847071
PMID: 28393285
Gut microbiota functions: metabolism of nutrients and other food components

1 Glenn Gibson,1 Almut Heinken,2 Karen Scott,3 Jonathan Swann,4 Ines Thiele,2 and Kieran Tuohy5
Author information Article notes Copyright and License information PMC Disclaimer
Abstract
The diverse microbial community that inhabits the human gut has an extensive metabolic repertoire that is distinct from, but complements the activity of mammalian enzymes in the liver and gut mucosa and includes functions essential for host digestion. As such, the gut microbiota is a key factor in shaping the biochemical profile of the diet and, therefore, its impact on host health and disease. The important role that the gut microbiota appears to play in human metabolism and health has stimulated research into the identification of specific microorganisms involved in different processes, and the elucidation of metabolic pathways, particularly those associated with metabolism of dietary components and some host-generated substances. In the first part of the review, we discuss the main gut microorganisms, particularly bacteria, and microbial pathways associated with the metabolism of dietary carbohydrates (to short chain fatty acids and gases), proteins, plant polyphenols, bile acids, and vitamins. The second part of the review focuses on the methodologies, existing and novel, that can be employed to explore gut microbial pathways of metabolism. These include mathematical models, omics techniques, isolated microbes, and enzyme assays.
인간의 장에 서식하는 다양한 미생물 군집은
간과 장 점막의 포유류 효소와는 구별되지만
포유류 효소의 활동을 보완하는 광범위한 대사 레퍼토리를 가지고 있으며
숙주의 소화에 필수적인 기능을 포함합니다.
따라서 장내 미생물은
식단의 생화학적 프로필을 형성하는 핵심 요소이며,
따라서 숙주의 건강과 질병에 미치는 영향에 영향을 미칩니다.
장내 미생물이
인간의 신진대사와 건강에 중요한 역할을 하는 것으로 밝혀지면서
다양한 과정에 관여하는 특정 미생물의 확인과
대사 경로,
특히 식이 성분 및 일부 숙주 생성 물질의 대사와
관련된 대사 경로의 규명에 대한 연구가 활발해졌습니다.
리뷰의 첫 번째 부분에서는
주요 장내 미생물,
특히
박테리아와 식이 탄수화물(단쇄 지방산 및 가스),
단백질,
식물 폴리페놀,
담즙산 및
비타민의 대사와 관련된 미생물 경로에 대해 설명합니다.
이 리뷰의 두 번째 부분에서는
장내 미생물의 대사 경로를 탐구하는 데 사용할 수 있는 기존 및 새로운 방법론에 초점을 맞춥니다.
여기에는 수학적 모델, 오믹스 기술, 분리 미생물 및 효소 분석이 포함됩니다.
Keywords: Gut microbiota, Microbiome, Microbial metabolism, Food components, Methodology
Introduction
The human colonic microbiota is a large and complex microbial community. In total, over 1000 bacterial species have been identified of which many remain uncultured, with about 160 species being found in the gut of any individual [1]. The gene set of the gut microbiota (the gut microbiome) is estimated to be about 3 million genes −150 times larger than that of the human genome [2]. This large and diverse microbial community has an equally extensive metabolic repertoire that complements the activity of mammalian enzymes in the liver and gut mucosa [3]. The gut microbiota makes an important contribution to human metabolism by contributing enzymes that are not encoded by the human genome, for example, the breakdown of polysaccharides, polyphenols and synthesis of vitamins. The evidence for the role of the microbiota in metabolism of dietary components and for its impact on health is derived from comparative studies in germ-free and conventional microbiota, or human microbiota-associated animals, and from in vitro studies using human faecal incubations or more complex continuous culture gut models. Furthermore, observational studies comparing the faecal microbiota of healthy subjects with those of patients strongly suggest that the gut microbiota plays a significant role in the aetiology and/or development of a range of gastrointestinal diseases and conditions such as inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), colon cancer, and antibiotic-associated diarrhoea. More recently evidence has been accumulating that the microbiota may also be involved in obesity and diabetes [4, 5].
인간의 대장 미생물은 크고 복잡한 미생물 군집입니다.
총 1,000여 종의 박테리아가 확인되었으며,
이 중 상당수는 아직 배양되지 않은 상태로 남아 있으며,
약 160여 종은 모든 개인의 장에서 발견됩니다[1].
장내 미생물 군집(장내 미생물군)의 유전자 세트는
인간 게놈의 150배에 달하는
약 300만 개의 유전자로 추정됩니다[2].
이 크고 다양한 미생물 군집은
간과 장 점막에서 포유류 효소의 활동을 보완하는
광범위한 대사 레퍼토리를 가지고 있습니다[3].
장내 미생물은
다당류,
폴리페놀 분해,
비타민 합성 등
인간 게놈에 암호화되지 않은 효소를 제공함으로써
인간의 신진대사에 중요한 기여를 합니다.
식이 성분의 대사에서 미생물총의 역할과 건강에 미치는 영향에 대한 증거는
무균 및 기존 미생물총 또는
인간 미생물총 관련 동물의 비교 연구와
인간 대변 배양 또는 보다 복잡한 연속 배양 장 모델을 사용한 체외 연구로부터 도출됩니다.
또한 건강한 피험자의 분변 미생물과 환자의 분변 미생물을 비교한 관찰 연구에 따르면
장내 미생물이
염증성 장 질환(IBD),
과민성 대장 증후군(IBS),
대장암,
항생제 관련 설사 등 다양한 위장 질환 및 상태의 원인 및/또는
발병에 중요한 역할을 하는 것으로 나타났습니다.
최근에는
장내 미생물이 비만과 당뇨병에도 관여할 수 있다는 증거가 축적되고 있습니다[4, 5].
The critical role that the gut microbiota appears to play in human metabolism and health has stimulated research to identify the microorganisms involved and their functionality, in relation to metabolic pathways, particularly those associated with metabolism of dietary components. These areas are the focus of the present review.
장내 미생물이
인간의 신진대사와 건강에 중요한 역할을 하는 것으로 밝혀지면서
대사 경로, 특히
식이 성분의 대사와 관련된 경로와 관련하여
관련된 미생물과 그 기능을 규명하려는 연구가 활발해졌습니다.
이번 리뷰에서는 이러한 분야를 중점적으로 살펴봅니다.
Carbohydrates
Bacteria in the large intestine mainly rely on dietary substrates that are undigested in the upper digestive tract for survival. Saccharolytic bacterial fermentation produces generally beneficial metabolites, whereas if there is limited carbohydrate, bacteria turn to alternative energy sources resulting in the production of other metabolites that may be more detrimental to human health [6]. The key bacterial fermentation products following the fermentation of dietary carbohydrates are short chain fatty acids and gases (Fig. 1).
대장의 박테리아는
생존을 위해 주로 상부 소화관에서 소화되지 않은
식이 기질에 의존합니다.
당분해성 박테리아 발효는
일반적으로 유익한 대사산물을 생성하는 반면,
탄수화물이 제한되면
박테리아는 대체 에너지원으로 전환하여
인체 건강에 더 해로울 수 있는 다른 대사산물을 생성하게 됩니다[6].
식이 탄수화물의 발효에 따른 주요 박테리아 발효 생성물은
단쇄 지방산과 가스입니다(그림 1).
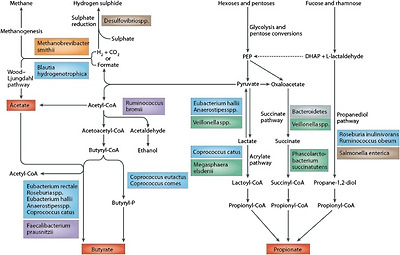
Pathways of carbohydrate metabolism [156]
Short chain fatty acids (SCFAs)
The three most abundant SCFAs detected in faeces are acetate, propionate, and butyrate, normally present in molar ratios ranging from 3:1:1 to 10:2:1. This ratio is consistent with values observed within the intestine in early sudden death victims [7]. These three main SCFAs perform very different but important roles in the human body. Butyrate is arguably the most important SCFA for human health. It forms the key energy source for human colonocytes and also has potential anti-cancer activity via the ability to induce apoptosis of colon cancer cells and its ability to regulate gene expression by inhibiting histone deacetylases [8]. There is also evidence that butyrate can activate intestinal gluconeogenesis (IGN) via a cAMP-dependent mechanism with beneficial effects on glucose and energy homeostasis [9]. Propionate is also an energy source for the epithelial cells, but is also transferred to the liver where it also plays a role in gluconeogenesis. It is also increasingly thought to be an important molecule in satiety signalling due to interaction with the gut receptors (G protein-coupled receptor, GPR) GPR 41 and GPR 43, also known as Fatty Acid Receptors FFAR2 and FFAR3, which may, in turn, activate intestinal IGN [9–11]. The conversion of propionate to glucose in intestinal gluconeogenesis directly promotes energy homeostasis by reducing the production of hepatic glucose, and consequently reduces adiposity [9]. Acetate is the most abundant SCFA, and is an essential co-factor/metabolite for the growth of other bacteria. For instance, Faecalibacterium prausnitzii will not grow in pure culture in the absence of acetate [12]. Within the human body, acetate is transported to the peripheral tissues and used in cholesterol metabolism and lipogenesis, and recent evidence from studies in mice indicates that it also plays a significant role in central appetite regulation [13].
대변에서 가장 많이 검출되는 세 가지 SCFA는
아세테이트,
프로피온산,
부티레이트이며,
일반적으로 3:1:1에서 10:2:1의 몰 비율로 존재합니다.
이 비율은 돌연사 초기 환자의 장내에서 관찰된 수치와 일치합니다[7].
이 세 가지 주요 SCFA는
인체에서 매우 다르지만 중요한 역할을 수행합니다.
부티레이트는
인체 건강에 가장 중요한 SCFA입니다.
부티레이트는
인간 대장 세포의 주요 에너지원을 형성하며
대장암 세포의 세포 사멸을 유도하는 능력과
히스톤 탈아세틸화 효소를 억제하여
유전자 발현을 조절하는 능력을 통해 잠재적인 항암 작용도 가지고 있습니다 [8].
또한
부티레이트가
포도당과 에너지 항상성에 유익한 영향을 미치는
cAMP 의존적 메커니즘을 통해
장내 포도당 생성(IGN)을 활성화할 수 있다는 증거도 있습니다 [9].
프로피오네이트는
상피 세포의 에너지원이기도 하지만
간으로 전달되어 포도당 생성에도 중요한 역할을 합니다.
또한 프로피오네이트는
지방산 수용체 FFAR2 및 FFAR3로도 알려진
장 수용체(G 단백질 결합 수용체, GPR) GPR 41 및
GPR 43과의 상호작용으로 인해
포만 신호에 중요한 분자로 여겨지고 있으며,
이는 결과적으로 장내 IGN(포도당생성)을 활성화할 수 있습니다 [9-11].
장내 포도당 생성에서
프로피오네이트가 포도당으로 전환되면
간 포도당 생성을 감소시켜
에너지 항상성을 직접적으로 촉진하고
결과적으로 지방을 감소시킵니다[9].
아세테이트는
가장 풍부한 SCFA이며
다른 박테리아의 성장에 필수적인 보조 인자/대사 산물입니다.
예를 들어,
페칼리박테리움 프라우스니치균은
아세테이트가 없으면 순수 배양에서 성장하지 않습니다 [12].
인체 내에서 아세테이트는
말초 조직으로 운반되어
콜레스테롤 대사와 지방 생성에 사용되며,
최근 생쥐를 대상으로 한 연구에 따르면
중추 식욕 조절에도 중요한 역할을 하는 것으로 나타났습니다 [13].
Bacterial cross-feeding
Bacteria produce intermediate fermentation products including fumarate, succinate, and lactate, but these are normally detected at low levels in faeces from healthy individuals due to extensive utilization of them by other bacteria. For example, lactate is typically converted into either propionate or butyrate by other bacteria, and is thus present at negligible levels in adult faeces. However, in patients with ulcerative colitis, lactate can be detected in significantly higher amounts [14] and could potentially be an indicator of disease. Co-culture cross-feeding studies illustrate the impact of bacterial interactions on final SCFA detection. Lactate produced by Bifidobacterium longum during growth in pure culture on fructo-oligo-saccharides (FOS) completely disappeared in co-culture with Eubacterium hallii, and was replaced by significant butyrate levels- despite the fact that E. hallii alone could not grow on the carbohydrate substrate [15]. Growth of Roseburia intestinalis is stimulated by acetate and in co-culture with a different strain of B. longum, growth of the R. intestinalis on FOS was delayed until sufficient acetate, produced by B. longum, accumulated in the growth medium [16].
박테리아는
푸마르산염, 숙신산염, 젖산염을 포함한 중간 발효 산물을 생성하지만,
다른 박테리아가 이를 광범위하게 이용하기 때문에
건강한 사람의 대변에서는 일반적으로 낮은 수준으로 검출됩니다.
예를 들어 젖산염은
일반적으로 다른 박테리아에 의해
프로피오네이트 또는 부티레이트 형태로 전환되므로
성인 대변에서는 무시할 수 있는 수준으로 존재합니다.
그러나
궤양성 대장염 환자의 경우
젖산염이 상당히 많은 양으로 검출될 수 있으며[14]
잠재적으로 질병의 지표가 될 수 있습니다.
공동 배양 교차 수유 연구는
박테리아 상호 작용이
최종 SCFA 검출에 미치는 영향을 보여줍니다.
비피도박테리움 롱검이
프락토올리고당(FOS)에 대한 순수 배양에서 성장하는 동안
생성된 젖산염은
유박테리움 할리와 공배양에서 완전히 사라지고
상당한 부티레이트 수치로 대체되었는데,
이는 E. 할리 단독으로는 탄수화물 기질에서 성장할 수 없음에도 불구하고 [15] 나타났습니다.
로즈부리아 인테스티날리스의 성장은
아세테이트에 의해 자극되며,
다른 균주인 B. 롱검과의 공배양에서는
B. 롱검에 의해 생성된 충분한 아세테이트가
성장 배지에 축적될 때까지
FOS에서 R. 인테스티날리스의 성장이 지연되었습니다 [16].
Specificity of SCFA production by intestinal species
Acetate is produced by many bacteria, but propionate and butyrate tend to be produced by specific bacteria [17, 18;]. Within the gastrointestinal environment, the predominant butyrate producers are Firmicutes including some Lachnospiraceae and also Faecalibacterium prausnitzii, whilst propionate is produced by Bacteroides species, Negativicutes, and also some Clostridium species. Metagenomic screening of more than 3000 sequenced bacterial genomes identified many other species containing butyrate production pathways, with no consistency within families [19]. Since the production of SCFA is not defined by bacterial phylogeny, different methods targeting key genes are required to enumerate bacteria with specific metabolic activities. Louis and co-workers identified two main routes of butyrate production [20], and three pathways for propionate production [18], amongst the colonic microbiota. The primers designed against key metabolic genes in these pathways can help to enumerate functional groups of bacteria in different cohorts. This approach may prove more useful than the current focus on the 16S rRNA gene, which provides information about the bacterial composition but indicates nothing about fluctuations in metabolic activities.
아세테이트는
많은 박테리아에 의해 생성되지만
프로피온산과 부티레이트는 특정 박테리아에 의해 생성되는 경향이 있습니다 [17, 18;].
위장 환경에서
부티레이트의 주요 생산자는
일부 라크노스피라세아과와 페칼리박테리움 프라우스니치균을 포함한 펌리쿠테스(Firmicutes)이며,
프로피오네이트는
박테로이데스 종, 네거티비쿠테스, 일부 클로스트리디움 종에서 생산됩니다.
3000개 이상의 시퀀싱된 박테리아 게놈에 대한 메타게놈 스크리닝을 통해
부티레이트 생산 경로를 포함하는 다른 많은 종을 확인했지만,
계열 내 일관성은 없었습니다[19].
SCFA의 생산은
박테리아 계통 발생에 의해 정의되지 않기 때문에
특정 대사 활동을 가진 박테리아를 열거하려면 핵심 유전자를 표적으로 하는 다양한 방법이 필요합니다.
루이스와 동료들은
대장 미생물군에서
부티레이트 생산의 두 가지 주요 경로[20]와
프로피온산 생산의 세 가지 경로[18]를 확인했습니다.
이러한 경로의 주요 대사 유전자에 대해 설계된 프라이머는 다양한 코호트에서 박테리아의 기능적 그룹을 열거하는 데 도움이 될 수 있습니다. 이 접근법은 박테리아 구성에 대한 정보는 제공하지만 대사 활동의 변동에 대해서는 아무것도 알려주지 않는 현재 16S rRNA 유전자에 초점을 맞추고 있는 것보다 더 유용할 수 있습니다.
It is important to note that propionate and butyrate are also formed from peptide and amino-acid fermentation by certain Bacteroidetes and Firmicutes species [21]. In vitro studies indicate that aspartate, alanine, threonine, and methionine are the main sources of propionate, whereas butyrate is predominantly derived from fermentation of glutamate, lysine, histidine, cysteine, serine, and methionine.
프로피오네이트와 부티레이트는
특정 박테로이데테스 및 피르미쿠테스 종에 의한
펩타이드 및
아미노산 발효에서도
형성된다는 점에 유의하는 것이 중요합니다[21].
시험관 내 연구에 따르면
아스파르트산염,
알라닌,
트레오닌,
메티오닌이 프로피오네이트의 주요 공급원인 반면
부티레이트는
글루타메이트,
라이신,
히스티딘,
시스테인,
세린,
메티오닌의 발효에서 주로 파생되는 것으로 나타났습니다.
A targeted gene approach revealed that most bacteria either had the capability to produce propionate or butyrate—very few had genetic capacity to produce both [18]. Some bacteria can, however, alter their fermentation and produce different SCFA under different, substrate-dependent, growth conditions. Roseburia inulinivorans is a butyrate producer, but during growth on fucose, it is able to completely change its gene expression pattern, switching on a set of genes capable of utilizing fucose as an energy source, and producing propionate and propanol via a propanediol utilization pathway [22]. Ruminococcus obeum produces acetate, formate, and lactate during growth on glucose on pure culture, but additionally produces propionate during growth on fucose, again using the propanediol utilization pathway [18]. Fucose is a particularly important alternative dietary substrate, since many of the epithelial glycoconjugates are fucosylated. The ability of a bacterium to flick a metabolic switch and change its metabolism, and metabolic products, may give the bacterium a competitive advantage during times of low substrate availability. In Bacteroides thetaiotaomicron, the presence of fucose as a growth substrate not only stimulates expression of genes involved in fucose metabolism, but intracellular fucose levels are also critical in activating a signalling mechanism to the host, increasing synthesis of fucosylated glycans [23] and thus ensuring a continued supply of substrate to the bacterium. This entire alternative metabolism is upregulated during periods of nutrient depletion, and it may also be important in early colonization events in the infant gut [24].
표적 유전자 접근법에 따르면
대부분의 박테리아는
프로피온산 또는 부티레이트를 생산할 수 있는 능력을 가지고 있으며,
극소수의 박테리아는 두 가지를 모두 생산할 수 있는 유전적 능력을 가지고 있습니다[18].
그러나
일부 박테리아는 발효를 변경하여
기질에 따라 다른 성장 조건에서
다른 SCFA를 생산할 수 있습니다.
로즈부리아 이눌리니보란스는
부티레이트 생산자이지만,
포도당에서 성장하는 동안 유전자 발현 패턴을 완전히 바꾸어
포도당을 에너지원으로 활용할 수 있는 유전자 세트를 켜고
프로판디올 활용 경로를 통해
프로피오네이트와 프로판올을 생산할 수 있습니다[22].
루미노코커스 오붐은
순수 배양에서 포도당으로 성장하는 동안
아세테이트, 포름산염, 젖산염을 생산하지만,
포도당으로 성장하는 동안 프로판디올 활용 경로를 사용하여
프로피오네이트를 추가로 생산합니다 [18].
많은 상피 글리코콘쥬게이트가 푸코실화되어 있기 때문에
포도당은 특히 중요한 대체 식이 기질입니다.
박테리아가 대사 스위치를 켜고
신진대사와 대사 산물을 변화시키는 능력은
기질 가용성이 낮은 시기에
박테리아에 경쟁 우위를 제공할 수 있습니다.
박테로이데스 테타이오토마이크론에서
성장 기질로 포도당이 존재하면
포도당 대사에 관여하는 유전자의 발현이 자극될 뿐만 아니라
세포 내 포도당 수준은
숙주에 대한 신호 메커니즘을 활성화하여
푸코실화된 글리칸의 합성을 증가시켜[23]
박테리아에 기질을 지속적으로 공급하는 데도 매우 중요합니다.
이러한 전체 대체 대사는
영양소가 고갈되는 기간 동안 상향 조절되며,
유아 장내의 초기 식민지화 현상에서도 중요할 수 있습니다[24].
Altering the carbohydrate content of the diet can also alter the faecal SCFA profile by affecting the bacterial composition. Reducing the carbohydrate content of the diet significantly reduced both faecal butyrate concentrations and numbers of the Roseburia/E. rectale group in human studies [25], while wheat bran supplementation (consisting of >70% arabinoxylan oligo-saccharides; AXOS) increased the abundance of all three predominant SCFAs and thus also total SCFA concentrations [26]. However, it is probable that the indiscriminate increases in faecal SCFA concentrations observed in studies where the fibre content of the diet is increased are at least partly caused by the increased faecal bulking and reduced transit time resulting in decreased colonic absorption of SCFAs. The FODMAP diet, a diet low in Fermentable Oligo-saccharides, Disaccharides, Monosaccharides And Polyols, and thus designed to reduce large intestinal bacterial fermentation, is increasingly used as an effective therapy to treat IBS. Although the diet is associated with increased faecal pH, presumably due to less bacterial fermentation, the actual faecal concentration of different SCFAs is similar to the control diet [27], illustrating the complex association between SCFA production, absorption, and excretion. Total numbers of bacteria declined on the FODMAP diet compared to the habitual Australian diet, with the proportion of a few specific bacterial groups significantly affected [27].
식단의 탄수화물 함량을 변경하면
박테리아 구성에 영향을 미쳐 분변
SCFA 프로필도 달라질 수 있습니다.
인간 대상 연구에서
탄수화물 함량을 줄이면
분변 부티레이트 농도와 로즈부리아/E. 렉탈 그룹의 수가 크게 감소한 반면[25],
밀기울 보충제(아라비녹실란 올리고당 70% 이상으로 구성; AXOS)는
세 가지 주요 SCFA의 풍부도를 모두 증가시켜
총 SCFA 농도도 증가시켰습니다[26].
그러나
식이 섬유 함량이 증가한 연구에서
관찰된 분변 SCFA 농도의 무차별적인 증가는
적어도 부분적으로는 대변의 부피 증가와
통과 시간 감소로 인한
SCFA의 대장 흡수 감소로 인한 것일 가능성이 높습니다.
발효성 올리고당, 이당류, 단당류 및 다당류가 적고
대장 박테리아 발효를 줄이기 위해 고안된 식단인 FODMAP 식단은
IBS 치료에 효과적인 치료법으로 점점 더 많이 사용되고 있습니다.
이 식단은
박테리아 발효가 적기 때문에
분변 pH 증가와 관련이 있지만,
다른 SCFA의 실제 분변 농도는 대조 식단과 유사하여[27]
SCFA 생산, 흡수 및 배설 사이의 복잡한 연관성을 보여줍니다.
총 박테리아 수는 습
관적인 호주식 식단에 비해 FODMAP 식단에서 감소했으며,
몇 가지 특정 박테리아 그룹의 비율이 크게 영향을 받았습니다 [27].
Bacterial gas production in the intestinal tract
Gas is an inevitable product of microbial fermentation in anaerobic ecosystems, including the alimentary tract. For example, hydrogen is an important fermentation intermediate and interspecies hydrogen transfer occurs when electron flow shifts from reduced organic products towards proton reduction. This can be achieved through the production of further gases like H2S or methane. This disposes of excess reducing power generated in reactions involving the oxidation of organic material.
Gas formation is not a universal trait among bacteria growing anaerobically and the biochemistry of some species involves no gas generation at all [28]. This is the case for common probiotics like lactobacilli and bifidobacteria. It is, therefore, theoretically feasible that probiotic or prebiotic use may reduce gas occurrence in the gut and also help negate odoriferous problems. Gas generated as a consequence of anaerobic bacterial fermentation may be partially excreted via the lungs or as flatus. In the healthy human, investigations have indicated that the volume of flatus excreted can reach up several litres per day [29]. The majority of bacterially generated gas comprises hydrogen, carbon dioxide, and methane, all odourless gases. In general, less than 1% of flatus is oxygen, which together with nitrogen, accounts for only about 26% of flatus [30]. While odoriferous gases constitute less than 1% of total flatus and include NH3, hydrogen sulphide, indole, skatole, and volatile amines, their accumulation is certainly noticeable. Key noxious, as well as potentially toxic, constituents are the sulphides, which also act as precursors for other S-based components like mercaptans.
가스는
소화관을 포함한 혐기성 생태계에서
미생물 발효의 필연적인 산물입니다.
예를 들어,
수소는
중요한 발효 중간체이며
종간 수소 이동은 전자의 흐름이 환원된 유기물에서 양성자 환원 쪽으로 이동할 때 발생합니다.
이는 H2S나 메탄과 같은
추가 가스의 생성을 통해 이루어질 수 있습니다.
이렇게 하면 유기 물질의 산화와 관련된 반응에서 생성된 과도한 환원 전력을 처리할 수 있습니다.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5023273/

가스 형성은
혐기성으로 성장하는 박테리아의 보편적인 특성이 아니며
일부 종의 생화학에서는 가스 생성이 전혀 일어나지 않습니다[28].
유산균이나 비피더스균과 같은 일반적인 프로바이오틱스가 이에 해당합니다.
따라서
프로바이오틱스 또는
프리바이오틱스를 사용하면
장내 가스 발생을 줄이고 악취 문제를 없애는 데 도움이 될 수 있다는 것은 이론적으로 가능합니다.
혐기성 박테리아 발효의 결과로 생성된 가스는
부분적으로 폐를 통해 배출되거나 방귀로 배출될 수 있습니다.
건강한 사람의 경우, 조사에 따르면 배설되는 방귀의 양이
하루에 최대 수 리터에 달할 수 있다고 합니다[29].
박테리아에 의해 생성되는 가스의 대부분은
수소,
이산화탄소,
메탄으로 구성되어 있으며
모두 무취의 기체입니다.
일반적으로
방귀의 1% 미만은
산소이며,
질소와 함께 방귀의 약 26%만을 차지합니다[30].
냄새가 나는 가스는
전체 방귀의 1% 미만을 차지하며
NH3, 황화수소, 인돌, 스카톨 및 휘발성 아민을 포함하지만,
그 축적은 확실히 눈에 띄게 나타납니다.
잠재적으로
독성이 있는 주요 유해 성분은 황화물이며,
황화물은 메르캅탄과 같은 다른 S계 성분의 전구체 역할을 하기도 합니다.
Hydrogen
The hydrogen composition of flatus ranges up to 40% and it seems that it is exclusively of microbial origin [31, 32]. Hydrogen is produced by a variety of gut bacteria, the most predominant including Bacteroides and Clostridium. These genera are common components of the microbiota, implying that they are the principal source of microbial gas [33]. The theoretical rate of hydrogen production far exceeds what is actually excreted, as it can be re-utilized by the gut microbiota. The removal of hydrogen allows a more complete oxidation of organic substrates and, therefore, a higher energy yield from anaerobic fermentation.
There are three main microbial routes by which hydrogen can be removed to enable a depletion of electron sink products such as lactate, succinate, and ethanol, and allow more efficient energy recovery from organic substrates. These are dissimilatory sulphate reduction, methanogenesis, and acetogenesis.
방귀의 수소 구성은
최대 40%에 이르며,
이는 전적으로 미생물에서 기인하는 것으로 보입니다 [31, 32].
수소는
다양한 장내 박테리아에 의해 생성되며,
박테로이데스와 클로스트리듐이 가장 우세합니다.
이 속은 미생물 군집의 일반적인 구성 요소로,
미생물 가스의 주요 공급원임을 의미합니다 [33].
이론적인 수소 생산 속도는
장내 미생물에 의해 재이용될 수 있기 때문에
실제로 배설되는 양을 훨씬 초과합니다.
수소를 제거하면
유기 기질을 더 완벽하게 산화할 수 있으므로
혐기성 발효에서 더 높은 에너지 생산량을 얻을 수 있습니다.
수소를 제거하여
젖산염, 숙신산염, 에탄올과 같은 전자 싱크 생성물을 고갈시키고
유기 기질에서 더 효율적으로 에너지를 회수할 수 있는
미생물 경로에는 세 가지 주요 경로가 있습니다.
이러한
황산염 환원,
메탄 생성,
아세트 생성에는
용해성 황산염 환원, 메탄 생성, 아세트 생성이 있습니다.
Dissimilatory sulphate reduction is carried out by sulphate reducing bacteria (SRB). These microorganisms utilize sulphate (as opposed to oxygen used in the conventional aerobic respiration) as an electron acceptor for the dissimilation of organic compounds and hydrogen [32]. The main genus of gut SRB is Desulfovibrio [31]. Sulphate can be provided in the diet or released following microbial metabolism of sulphated mucins. These are glycoproteins that line the gastrointestinal tract, acting as lubricant as well as a protective barrier between the mucosal surface and the luminal contents.
The utilization of hydrogen to reduce sulphate to sulphide has effects on overall colonic gas production by reducing the amount of free hydrogen in the colon, thereby helping to prevent excessive gas build up:
4H2 + SO42- + H+ → HS- + 4H2O.
용해성 황산염 환원은
황산염 환원 박테리아(SRB)에 의해 수행됩니다.
이 미생물은
유기 화합물과 수소를 분해하기 위한 전자 수용체로
황산염(기존의 호기성 호흡에 사용되는 산소와 반대)을 사용합니다[32].
장내 SRB의 주요 속은 데설포비브리오입니다 [31].
황산염은
식단에서 제공되거나
황산화된 뮤신의 미생물 대사에 따라 방출될 수 있습니다.
뮤신은
위장관을 감싸고 있는 당단백질로,
점막 표면과 내강 내용물 사이의 보호 장벽이자 윤활제 역할을 합니다.
황산염을 황화물로 환원하기 위해 수소를 사용하면
대장 내 유리 수소의 양을 줄여
과도한 가스 축적을 방지함으로써
전반적인 대장 가스 생성에 영향을 미칩니다:
However, the highly toxic nature of the hydrogen sulphide that is generated can have pathological consequences for the host.
Methanogenesis is a further mechanism of hydrogen disposal in the colon, also reducing overall gas accumulation, which is carried out as follows:
4H2 + CO2 → CH4 + 2H2O.
그러나
생성되는 황화수소의 독성이 매우 강하기 때문에
숙주에게 병적인 결과를 초래할 수 있습니다.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8861923/
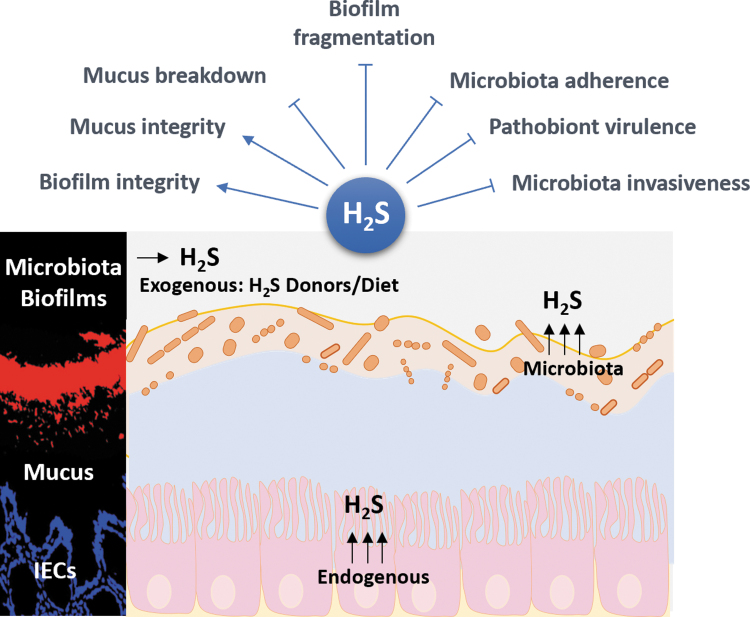
메탄 생성은
결장에서 수소를 처리하는 또 다른 메커니즘으로,
다음과 같이 수행되는 전체 가스 축적을 줄입니다:
4H2 + CO2 → CH4 + 2H2O.
Methanogens and sulphate reducers thus compete for hydrogen in the gut and the process that dominates is dependent on the amount of sulphate available [34, 35]. When sufficient sulphate is available SRB out-compete methanogens for hydrogen due to their greater substrate affinity. While methanogenesis and dissimilatory sulphate reduction are the principle means by which hydrogen is utilized, when either of these mechanisms are in play acetogenesis (the third mechanism of hydrogen utilization) is also feasible. In terms of host health, acetogenesis is likely to be the most favourable mode of hydrogen recycling. The reason for this lies in the fact that, in this process, carbon dioxide and hydrogen are converted into acetate with no evolution of gas [33]:
4H2 + CO2 → CH3COOH + 2H2O.
However, this reaction is energetically less favourable than dissimilatory sulphate reduction or methanogenesis.
따라서
메탄가스와 황산염 환원제는
장내에서 수소를 놓고 경쟁하며,
어떤 과정이 우세한지는 사용 가능한 황산염의 양에 따라 달라집니다[34, 35].
충분한 황산염을 사용할 수 있는 경우
SRB는 기질 친화성이 더 높기 때문에 수소를 놓고 메탄 생성 물질과 경쟁합니다.
메탄 발생과 황산염 환원이 수소가 활용되는 주요 수단이지만,
이 두 가지 메커니즘이 작동하는 경우 아세트 발생(수소 활용의 세 번째 메커니즘)도 가능합니다.
숙주의 건강 측면에서 보면
아세토제너레이션이 가장 유리한
수소 재활용 방식이 될 가능성이 높습니다.
그 이유는 이 과정에서
이산화탄소와 수소가 기체의 진화 없이
아세트산염으로 전환되기 때문입니다[33]:
4H2 + CO2 → CH3COOH + 2H2O.
그러나 이 반응은 황산염 환원이나 메탄 생성보다 에너지적으로 덜 유리합니다.
Carbon dioxide
Carbon dioxide is another quantitatively significant gas that is expelled in flatus. Carbon dioxide can account for between 5 and 50% of the total flatus volume and as shown above is recycled with hydrogen via methanogenesis and, to a lesser extent, acetogenesis [36].
이산화탄소는
방귀로 배출되는 또 다른 정량적으로 중요한 가스입니다.
이산화탄소는
전체 방귀 부피의 5~50%를 차지할 수 있으며,
위에 표시된 것처럼 메탄 생성과 아세트 생성을 통해 수소와 함께 재활용됩니다[36].
In contrast to hydrogen and methane, carbon dioxide can be generated by a number of processes, not just bacterial metabolism. Three potential sources of carbon dioxide include its diffusion from the blood into the colonic lumen, the acidification of bicarbonate in the upper gastrointestinal tract, and bacterial metabolism [30]. Some species of clostridia (e.g., C. sporogenes, C. butyricum, and C. perfringens) produce both carbon dioxide and hydrogen in their metabolic pathways.
수소 및 메탄과 달리 이산화탄소는
박테리아 대사뿐만 아니라
다양한 과정을 통해 생성될 수 있습니다.
이산화탄소의 세 가지 잠재적 공급원에는
혈액에서 대장 내강으로의 확산,
상부 위장관에서의 중탄산염의 산성화,
박테리아 대사가 포함됩니다 [30].
클로스트리듐의 일부 종(예: C. 스포로제네스, C. 부티리쿰, C. 퍼프린젠스)은 대사 경로에서 이산화탄소와 수소를 모두 생성합니다.
Clinical aspects
Gas production by the colonic microbiota can exert clinical consequences for the host. For example, a common feature of IBS is excessive gas production and flatus, and is associated with bloating and abdominal distension. An absence of bacterial hydrogen recycling can lead towards pneumatosis cystoides intestinalis which is characterized by excessive gas production and the presence of gas filled cysts on the colonic wall [37]. In this instance, an absence of SRB, methanogenic bacteria, and acetogens causes the individual to produce between 5 and 10 times more gas than is usual.
대장 미생물에 의한 가스 생성은
숙주에게 임상적인 결과를 초래할 수 있습니다.
예를 들어,
과민성 대장 증후군의 일반적인 특징은
과도한 가스 생성 및 방귀이며,
복부 팽만감 및 복부 팽창과 관련이 있습니다.
박테리아 수소 재활용이 없으면
과도한 가스 생산과 대장 벽에 가스로 채워진 낭종이 존재하는
장내 낭포증으로 이어질 수 있습니다 [37].
https://www.thelancet.com/journals/lancet/article/PIIS0140673607603626/fulltext
Recycling of hydrogen via dissimilatory sulphate reduction generates hydrogen sulphide, which is a cell signalling molecule of emerging physiological importance [38], but also is highly toxic to colonic cells and is potentially implicated in inflammatory bowel disease, since sufferers of ulcerative colitis have a universal carriage of SRB [39, 40]. The presence of methane in the colon has been linked with colorectal cancer, although the association may be a consequence of the disease rather than causal, since patients with the condition have slower colonic transit times [41]. This would assist growth of methanogens in the gut due to their slow growing nature. Individuals with lactose intolerance have increased gas production, since the defective absorption of lactose in the upper GIT means that lactose reaches colonic bacteria, and is fermented forming gas.
이 경우
SRB,
메탄 생성 박테리아 및
아세토겐이 없으면
평소보다 5~10배 많은 가스를 생성하게 됩니다.
황산염 환원을 통한 수소 재활용은
황화수소를 생성하는데,
이는 생리적으로 중요한 세포 신호 분자인 동시에[38]
대장 세포에 매우 독성이 강하며
궤양성 대장염 환자는 SRB를 보편적으로 운반하기 때문에[39, 40]
염증성 장 질환과 관련이 있을 가능성이 있습니다.
대장 내 메탄의 존재는
대장암과 관련이 있지만,
대장암 환자는 대장 통과 시간이 더 느리기 때문에
인과관계보다는 질병의 결과일 수 있습니다 [41].
https://www.nature.com/articles/srep12693
이는 장에서 천천히 성장하는 메탄올의 특성으로 인해
장내 메탄올의 성장을 도울 수 있습니다.
유당 불내증이 있는 사람은
가스 생성이 증가하는데,
이는 상부 위장관에서 유당이 제대로 흡수되지 않아
유당이 대장 박테리아에 도달하여
발효되어 가스를 형성하기 때문입니다.
Proteins
Early work with human gut contents by Macfarlane and Cummings [42] showed that the colonic microbiota has considerable proteolytic power, converting ingested dietary protein and endogenous protein from host enzymes, mucin, and sloughed off intestinal cells into shorter peptides, amino acids and derivatives, short and branched-chain fatty acids, and gases, including ammonia, H2, CO2, and H2S [42]. This early work was limited at the time to culture-based microbiology techniques, but the authors identified Bacteroides and Propionibacterium species as the predominant proteolytic species in faecal samples, with proteolysis common also amongst clostridia, streptococci, staphylococci, and Bacillus species. Gibson et al. [43] showed that the proteolytic activity of the faecal microbiota differed, both in quantity and quality of protein degradation, from that in the ileum. Faecal proteolyis was more efficacious at degrading the highly globular protein bovine serum albumin, despite having lower overall proteolytic activity compared to ileal effluent. In 1996, the Macfarlane team also provided some of the only information on the metabolic processes governing amino-acid fermentation by gut bacteria using both pure cultures of intestinal bacteria and in vitro gut models inoculated with human faeces [44].
맥팔레인과 커밍스의 인간 장 내용물에 대한 초기 연구[42]에 따르면
대장 미생물은
상당한 단백질 분해력을 가지고 있어
섭취한 식이 단백질과 숙주 효소, 뮤신, 장 세포에서 떨어져 나온 내인성 단백질을
더 짧은 펩티드,
아미노산 및 유도체,
단쇄 및 분지쇄 지방산,
암모니아,
H2, CO2, H2S 등의 기체로 변환하는 것으로 나타났습니다 [42].
이 초기 연구는 당시 배양 기반 미생물학 기술로 제한되었지만 저자들은
박테로이데스와 프로피오니박테리움 종을
분변 샘플의 주요 단백질 분해 종으로 확인했으며
클로스트리디움, 연쇄상구균, 포도상구균 및 바실러스 종에서도
단백질 분해가 공통적으로 발생한다는 사실을 밝혀냈습니다.
깁슨 등[43]은
분변 미생물의 단백질 분해 활성은
단백질 분해의 양과 질 모두에서 회장 내 미생물과 차이가 있음을 보여주었습니다.
분변 단백질 분해효소는
회장 유출물에 비해 전반적인 단백질 분해 활성은 낮았지만
구형 단백질인 소 혈청 알부민을 분해하는 데 더 효과적이었습니다.
1996년 맥팔레인 팀은 장내 세균의 순수 배양과 사람의 대변을 접종한 시험관 내 장 모델을 사용하여 장내 세균의 아미노산 발효를 관장하는 대사 과정에 대한 유일한 정보를 제공하기도 했습니다[44].
They successfully characterized dissimilatory aromatic amino-acid fermentation by these bacteria and measured their production of phenols and indoles upon fermentation of aromatic amino acids using GC-MS. They also measured the impact of pH, carbohydrate availability, and gut model retention time on this activity, and found a preference for amino-acid fermentation at higher ranges of colonic pH and a 60% reduction in this fermentation and end product production (phenols and indoles) when fermentable carbohydrate was available [44]. In seminal work involving intestinal contents from two sudden death victims, Macfarlane et al. [7] reported the metabolic potential of different regions of the colon. The proximal colon was predominantly saccharolytic by nature, whereas protein fermentation increased distally, as did pH, through the transverse colon and into the distal colon. This protein fermentation was associated with increased concentrations of branched-chain fatty acids, phenol, and indole derivatives of amino-acid fermentation and ammonia.
연구팀은
이러한 박테리아에 의한 방향족 아미노산 발효를 성공적으로 특성화하고,
방향족 아미노산 발효 시 페놀과 인돌의 생성을
GC-MS를 사용하여 측정했습니다.
또한 pH, 탄수화물 가용성 및 장내 모델 유지 시간이 이 활동에 미치는 영향을 측정한 결과,
높은 범위의 대장 pH에서
아미노산 발효를 선호하고
발효 가능한 탄수화물이 있을 때
이 발효와 최종 생성물(페놀 및 인돌) 생산이
60% 감소한다는 것을 발견했습니다[44].
맥팔레인 등[7]은 두 명의 돌연사 희생자의 장 내용물을 대상으로 한 연구에서
대장의 여러 부위의 대사 잠재력을 보고했습니다.
근위 결장은
본질적으로 당분 분해가 우세한 반면,
단백질 발효는
횡행 결장을 거쳐 원위 결장까지 pH와 마찬가지로
원위부에서 증가했습니다.
이러한 단백질 발효는
분지 사슬 지방산,
페놀,
아미노산 발효 및 암모니아의 인돌 유도체 농도 증가와 관련이 있었습니다.
Recently, evidence has emerged that aromatic amino acids (phenylalanine, tyrosine, and tryptophan) can be fermented to phenylpropanoid metabolites, phenylacetic acid, and 4-hydroxyphenyl-acetic acid, which are abundant in faeces [45]. The organisms involved include several species of Bacteroides, Eubacterium hallii, and Clostridium barlettii. Interestingly, these phenolic compounds are the same as those generated by microbial breakdown of plant polyphenols.
최근에는
방향족 아미노산(페닐알라닌, 티로신, 트립토판)이
대변에 풍부한 페닐프로파노이드 대사산물,
페닐아세트산, 4-하이드록시페닐아세트산으로
효될 수 있다는 증거가 나타났습니다 [45].
관련된 유기체에는
여러 종의 박테로이데스, 유박테리움 할리, 클로스트리디움 바렛티가 포함됩니다.
흥미롭게도
이러한 페놀 화합물은
식물 폴리페놀의 미생물 분해에 의해 생성되는 것과
동일합니다.
The complexities of amino-acid utilization and subsequent availability to the host are now becoming apparent and warrant more in-depth scrutiny in specifically designed mechanistic studies (Fig. 2; [46]). Dai et al. [47] showed that bacterial conversion of free amino acids into polypeptides contributes considerably to amino-acid metabolism and bioavailability in the mammalian gut. They also found that the relative concentrations of different amino acids available to intestinal bacteria can impact greatly on overall amino-acid utilization at the community level [48]. For example, they found that L-glutamine regulates small intestinal bacterial metabolism of arginine, serine, and aspartate, and reduced the catabolism of essential and non-essential amino acids. This is especially relevant given the fact that modern food processing has a dramatic effect on the relative concentrations of amino acids present in commonly consumed processed foods and recent evidence for important physiological roles for both essential (e.g., tryptophan) and non-essential amino acids in mammalian nutrition [49–51].
아미노산 이용의 복잡성과
숙주에 대한 후속 가용성은
이제 분명해지고 있으며
특별히 설계된 기계론적 연구에서 더 심층적인 조사가 필요합니다(그림 2; [46]).
다이 등[47]은
유리 아미노산을 폴리펩타이드로 전환하는 박테리아가
포유류 장내에서 아미노산 대사와 생체 이용률에 상당히 기여한다는 것을 보여주었습니다.
또한
장내 박테리아가 이용할 수 있는 다양한 아미노산의 상대적 농도가
커뮤니티 수준에서 전반적인 아미노산 이용률에
큰 영향을 미칠 수 있음을 발견했습니다[48].
예를 들어, 연구진은
L-글루타민이
아르기닌,
세린,
아스파르트산염의 소장 박테리아 대사를 조절하고
필수 아미노산과 비필수 아미노산의 이화 작용을 감소시킨다는 사실을 발견했습니다.
이는 현대 식품 가공이 일반적으로 소비되는 가공식품에 존재하는 아미노산의 상대적 농도에 극적인 영향을 미친다는 사실과 포유류 영양에서 필수(예: 트립토판) 및 비필수 아미노산의 중요한 생리적 역할에 대한 최근 증거를 고려할 때 특히 관련이 있습니다 [49-51].
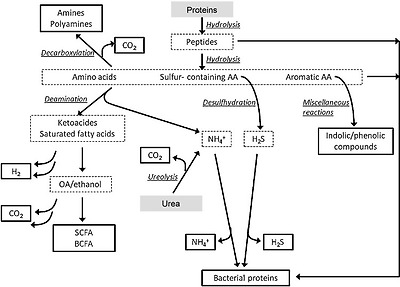
Pathways of gut microbial protein degradation [46]
참고) Among the 20 standard amino acids, phenylalanine, tryptophan and tyrosine are classified as aromatic.[1] In some cases, histidine is included in the list of aromatic amino acids,[2] even though the aromaticity of the imidazole side chain is questionable.
참고)
Vitamin synthesis
It has been known for over 40 years, via studies in germ-free and the conventional rodents and in human volunteers, that the gut microbiota can synthesize certain vitamins, notably vitamin K, and B group vitamins including biotin, cobalamin, folates, nicotinic acid, panthotenic acid, pyridoxine, riboflavin, and thiamine [52]. These vitamins are clearly important for bacterial metabolism, but there is evidence for the metabolic and physiological significance of some of these pathways in mammals. For example, germ-free rats reared without a dietary supplement of vitamin K have low prothrombin levels and develop haemorrhages, while their conventional counterparts have normal prothrombin levels and normal clotting activity [53]. Furthermore, human subjects on low vitamin K diets for 3–4 weeks did not develop vitamin deficiency, but those treated with a broad-spectrum antibiotic to suppress the microbiota showed a significant decrease in plasma prothrombin levels [54]. Metagenomic sequencing has recently been used to provide insight into pathways for vitamin synthesis by the gut microbiota. Le Blanc et al. [55] explored the metabolic potential of gut microbial sequences from two subjects and found that their microbiomes were enriched for a variety of clustered orthologous groups (COGs) involved in the synthesis of deoxyxylulose-5-phosphate, a precursor of thiamine and pyridoxal.
장내 미생물이
특정 비타민,
특히 비오틴, 코발라민, 엽산, 니코틴산, 판토텐산, 피리독신, 리보플라빈, 티아민을 포함한
비타민 K와 비타민 B군[52]을 합성할 수 있다는 사실은
40년 이상 무균 및 일반 설치류와 인간 지원자에 대한 연구를 통해 알려져 왔습니다[52].
이러한 비타민은
박테리아 대사에 분명히 중요하지만
포유류에서 이러한 경로 중 일부의 대사 및 생리적 중요성에 대한 증거가 있습니다.
예를 들어,
비타민 K를 보충하지 않고
사육한 무균 쥐는 프로트롬빈 수치가 낮고 출혈이 발생하는 반면,
일반 쥐는 프로트롬빈 수치가 정상이고 응고 활동이 정상입니다 [53].
또한, 3-4주 동안 저 비타민 K 식단을 섭취한 피험자는
비타민 결핍이 발생하지 않았지만,
미생물을 억제하기 위해 광범위한 항생제로 치료한 피험자는
혈장 프로트롬빈 수치가 현저히 감소한 것으로 나타났습니다 [54].
메타게놈 시퀀싱은
최근 장내 미생물에 의한 비타민 합성 경로에 대한 통찰력을 제공하는 데 사용되었습니다.
Le Blanc 등[55]은 두 피험자의 장내 미생물 서열의 대사 잠재력을 조사한 결과,
이들의 미생물군이 티아민과 피리독살의 전구체인 데옥시실룰로스-5-포스페이트의 합성에 관여하는
다양한 클러스터된 상동 그룹(COG)이 풍부하다는 사실을 발견했습니다.
Magnusdottir et al. [56] have systematically explored the genomes of 256 common gut bacteria for the presence of biosynthetic pathways for eight B vitamins, namely biotin, cobalamin, folate, niacin, pantothenate, pyridoxine, riboflavin, and thiamin. This allowed the authors to predict the proportion of each phylum containing potential producers of each vitamin. Some genomes contained all eight pathways, others none. The most commonly synthesised vitamins were riboflavin (166 potential producers) and niacin (162 producers). For riboflavin and biotin, virtually all microbes from the phyla Bacteroidetes, Fusobacteria and Proteobacteria possessed the necessary pathways, with a much smaller proportion of the Firmicutes and Actinobacteria having the potential for vitamin B biosynthesis. In the case of vitamin B12, all the Fusobacteria, compared with 10–50% of the other four phyla were predicted to be producers. Overall, Bacteroidetes appeared to be the phylum with the greatest number of predicted B vitamin producers. Excluding vitamin B12, over 90% of Bacteroidetes were predicted to be producers.
Magnusdottir 등[56]은
비오틴, 코발라민, 엽산, 니아신, 판토텐산, 피리독신, 리보플라빈, 티아민 등
8가지 비타민 B의 생합성 경로가 존재하는지
256개의 일반적인 장내 세균의 게놈을 체계적으로 조사했습니다.
이를 통해 저자들은
각 비타민의 잠재적 생산자를 포함하는
각 문(phylum)의 비율을 예측할 수 있었습니다.
어떤 게놈은 8가지 경로를 모두 포함하고 있었고,
어떤 게놈은 전혀 포함하지 않았습니다.
가장 일반적으로 합성되는 비타민은
리보플라빈(166개의 잠재적 생산자)과
니아신(162개의 생산자)이었습니다.
리보플라빈과 비오틴의 경우
박테로이데테아, 푸소박테리아, 프로테오박테리아 문에 속하는
거의 모든 미생물이 필요한 경로를 가지고 있었으며,
피르미쿠테스와 액티노박테리아는
훨씬 적은 비율로 비타민 B 생합성에 대한 잠재력을 가지고 있었습니다.
비타민 B12의 경우, 다
른 네 문(門)의 10~50%가 생산자로 예측된 것과 달리
모든 푸소박테리아가 생산자로 예측되었습니다.
전반적으로
박테로이데테스는
예상되는 비타민 B 생산자 수가 가장 많은 문(門)인 것으로 나타났습니다.
비타민 B12를 제외한 90% 이상의 박테로이데테아과가 생산자로 예측되었습니다.
Interestingly, the authors identified several pairs of organisms whose vitamin synthesis pathway patterns complemented each other [56]. This implies cross-feeding between gut microbes, providing essential vitamins for growth. This, in turn, suggests that a major proportion of the microbially produced vitamins are utilized by other non-vitamin producing bacteria. Such utilization limits their availability for the host. The authors estimated the percentage of human daily reference intake of each vitamin obtained from the gut bacteria [56]. Of the eight studied, without considering bacterial utilization, the gut microbiota were estimated to contribute over a quarter of the suggested dietary intake for four vitamins (Table 1, [56]). In addition, there is evidence from studies using a various human and animal colon preparations that the colonic epithelium can absorb a range of B vitamins, including folate, riboflavin, biotin, niacin and thiamine, via specific carrier-mediated mechanisms [57].
흥미롭게도 저자들은
비타민 합성 경로 패턴이 서로 보완되는
여러 쌍의 유기체를 확인했습니다 [56].
이는
장내 미생물 간의 교차 먹이를 통해
성장에 필수적인 비타민을 공급한다는 것을 의미합니다.
이는 결국
미생물이 생산한 비타민의 상당 부분이
비타민을 생산하지 않는
다른 박테리아에 의해 활용된다는 것을 시사합니다.
이러한 활용은
숙주에 대한 비타민의 가용성을 제한합니다.
저자들은 장내 박테리아에서 얻은 각 비타민의 일일 기준 섭취량 비율을 추정했습니다[56].
박테리아 활용을 고려하지 않고
연구한 8가지 중
장내 미생물은
4가지 비타민에 대한 권장 식이 섭취량의 1/4 이상을 기여하는 것으로 추정되었습니다(표 1, [56]).
또한 다양한 인간 및 동물 결장 제제를 사용한 연구에서
대장 상피가 특정 운반체 매개 메커니즘을 통해
엽산, 리보플라빈, 비오틴, 니아신 및 티아민을 포함한
다양한 비타민 B군을 흡수할 수 있다는 증거가 있습니다[57].
Table 1
Estimated maximal % of daily reference intake (DRI) of B vitamins that could be provided by the gut microbiota (from Magnusdottir et al. [56])
VitaminIntracellular concentration (mmol/gDW)DRIa (mg/day)HGMratio%DRI from HGM
| Biotin | 9.0 × 10− 7 | 0.03 | 0.40 | 4.5 |
| Cobalamin | 8.5 × 10−8 | 0.0024 | 0.42 | 31 |
| Folateb | 5.0 × 10−5 | 0.4 | 0.43 | 37 |
| Niacinb | 3.3 × 10−3 | 15 | 0.63 | 27 |
| Pantothenate | 2.3 × 10−6 | 5 | 0.51 | 0.078 |
| Pyridoxineb | 5.8 × 10−4 | 1.3 | 0.50 | 86 |
| Riboflavin | 9.0 × 10−6 | 1.2 | 0.65 | 2.8 |
| Thiaminb | 8.7 × 10−6 | 1.15 | 0.56 | 2.3 |
aDietary reference intakes (Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and Its Panel on Folate, Other B Vitamins, and Choline, 1998). Values averaged for male and female references intakes (ages 19–50)
bAtomic mass for dihydrofolic acid, nicotinic acid, pyridoxine 5′-phosphate, and thiamine monophosphate
Bile acids
Bile acids are classical examples of trans-genomic metabolites arising from the interactive metabolism between the host genome and the gut microbiome. As outlined in Fig. 3, bile acids are synthesised in the liver from cholesterol to form the two primary bile acids cholic acid (CA) and chenodeoxycholic acid (CDCA). Prior to secretion into bile, N-acyl amidation occurs conjugating the carboxyl group of the bile acids to a molecule of either taurine or glycine. This conjugation step produces a molecule that is fully ionised at physiologic pH, enhancing the amphipathic nature and, therefore, detergent properties of the molecule. Upon ingestion of a meal, bile acids stored in the gall bladder are secreted into the small intestine to facilitate lipid digestion and absorption. While the majority of bile acids are actively absorbed in the distal ileum and recycled back to the liver, a small fraction (1–5%; 200–800 mg daily in humans) escapes this enterohepatic circulation and enters the colon. It is here that a bidirectional relationship exists between the gut microbiota and the bile acids. The colonic microbiota are able to modify the structure and properties of the bile acids, while the bile acids possess antimicrobial characteristics and can exert selection pressures on the community structure of the gut microbiota. These characteristics include detergent effects on bacterial cell membranes and the ability to induce DNA damage and disruption to protein structures [58–60].
담즙산은
숙주 게놈과 장내 미생물군집 사이의 상호작용 대사에서 발생하는
대표적인 초유전체 대사산물의 예입니다.
그림 3에 설명된 바와 같이
담즙산은
간에서 콜레스테롤로부터 합성되어
두 가지 주요 담즙산인 콜린산(CA)과 케노데옥시콜산(CDCA)을 형성합니다.
담즙으로 분비되기 전에
담즙산의 카르복실기를
타우린 또는 글리신 분자에 접합하는
N-아실 아미데이션이 발생합니다.
이 접합 단계는
생리적 pH에서 완전히 이온화된 분자를 생성하여
양친매성 특성을 향상시켜 분자의 세제 특성을 향상시킵니다.
음식을 섭취하면
담낭에 저장된 담즙산이
소장으로 분비되어
지질의 소화와 흡수를 촉진합니다.
담즙산의 대부분은
회장 말단부에서 활발하게 흡수되어
간으로 다시 재활용되지만,
소량(1-5%, 사람의 경우 매일 200-800mg)은
이러한 간내 순환을 빠져나와 결장으로 들어갑니다.
여기서
장내 미생물과 담즙산 사이에는
양방향 관계가 존재합니다.
대장 미생물은
담즙산의 구조와 특성을 수정할 수 있고,
담즙산은 항균 특성을 가지고 있으며
장내 미생물의 군집 구조에 선택 압력을 가할 수 있습니다.
이러한 특성에는
박테리아 세포막에 대한
세제 효과와 DNA 손상 및
단백질 구조 파괴를 유도하는 능력이 포함됩니다[58-60].
Interestingly, the potency of deoxycholic acid (DCA), a microbially derived secondary bile acid, is tenfold greater than that of its precursor, CA, due to its greater detergent properties [61]. Hence, greater microbial interaction with the enterohepatic circulation enhances its antimicrobial properties, potentially providing a feedback mechanism to control bacterial populations.
흥미롭게도
미생물 유래 이차 담즙산인
데옥시콜산(DCA)의 효능은 전구체인 CA의 효능보다 10배 더 큰데,
이는 세제 특성이 더 크기 때문입니다[61].
따라서
장간 순환과 미생물의 상호작용이 증가하면
항균성이 향상되어
잠재적으로 박테리아 개체군을 제어하는 피드백 메커니즘을 제공할 수 있습니다.
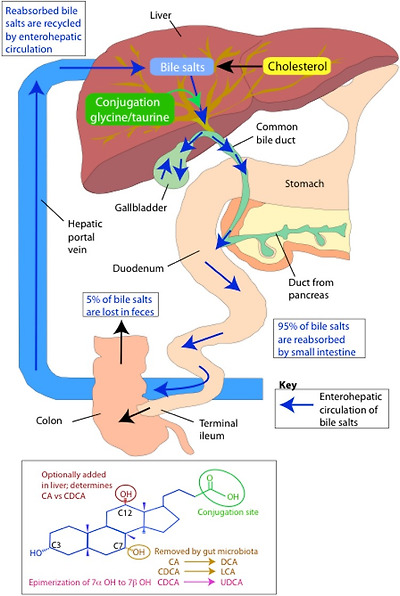
Bile acid metabolism
(adapted from [167])
Microbial biotransformation of bile acids includes modification to both the side chain and the steroid nucleus (Fig. 3). At the side chain, a number of gut bacteria possess bile salt hydrolase (BSH) enzymes that are capable of hydrolysing the amide bond between the bile acid and its conjugated amino acid [62]. BSH genes have been identified in the main bacterial genera of the microbiota including Bacteroides, Bifidobacterium, Clostridium, Lactobacillus, and Listeria [63], and most hydrolyse both glyco and tauro-conjugates. This deconjugation step provides bacteria with a mechanism to reduce the toxicity of the bile acids and is a source of nitrogen, sulphur and carbon atoms [64, 65]. Deconjugated bile acids can be absorbed and returned to the liver for re-conjugation before re-entering the enterohepatic circulation or undergo further bacterial processing.
담즙산의 미생물 생체 변형에는
측쇄와 스테로이드 핵 모두에 대한 변형이 포함됩니다(그림 3).
측쇄에서 많은 장내 세균은
담즙산과 공액 아미노산 사이의 아미드 결합을 가수분해할 수 있는
담즙산염 가수분해효소(BSH) 효소를 보유하고 있습니다[62].
BSH 유전자는
박테로이데스, 비피도박테리움, 클로스트리디움, 락토바실러스, 리스테리아 등
미생물총의 주요 세균속에서 확인되었으며[63],
대부분 글리코와 타우로 공액체를 모두 가수분해합니다.
이 분해 단계는
담즙산의 독성을 줄이는 메커니즘을
박테리아에 제공하며
질소, 황 및 탄소 원자의 공급원입니다[64, 65].
분해된 담즙산은
흡수되어 간으로 되돌아가 재접합된 후
장내 순환으로 다시 들어가거나
추가적인 박테리아 처리를 거칠 수 있습니다.
At the steroid nucleus, a range of microbially mediated modifications can occur resulting in secondary bile acids. Following deconjugation, the C7 hydroxyl group of the bile acid becomes available for microbial dehydroxylation. Genera such as Clostridium and Eubacterium transform CDCA and CA to the secondary bile acids lithocholic acid (LCA) and DCA, respectively [66]. This 7α dehydroxylation activity is thought to provide these bacteria with an ancillary electron acceptor [65, 67]. These secondary bile acids are potentially cytotoxic for the host and have been associated with colon cancer and cholesterol gallstone formation [68, 69]. To reduce their toxicity, these secondary bile acids undergo further processing in the liver. The inability of the liver to re-hydroxylate secondary bile acids preserves the diversity of the bile acid pool instead, and secondary bile acids are detoxified through conjugation with glycine or taurine, and in some instances sulphate [70]. Another bacterial modification to bile acids is the epimerization of hydroxyl groups from the α to β orientation. Ursodeoxycholic acid (UDCA) is the most common secondary bile acid produced through this action following the epimerization of the 7α hydroxyl group on CDCA [71]. This action decreases the toxicity of the bile acid producing a more favourable microenvironment for the bacteria.
스테로이드 핵에서는
미생물을 매개로 한 다양한 변형이 일어나
2차 담즙산이 생성될 수 있습니다.
디컨쥬게이션 후 담즙산의 C7 수산기는 미생물의 탈수산화 작용에 사용할 수 있게 됩니다. 클로스트리디움과 유박테리움 같은 속은 CDCA와 CA를 각각 이차 담즙산인 리토콜산(LCA)과 DCA로 변환합니다[66]. 이 7α 탈수산화 활성은 이러한 박테리아에 보조 전자 수용체를 제공하는 것으로 생각됩니다 [65, 67].
이러한 이차 담즙산은
숙주에게 잠재적으로 세포 독성이 있으며
대장암 및 콜레스테롤 담석 형성과 관련이 있습니다 [68, 69].
독성을 줄이기 위해 이러한 이차 담즙산은
간에서 추가 처리를 거칩니다.
간에서 이차 담즙산을 재수산화할 수 없기 때문에
담즙산 풀의 다양성이 유지되며,
이차 담즙산은
글리신이나 타우린, 경우에 따라 황산염과의 접합을 통해 해독됩니다 [70].
담즙산에 대한 또 다른 박테리아 변형은
α 방향에서 β 방향으로 수산기가 에피머화되는 것입니다.
우르소데옥시콜산(UDCA)은 CDCA에서 7α 수산기가 에피머화된 후 이 작용을 통해 생성되는 가장 흔한 이차 담즙산입니다[71]. 이 작용은 담즙산의 독성을 감소시켜 박테리아에 더 유리한 미생물 환경을 조성합니다.
Overall, the circulating and hepatic bile acid pool contains more than 30 known bile acids and the gut microbiome is responsible for driving the majority of this diversity [72]. Variation in bile acid composition has potential to modulate the physico-chemical properties of the overall pool. Bacterial deconjugation reduces the efficiency of bile acids for the emulsification of dietary lipids and micelle formation. This can modify the digestive function of the host as bile acids have key roles facilitating the absorption of dietary lipids, nutrients, and lipid-soluble vitamins. Bile acids are also recognised as important signalling molecules serving as ligands for the nuclear receptor farnesoid X receptor (FXR), and the plasma membrane bound GPR TGR5 [73, 74]. Through binding to these receptors, bile acids can regulate genes critical to their synthesis, conjugation, transport, and detoxification [75–77] as well as lipid [78, 79] and glucose metabolism [80, 81] and energy homeostasis [82]. Variation in the bile acid signature induced by the gut microbiota can, therefore, have downstream effects on a range of host metabolic processes. The global signalling function of bile acids throughout the host metabolic system is suggested by the expression of receptors, transporters, and tissue-specific bile acid signatures outside of the enterohepatic circulation, including in the kidney and heart [83]. These observations demonstrate the systemic regulatory role of bile acids providing a biochemical bridge for the gut microbiome to influence the metabolic status of the host.
전반적으로 순환 및 간 담즙산 풀에는
30개 이상의 알려진 담즙산이 포함되어 있으며
장내 미생물은 이러한 다양성의 대부분을 주도합니다 [72].
담즙산 구성의 변화는 전
체 담즙산 풀의 물리화학적 특성을 조절할 수 있는 잠재력을 가지고 있습니다.
박테리아 분해는
식이 지질의 유화 및 미셀 형성을 위한
담즙산의 효율을 감소시킵니다.
담즙산은
식이 지질, 영양소 및 지용성 비타민의 흡수를 촉진하는 중요한 역할을 하므로
숙주의 소화 기능을 변경할 수 있습니다.
담즙산은 또한 핵 수용체 파네소이드 X 수용체(FXR)와 혈장막 결합 GPR TGR5의 리간드 역할을 하는 중요한 신호 분자로 인식되고 있습니다[73, 74]. 담즙산은 이러한 수용체와의 결합을 통해 지질[78, 79] 및 포도당 대사[80, 81], 에너지 항상성[82]뿐만 아니라 합성, 접합, 수송, 해독[75-77]에 중요한 유전자를 조절할 수 있습니다.
따라서
장내 미생물에 의해 유도된 담즙산 시그니처의 변화는
다양한 숙주 대사 과정에 하류 영향을 미칠 수 있습니다.
숙주 대사 시스템 전반에 걸친 담즙산의 전반적인 신호 기능은
신장과 심장을 포함한 장내 순환 외부의 수용체,
수송체 및 조직 특이적 담즙산 시그니처의 발현을 통해 알 수 있습니다 [83].
이러한 관찰은
담즙산이 장내 미생물이 숙주의 대사 상태에 영향을 미칠 수 있도록
생화학적 다리를 제공하는
담즙산의 전신 조절 역할을 보여줍니다.
Phytochemicals/polyphenols
Polyphenols from fruits and vegetables are the subject of intensive research due to their putative bioactivities and their relatively high intake levels, about 820 mg/day [84]. Most polyphenols are poorly absorbed in the small intestine and pass into the colon [85]. Studies in germ-free and human microbiota-associated animals and in vitro faecal incubations provide evidence that parent polyphenols are extensively metabolized by the colonic microbiota, which can affect their bioactivity [86, 87].
Polyphenols exhibit structural diversity, which impacts on bioavailability, metabolism, and bioactivity [85]. The main groups comprise phenolic acids, flavonoids (flavonols, flavones, isoflavones, flavanones, anthocyanidins, and flavonols), stilbenes, lignans, and secoiridoids (Table 2, [88]). Most polyphenols are present in food as glycosides (especially flavonoids), i.e., conjugated to various sugars including glucose, galactose, rhamnose, and rutinose. The hydroxycinnamic acids are usually esterified with sugars, organic acids, or lipids. Other polyphenols such as proanthocyandins and ellagitannins are in the form of high molecular weight oligomers and polymers. These conjugated and polymeric forms are generally poorly bioavailable and must be converted to aglycones before absorption. Although for some glucosides, this can be catalysed by intestinal mucosal enzymes, the majority of conjugates, and esters are not absorbed and pass into the colon where they are hydrolyzed by the colonic microbiota [88]. Microbial species involved in hydrolysis include Bacteroides distasonis, Bacteroides uniformis, Bacteroides ovatus, Enterococcus casseliflavus, Eubacterium cellulosolvens, Lachnospiraceae CG19-1, and Eubacterium ramulus [88, 89].
과일과 채소의 폴리페놀은
추정되는 생리 활성과 하루 약 820㎎의 비교적 높은 섭취량으로 인해
집중적인 연구 대상이 되고 있습니다 [84].
대부분의 폴리페놀은
소장에서 잘 흡수되지 않고
결장으로 전달됩니다 [85].
무균 및 인간 미생물총 관련 동물과 시험관 내 대변 배양에 대한 연구는
모 폴리페놀이 대장 미생물총에 의해 광범위하게 대사되어
생리활성에 영향을 미칠 수 있다는 증거를 제공합니다 [86, 87].
폴리페놀은
생체 이용률,
대사 및 생리 활성에 영향을 미치는 구조적 다양성을 나타냅니다 [85].
주요 그룹은
페놀산,
플라보노이드(플라보놀, 플라본, 이소플라본, 플라바논, 안토시아니딘, 플라보놀),
스틸벤,
리그난,
세카이리도이드로 구성됩니다(표 2, [88]).
대부분의 폴리페놀은 식
품에 배당체(특히 플라보노이드),
즉 포도당, 갈락토스, 람노스, 루티노스를 포함한 다양한 당에 결합된 형태로 존재합니다.
하이드록시신남산은
일반적으로 당, 유기산 또는 지질과 에스테르화됩니다.
프로안토시아닌과 엘라기탄닌과 같은 다른 폴리페놀은
고분자 올리고머와 폴리머 형태로 존재합니다.
이러한 공액 및 고분자 형태는
일반적으로 생체이용률이 낮으며
흡수되기 전에 아글리콘으로 전환되어야 합니다.
일부 글루코사이드의 경우 장 점막 효소에 의해 촉매될 수 있지만,
대부분의 공액과 에스테르는 흡수되지 않고
결장으로 전달되어 결장 미생물에 의해 가수분해됩니다[88].
가수분해에 관여하는 미생물 종에는
박테로이데스 디스타소니스, 박테로이데스 유니베니스, 박테로이데스 오바투스, 엔테로코커스 카셀리플라부스, 유박테리움 셀룰로솔벤스, 라크노스피라세아 CG19-1, 유박테리움 라물루스 등이 있습니다 [88, 89].
Table 2
Polyphenol metabolism by gut microbiota (after Marin et al. [88])
Polyphenol groupExamplesPrincipal metabolitesMicrobial typesReference
| Phenolic acids | ||||
| Benzoic acids | Gallic acid Ellagitannins | Urolithins A & B, isourolithins A & B | Gordonibacter urolithinfaciens, Gordonibacter pamelaeae | [157, 158] |
| Hydroxycinnamic acids | Chlorogenic acid Caffeic acid Ferulic acid | 3-(3,4-dihydroxyphenyl)-propionic acid 3-(3-hydroxyphenyl)-propionic acid 3-(4-hydroxyphenyl)-propionic acid Hydroxyphenyl-ethanol Phenylacetic acidBenzoic acid | Escherichia coli, Bifidobacterium lactis, Lactobacillus gasseri | [90, 97, 159] |
| Flavonoids | ||||
| Flavonols | Kaempferol Quercetin Myricetin | 2-(4-Hydroxyphenyl)propionic acid 3-(3,4-Dihydroxyphenyl)-acetic acid 2-(3-Hydroxyphenyl)acetic acid 3-(3,4-Dihydroxyphenyl)propionic acid 3-(3-Hydroxyphenyl)propionic acid 2-(3,5-Dihydroxyphenyl)acetic acid 2-(3-Hydroxyphenyl)acetic acid | Clostridium orbiscidens Clostridium orbiscidens, Eubacterium.oxidoreducens Eubacterium ramulus Enterococcus casseliflavus Clostridium orbiscidens, E. oxidoreducens | [90] |
| Flavanones | Naringenin | 3-(4-Hydroxyphenyl)propionic acid 3- phenylpropionic acid | Clostridium strains Eubacterium ramulus | [85, 90] |
| Isoxanthohumol (from hops) | 8-Prenyl-naringenin | Eubacterium limosum | [160] | |
| Flavan-3-ols | Catechin | 3-(3-Hydroxyphenyl)propionic acid 5-(3′,4′-Dihydroxyphenyl)--valerolactone | Clostridium coccoides, Bifidobacterium infantis | [88] |
| Epicatechin | 5-(3,4-Dihydroxyphenyl)valeric acid 3-(3,4-Dihydroxyphenyl)propionic acid | |||
| Epigallocatechin | 5-(3′,4′-Dihydroxyphenyl)--valerolactone 5-(3′,5′-Dihydroxyphenyl)--valerolactone | |||
| Flavones | Luteolin Apigenin | 3-(3,4-Dihydroxyphenyl)-propionic acid 3-(4-hydroxyphenyl)-propionic acid 3-(3-hydroxyphenyl)-propionic acid, and 4-hydroxycinnamic acid, phloretin | Clostridium. orbiscindens, Enterococcus avium Eubacterium ramulus Bacteroides distasonis | [161] |
| Isoflavones | Daidzein | Equol O-desmethylangolensin | Bacteroides ovatus, Streptococcus intermedius, Ruminococcus productus Eggerthella sp.Julong 732, Slakia isoflavoniconvertens, Slakia equolifaciens, Adlercreutzia equolifaciens Consortium of Lactobacillus mucosae Enterococcus faecium Finegoldia magna, Veillonella sp Clostridium spp. HGHA136, Eubacterium ramulus | [88, 96, 98, 102, 162, 163] |
| Genistein | 6′-OH-O-desmethylangolensin 2-(4-hydroxyphenyl)propionic acid | Eubacterium ramulus | [163] | |
| Anthocyanidins | Cyanidin Pelargonidin Malvidin | 3,4-Dihydroxybenzoic acid 3-hydroxycinnamic acid 4-Hydroxybenzoic acid 3,4-Dimethoxybenzoic acid | Clostridium saccharogumia Eubacterium ramulus | [164] |
| Lignans | Secoisolaricinresinol diglucoside | Enterodiol Enterolactone | Bact. distasonis, Bact. fragilis, Bact. ovatus, Clostridium cocleatum, Clostridium.sp SDG-MT85-3Db, Butyribacterium methylotrophicum, Eubacterium callanderi, Eubacterium limosum, Peptostreptococcus productus, Clostridium scindens Eggerthella lenta, ED-Mt61/PY-s6 | [92] |
| Secoiridoids | Oleuropein Ligstroside | Tyrosol Hydroxytyrosol | Not studied | [165, 166] |
Once the polyphenols have been metabolized to their aglycones or the polymers have been converted to monomers, they are extensively degraded by other components of the colonic microbiota via dehydroxylation, decarboxylation, and ring breakage ultimately generating simpler phenolic compounds, such as hydroxyphenyl-acetic acids and hydroxyphenylpropionic acids. An example of such a pathway is shown for quercetin (Fig. 4; [90]), but equivalent reactions are seen for other flavonoids, phenolic acids and lignans [89–91]. In Table 1, organisms identified as participating in these reactions are shown. It should also be noted that these phenylacetic- and hydroxyphenyl-acetic acids can also be derived from fermentation of aromatic amino acids ([45]; see “Protein” section).
폴리페놀이
아글리콘으로 대사되거나 폴리머가 단량체로 전환되면
탈수산화,
탈카르복실화 및
고리 파괴를 통해 대장 미생물의 다른 성분에 의해 광범위하게 분해되어
궁극적으로 하이드록시페닐-아세트산 및
하이드록시페닐프로피온산과 같은
더 단순한 페놀 화합물을 생성합니다.
이러한 경로의 예는
케르세틴(그림 4; [90])에 대해 표시되어 있지만
다른 플라보노이드,
페놀산 및 리그난에서도
동일한 반응이 나타납니다 [89-91].
표 1에는 이러한 반응에 참여하는 것으로 확인된 유기체가 나와 있습니다. 또한 이러한 페닐아세트산과 히드록시페닐아세트산은 방향족 아미노산의 발효로부터도 파생될 수 있습니다([45], "단백질" 섹션 참조).

Scheme of gut microbial degradation of rutin [90]
It is evident from several studies that the complete metabolism of polyphenol glycosides in the gut requires the involvement of a consortium of microbes. For example, in the case of the lignan, secoisolariciresinol diglucoside, the initial deglycosylation is catalysed by three Bacteroides species (B. distasonis, B. fragilis, and B. ovatus) and two strains of Clostridium (C. cocleatum, C. saccharogumia). Demethylation of the lignan aglycone involves strains of Butyribacterium methylotrophicum, Eubacterium callanderi, Eubacterium limosum, Blautia producta, and Peptostreptococcus productus. Dehydroxylation of secoisolariciresinol is catalysed by Clostridium scindens and Eggerthella lenta and the final step, dehydrogenation of enterodiol to enterolactone, and closure of the lactone ring is catalysed by subdominant populations of Clostridiales, in particular Lactonifactor longoviformis (Fig. 5; [92–94]).
여러 연구에 따르면
장에서 폴리페놀 배당체의 완전한 대사를 위해서는
미생물 컨소시엄의 참여가 필요하다는 것이 분명합니다.
예를 들어,
리그난인 세코이솔라리시놀 디글루코사이드의 경우,
초기 탈당화는 세 가지 박테로이데스 종(B. 디스타소니스, B. 프라길리스, B. 오바투스)과
두 가지 클로스트리디움 균주(C. 코클리아툼, C. 사카로구미아)가 촉매를 통해 이루어집니다.
리그난 아글리콘의 탈메틸화에는
부티리박테리움 메틸로트로피쿰, 유박테리움 칼란데리, 유박테리움 리모섬, 블라우티아 프로덕타 및 펩토스트렙토코커스 프로덕투스 균주가 관여합니다.
세코이솔라리시놀의 탈수산화는
클로스트리디움 신덴스와 에거텔라 렌타에 의해 촉매되고,
마지막 단계인 엔테로디올의 엔테로락톤으로의 탈수소화 및 락톤 고리의 폐쇄는
클로스트리디움, 특히 락토니팩터 롱고비포르미스의 우세 집단이 촉매합니다(그림 5; [92-94]).
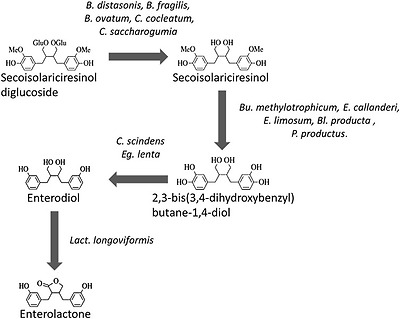
Gut bacterial metabolism of the lignan secoisolariciresinol diglucoside (after Clavel et al. [92]). Abbreviations of bacterial genus names: B.—Bacteroides; Bu.—Butyribacterium; Bl—Blautia; C.—Clostridium; E.—Eubacterium; Eg.—Eggerthella; Lact.—Lactonifactor; P.—Peptostreptococcus
Human dietary intervention trials and in vitro faecal metabolism studies with dietary plant polyphenols including flavonoids, isoflavones, lignans, hydroxycinnamic acids, ellagotannins, and anthocyanins have revealed large inter-individual variations in absorption, metabolism, and excretion which have been ascribed to differences in gut microbiota [90, 94–97]. The most well-studied example of such inter-individual variation is the microbial metabolism of the soy isoflavone daidzein which is metabolized by two different pathways depending on the gut microbiota of the subjects [96]. A majority of subjects convert daidzein to O-desmethylangolensin with a Clostridium species involved. However, about 30% of subjects convert daidzein to (S)-equol via dihydrodaidzein and tetrahydrodaidzein resulting from the activities of a wide range of organisms including Streptococcus intermedius, B. ovatus, Ruminococcus productus, Eggerthella sp. Julong732, Adlercreutzia equolifaciens, Slakia isoflavoniconvertens, and Slakia equolifaciens. One study [98] found that a consortium of Lactobacillus mucosae, Enterococcus faecium, and Finegoldia magna EPI3 Veillonella sp. was sufficient to effect the conversion.
Inter-individual variation is also apparent in the conversion of dietary ellagitannins and ellagic acid to the gut microbial derivatives urolithins. Subjects can be divided into three main phenotypic groups. Group A (25–80% of subjects depending on the trial) produces only urolithin A conjugates, and group B (10–50% of subjects) produces isourolithin A and/or B as well as urolithin A, whereas group 0 (5–25% of subjects) produces no detectable urolithins [97].
플라보노이드, 이소플라본, 리그난, 하이드록시신남산, 엘라고탄닌, 안토시아닌을 포함한 식이 식물 폴리페놀을
이용한 인간 식이 중재 시험과 체외 대변 대사 연구에 따르면
흡수, 대사 및 배설에 있어 장내 미생물의 차이에 기인하는
큰 개인 간 차이가 있는 것으로 나타났습니다 [90, 94-97].
이러한 개인 간 변이의 가장 잘 연구된 예는
대두 이소플라본 다이드제인의 미생물 대사로,
피험자의 장내 미생물에 따라 두 가지 경로로 대사됩니다 [96].
대부분의 피험자는 클
로스트리디움 종과 관련된 다이드제인을 O-데스메틸안골렌신으로 전환합니다.
그러나 약 30%의 피험자는
스트렙토코커스 인터미디우스, B. 오바투스, 루미노코커스 프로덕투스, 에거텔라 줄롱732, 아들레르크레우치아 에콜리파시엔스, 슬라키아 이소플라본콘버텐스, 슬라키아 에콜리파시엔스를 포함한 광범위한 유기체의 활동에 의해 디하이드로다이제인 및 테트라하이드로다이제인을 통해 다이드제인을 (S)-에콜로 전환합니다.
한 연구[98]에서는 락토바실러스 뮤코세, 엔테로코커스 페시움, 파인골디아 마그나 EPI3 베일로넬라 sp. 의 컨소시엄이 전환에 충분한 영향을 미친다는 사실을 발견했습니다.
식이 엘라기탄닌과 엘라그산이
장내 미생물 유도체인 우루리틴으로 전환되는 과정에서도
개인 간 편차가 뚜렷합니다.
피험자는 세 가지 주요 표현형 그룹으로 나눌 수 있습니다.
그룹 A(시험에 따라 피험자의 25~80%)는
우루리틴 A 접합체만 생성하고
그룹 B(피험자의 10~50%)는 우루리틴 A뿐만 아니라 이소우리틴 A 및/또는 B를 생성하는 반면,
그룹 0(피험자의 5~25%)은 검출 가능한 우루리틴을 생성하지 않습니다 [97].
There is growing evidence that the metabolism of polyphenols by the microbiota can influence their bioactivity; consequently, inter-individual variation in microbial metabolism could have significance for the health benefits of phytochemicals. Perhaps, the best example is again the soy isoflavone daidzein. There is evidence that equol is more bioactive than its parent isoflavone in a range of areas including oestrogenic and anti-oestrogenic activity, antioxidant capacity and potential anti-cancer effects [99]. Studies of equol producers versus non-producers have suggested that equol production may be important in determining benefits of soy consumption in terms of bone health, menopausal symptoms, and breast cancer, although the data are not consistent [100]. The major metabolites of ellagitannins and ellagic acid, urolithins, are better absorbed than the parent compounds and there is evidence that they are responsible for the health benefits of ellagitannin-containing foods [101]. Consequently, subjects in the phenotypic group 0 (see above) who do not produce urolithins might be expected not to benefit from intake of ellagotannins.
미생물에 의한 폴리페놀의 대사가
생리 활성에 영향을 미칠 수 있다는 증거가 점점 늘어나고 있으며,
결과적으로 미생물 대사의 개인 간 차이가
파이토케미컬의 건강 효능에 중요한 영향을 미칠 수 있습니다.
아마도 가장 좋은 예는
대두 이소플라본 다이드제인일 것입니다.
에콜은
에스트로겐 및 항에스트로겐 활성,
항산화 능력,
잠재적 항암 효과 등 다양한 영역에서 모체인 이소플라본보다
생리활성이 더 높다는 증거가 있습니다[99].
에콜 생산자와 비생산자를 대상으로 한 연구에 따르면
에콜 생산이
뼈 건강, 갱년기 증상, 유방암 측면에서
콩 섭취의 이점을 결정하는 데 중요할 수 있다고 하지만
데이터가 일관되지는 않습니다[100].
엘라기탄닌과 엘라그산의 주요 대사산물인
우루리틴은 모 화합물보다 흡수가 잘되며,
엘라기탄닌 함유 식품의 건강상의 이점을 제공한다는 증거가 있습니다 [101].
따라서
우루리틴을 생성하지 않는 표현형 그룹 0(위 참조)에 속하는 피험자는
엘라고탄닌 섭취로 인한 이점이 없을 것으로 예상할 수 있습니다.
Methodologies
Isolation of gut organisms involved in metabolizing dietary components
Studies on the metabolism of the soy isoflavone daidzein to equol provide good examples of methods commonly used to identify specific organisms involved in gut microbiota metabolism of dietary compounds. The identification of equol-producing organisms has been the subject of several studies as a consequence of the potential importance of equol in human health and the intriguing observation that only about 30% of people appear to be capable of its production.
Matthies et al. [102] isolated a novel strain from an equol-producing subject, by serial dilution of a faecal homogenate and incubation in a nutrient broth containing 100 uM daidzein and tetracycline. The latter inhibited the growth of the majority of the faecal microbiota without affecting the metabolism of daidzein. From the highest dilution that contained equol-producing microorganisms, further serial dilutions were prepared and repeated until a pure culture was obtained. On the basis of phenotypic and phylogenetic characterization, the culture was identified as a new species and named Slackia isoflavoniconvertans.
식이 성분 대사에 관여하는 장내 미생물의 분리
대두 이소플라본 다이드제인의 에콜 대사에 대한 연구는 식이 화합물의 장내 미생물 대사에 관여하는 특정 유기체를 식별하는 데 일반적으로 사용되는 방법의 좋은 예를 제공합니다. 에콜 생산 유기체의 확인은 인체 건강에서 에콜의 잠재적 중요성과 약 30%의 사람들만이 에콜을 생산할 수 있는 것으로 보인다는 흥미로운 관찰의 결과로 여러 연구의 대상이 되어 왔습니다.
Matthies 등[102]은 대변 균질액을 연속적으로 희석하고 100 uM 다이드제인 및 테트라사이클린을 함유한 영양액에서 배양하여 에콜을 생산하는 대상에서 새로운 균주를 분리했습니다. 후자는 다이드제인의 대사에 영향을 미치지 않고 대부분의 대변 미생물의 성장을 억제했습니다. 에콜 생성 미생물을 포함하는 가장 높은 희석액에서 순수한 배양이 얻어질 때까지 추가 연속 희석을 준비하고 반복했습니다. 표현형 및 계통 발생학적 특성 분석에 기초하여 이 배양균은 새로운 종으로 확인되었고 슬랙키아 이소플라본콘버탄스로 명명되었습니다.
In their study to identify equol-producing organisms, Decroos et al. [98] serially diluted a faecal sample from an equol producer and plated on a nutrient agar. Single colonies from the plates were tested for ability to metabolize daidzein. From one such colony a stable, mixed culture capable of converting daidzein to equol was obtained and shown to comprise four bacterial strains identified as Lactobacillus mucosae, Enterococcus faecium, Finegoldia magna, and a Veillonella sp. The first three were obtained as pure cultures, but interestingly, none was capable of producing equol in pure culture, and the complete consortium was required for the conversion. These isolation attempts illustrate the difficulties that can be experienced in obtaining a pure culture of a bacterium that is intimately dependent on another bacterial species/strain to provide essential growth co-factor(s).
Enrichment techniques have been used extensively in environmental microbiology to isolate organisms capable of degrading contaminants and other xenobiotics in the environment. These techniques usually involve either suspension batch cultures or continuous culture enrichment methods in which the mixed culture is incubated with the xenobiotic as a selection factor, usually as the sole carbon source [103]. These techniques would lend themselves to the isolation of organisms or consortia capable of metabolism dietary compounds, but they have not been widely used in the human gut microbiota area. A recent study by Ziemer [104] illustrates the potential of the technique as applied to the ruminant gut. In this study, continuous culture fermenters containing nutrient medium with cellulose or xylan-pectin as sole carbon sources were inoculated with cattle faeces and run for 8 weeks under operating conditions that modelled the caecum and colon of cattle. Samples were then serially diluted and plated onto carbohydrate-specific agar to isolate colonies that were then identified by 16S rRNA gene sequencing. The communities that arose during the enrichment had a broad microbial diversity representing six phyla (Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Synergistetes, and Fusobacteria). Many of the Firmicutes and Bacteroidetes isolates were related to species demonstrated to possess enzymes involved in fermenting plant cell wall components, but interestingly did not exhibit a high identity to cultured bacteria with sequences in the Ribosomal Database Project and so represented novel genera or species. In fact, over 98% of the isolates were not previously cultured. This methodology, therefore, could provide new opportunities to characterize the metabolic capacities of members of the gut microbiota.
에콜 생산 유기체를 확인하기 위한 연구에서 데크루스 등[98]은 에콜 생산자의 분변 샘플을 연속적으로 희석하여 영양 한천에 도금했습니다. 접시에서 나온 단일 콜로니를 대상으로 다이드제인 대사 능력을 테스트했습니다. 이러한 콜로니 중 하나에서 다이드제인을 에콜로 전환할 수 있는 안정적인 혼합 배양물을 얻었으며, 락토바실러스 뮤코세, 엔테로코커스 페이시움, 파인골디아 마그나, 바일로넬라 스프로 확인된 4개의 세균 균주로 구성된 것으로 나타났습니다. 처음 3개는 순수 배양으로 얻었지만 흥미롭게도 순수 배양에서 에콜 생산이 가능했던 것은 없었으며 전환을 위해 완전한 컨소시엄이 필요했습니다. 이러한 분리 시도는 필수 성장 보조 인자를 제공하기 위해 다른 박테리아 종/균주에 밀접하게 의존하는 박테리아의 순수 배양을 얻는 데 겪을 수 있는 어려움을 보여줍니다.
농축 기술은 환경 미생물학에서 환경 내 오염 물질 및 기타 외래 생물체를 분해할 수 있는 유기체를 분리하기 위해 광범위하게 사용되어 왔습니다. 이러한 기법에는 일반적으로 현탁 배치 배양 또는 혼합 배양물을 선택 인자로, 일반적으로 유일한 탄소 공급원으로 사용하여 배양하는 연속 배양 농축 방법이 포함됩니다[103]. 이러한 기술은 식이 화합물을 대사할 수 있는 유기체 또는 컨소시엄을 분리하는 데 적합하지만, 인간 장내 미생물 영역에서는 널리 사용되지 않았습니다. Ziemer의 최근 연구[104]는 반추동물 장에 적용된 이 기술의 잠재력을 보여줍니다. 이 연구에서는 셀룰로스 또는 자일란 펙틴을 유일한 탄소원으로 하는 영양 배지를 포함하는 연속 배양 발효기에 소의 분변을 접종하고 소의 맹장과 결장을 모델링한 작동 조건에서 8주 동안 실행했습니다. 그런 다음 샘플을 연속적으로 희석하고 탄수화물 전용 한천에 플레이트하여 콜로니를 분리한 다음 16S rRNA 유전자 시퀀싱을 통해 확인했습니다. 농축 과정에서 생성된 군집은 6개의 문(Firmicutes, 박테로이데테스, 프로테오박테리아, 액티노박테리아, 시너지스테테스, 푸소박테리아)을 대표하는 광범위한 미생물 다양성을 가졌습니다. 많은 피르미쿠테스 및 박테로이데테스 분리체는 식물 세포벽 성분 발효에 관여하는 효소를 가지고 있는 것으로 밝혀진 종과 관련이 있었지만, 흥미롭게도 리보솜 데이터베이스 프로젝트의 서열을 가진 배양 세균과 높은 동일성을 보이지 않아 새로운 속 또는 종으로 나타났습니다. 실제로 분리된 박테리아의 98% 이상이 이전에 배양된 적이 없는 박테리아였습니다. 따라서 이 방법론은 장내 미생물 군집의 대사 능력을 특성화할 수 있는 새로운 기회를 제공할 수 있습니다.
Although the approach of isolating strains capable of metabolizing dietary components provides insight into the potential microorganisms involved in vivo, there are drawbacks, in particular, it clearly focuses only on those gut microorganisms that can be cultured in vitro. Furthermore, the ability of a single strain to metabolize a compound in vitro may not translate into metabolism in the different physico-chemical conditions in the host-gut, and when in the presence of millions of other bacteria, which may be competing for the substrate or acting in partnership to degrade it [105].
Gut microbial enzyme activity
Much of the focus of recent microbiota research has utilized sequencing methods to describe the composition and relative abundance of the colonic community. Less attention has been paid to the assessment of specific microbial functions, which could be more useful in elucidating the gut metabolism of dietary components and links between the microbiota and health.
Measurement in faecal or colonic samples of the activity of enzymes involved in metabolism of dietary and endogenous compounds has been described for many years. The enzymes β-glycosidase (catalysing the hydrolysis of plant polyphenol glycosides), β-glucuronidase (cleavage of glucuronidated hepatic dietary metabolites), and various polysaccharide-degrading enzymes have been particularly well described [106]. In most cases, this approach of assaying enzyme activities ignores the contribution of individual bacterial types and focuses instead on overall activity in faecal samples. One of the limitations of this approach is that the activities are measured in vitro in faecal suspensions usually using model substrates so may not reflect the activity in vivo where the substrate concentrations and environmental conditions such as pH could be very different.
최근 미생물 군집 연구의 대부분은 염기서열 분석 방법을 사용하여 대장 군집의 구성과 상대적 풍부함을 설명하는 데 중점을 두고 있습니다. 식이 성분의 장내 대사와 미생물과 건강 사이의 연관성을 밝히는 데 더 유용할 수 있는 특정 미생물 기능 평가에 대한 관심은 덜 기울여졌습니다.
대변 또는 대장 샘플에서 식이 및 내인성 화합물의 대사에 관여하는 효소의 활성을 측정하는 방법은 수년 동안 설명되어 왔습니다. β-글리코시다제(식물 폴리페놀 배당체의 가수분해 촉매), β-글루쿠로니다제(글루쿠로니화 간식이 대사산물 분해) 및 다양한 다당류 분해 효소가 특히 잘 설명되어 있습니다 [106]. 대부분의 경우, 효소 활동을 분석하는 이 접근법은 개별 박테리아 유형의 기여도를 무시하고 대신 대변 샘플의 전반적인 활동에 초점을 맞춥니다. 이 접근법의 한계 중 하나는 일반적으로 모델 기질을 사용하여 대변 현탁액에서 시험관 내에서 활성을 측정하므로 기질 농도 및 pH와 같은 환경 조건이 매우 다를 수 있는 생체 내 활성을 반영하지 못할 수 있다는 것입니다.
Studies have also been conducted using a range of gut bacterial isolates to identify the main organisms involved. For example, Dabek et al. [107] screened 40 bacterial strains representative of the main bacterial groups in human faeces for β-glucosidase and β-glucuronidase activity. There was a higher prevalence of β-glucosidase producers (23/40 strains, including most of the Bifidobacterium spp. and Bacteroides thetaiotaomicron and over half of low G + C% Gram-positive Firmicutes) than β-glucuronidase producers (9/40 strains mainly members of clostridial clusters XIVa and IV). There was also evidence of dramatic strain specificity in β-glucuronidase activity in three F. prausnitzii isolates. The study also tested whether exposure to glycoside and glucuronide substrates induced enzyme activity. While there was no effect on most strains, a few exhibited several fold (4–12) increases in activity suggesting that changes in overall faecal enzyme activities in response to dietary exposure may be due to changes in the number of microbes possessing those activities and also enzyme induction in certain strains. McIntosh et al. [108] combined an enzymatic approach and a clone library analysis to study distribution of the β-glucuronidase genes gus and BG in the microbiota. Firmicutes accounted for 96% of amplified gus sequences, while 59% of BG sequences were attributed to Bacteroidetes.
It should be noted that measurement of enzyme activities of individual strains in vitro does not necessarily reflect activity in vivo where the environmental conditions, including pH, and relative abundance of the microbial types may be very different. For example, Cole et al. [105] compared the activity of enzymes measured after in vitro culture and also after the same strains has been introduced into germ-free rats and found significant differences.
More recently, a variety of molecular methods have been exploited to explore enzymatic diversity in the highly complex gut ecosystem. El Kaoutari et al. [109] have designed a custom microarray of non-redundant DNA probes for over 6500 genes coding for enzymes involved in dietary polysaccharide breakdown. It allows the detection of carbohydrate-degrading enzymes present in low abundance bacterial species in the gut. Alternatively, gene-specific primers can be used to enumerate all bacteria capable of performing a specific role in the gut, using qPCR, as has been done for butyrate producing bacteria [17]. However, both these techniques only identify the presence of genes, and not whether they are actively expressed at a specific time.
또한 다양한 장내 박테리아 분리물을 사용하여 관련된 주요 유기체를 확인하는 연구도 수행되었습니다. 예를 들어, Dabek 등[107]은 β-글루코시다아제 및 β-글루쿠로니다아제 활성에 대해 인간 대변의 주요 세균군을 대표하는 40개의 세균 균주를 선별했습니다. β-글루코시다제 생산자(23/40개 균주, 대부분의 비피도박테리움(Bifidobacterium spp.)과 박테로이데스 테타이오토미크론(Bacteroides thetaiotaomicron) 및 낮은 G + C% 그람 양성 피르미쿠테스(Firmicutes)의 절반 이상 포함)가 β-글루쿠로니다제 생산자(9/40개 균주, 주로 클로스트리디움 클러스터 XIVa 및 IV의 구성원)보다 더 많이 분포하고 있었습니다. 또한 세 개의 F. 프라우스니쯔이 분리균주에서 β-글루쿠로니다제 활성에서 균주 특이성이 극적으로 나타났다는 증거도 발견되었습니다. 이 연구에서는 글리코사이드 및 글루쿠로니드 기질에 노출되었을 때 효소 활성이 유도되는지 여부도 테스트했습니다. 대부분의 균주에는 영향이 없었지만 일부 균주는 몇 배(4-12)의 활성 증가를 보였는데, 이는 식이 노출에 따른 전체 분변 효소 활성의 변화가 이러한 활성을 가진 미생물 수의 변화와 특정 균주에서의 효소 유도 때문일 수 있음을 시사하는 결과입니다. 맥킨토시 등[108]은 효소적 접근법과 클론 라이브러리 분석을 결합하여 미생물총에서 β-글루쿠로니다제 유전자 gus와 BG의 분포를 연구했습니다. 증폭된 거스 서열의 96%는 피르미쿠테스(Firmicutes)가 차지했고, BG 서열의 59%는 박테로이데테스(Bacteroidetes)에 기인한 것으로 나타났습니다.
시험관 내에서 개별 균주의 효소 활성 측정이 pH를 포함한 환경 조건과 미생물 유형의 상대적 풍부도가 매우 다를 수 있는 생체 내 활성을 반드시 반영하는 것은 아니라는 점에 유의해야 합니다. 예를 들어, Cole 등[105]은 시험관 배양 후와 동일한 균주를 무균 쥐에 도입한 후 측정한 효소의 활성을 비교한 결과 상당한 차이가 있음을 발견했습니다.
Omics approaches
There is a growing awareness of the importance of the gut microbiome in the overall system of the host. This has led to the inclusion of top-down approaches studying the composition and functionality of the microbiota, so-called ‘-omics’ approaches. Metagenomics provides insight into the genes that could be expressed, while metatranscriptomics reveals information about regulatory networks and gene expression and combined with metaproteomics, and metabolomics informs about the functionality of the microbiota and, therefore, provides some strong insights into microbial activities in the gut.
Studies are performed in an unbiased fashion with the focus on hypothesis generation rather than hypothesis testing. This has proven particularly effective for studying the gut microbiota due to the relatively limited understanding of this multi-dimensional dynamic variable. Each ‘-omic’ technology provides its own unique perspective of the microbiota and its impact on the host, so to fully exploit their potential multiple ‘-omic’ approaches can be applied simultaneously and results integrated, preferably from the same sample. With the help of mathematical modelling, this enables a comprehensive understanding of the microbial ecosystem to be gleaned and its contribution to the overall biological system to be studied at the molecular level. This represents a significant technical and bioinformatic challenge, although a new methodological framework developed by Roume et al. [110] for the co-extraction of DNA, large and small RNA, proteins, and polar and non-polar metabolites from single samples of microbial communities represents a significant step in this process.
Metagenomics
Metagenomics has extensively been used to investigate differences in microbiota composition in disease states such as inflammatory bowel disease, obesity, and diabetes compared to healthy individuals, but it has also revealed novel changes in microbiota function in some diseases [111]. For example, Wei et al. [112] reported that the faecal microbiota of 20 patients with hepatitis B cirrhosis of the liver showed enrichment of metabolism of glutathione, branched-chain amino acids, nitrogen, lipids, and gluconeogenesis, and a decrease in aromatic amino acids and bile acid-related metabolism in comparison to control subjects.
Metagenomic analysis is being increasingly used to study functional genes of the gut microbiota. Jones et al. [63] used this methodology to study the distribution of BSH genes. Via metagenomic analyses, they identified functional BSH in all the main bacterial divisions and Archaea in the gut and demonstrated that BSH is a conserved adaptation to the amount of conjugated bile acids in the gut and exhibits a high level of redundancy. Of particular relevance to the present review is the approach taken in a recent paper by Mohammed and Guda [113]. The authors developed an ensemble of machine learning methods termed ECemble (Enzyme Classification using ensemble approach) to model and predict enzymes from protein sequences and identify enzyme classes and subclasses at high resolution. The method was then applied to predict enzymes encoded by the human gut microbiome from gut metagenomic samples, and to study the role of microbe-derived enzymes in the human metabolism. They identified 48 pathways that have at least one bacteria-encoded enzyme. The pathways were primarily involved in the metabolism of amino acids, lipids, co-factors, and vitamins. Subsequently, the methods were used to demonstrate differences in the profiles of gut microbiota-derived enzymes in lean and obese subjects and in patients with IBD. For example, the microbiota of obese subjects was enriched in polygalacturonase, which is encoded by Bacteroides and Prevotella species. In contrast, urease-encoding bacteria were found in fewer numbers in obese versus lean subjects.
A number of metagenomic studies have focused on so-called carbohydrate active enzymes (CAZymes) due to the critical role that the gut microorganisms play in the breakdown of dietary fibre and other non-absorbed carbohydrates in the gut. Such an approach is not restricted to the study of the enzymatic activity of cultivable microbes and has revealed a wide diversity of CAZymes of at least 81 families of glycoside-hydrolases. For example, Tasse et al. [114] using in-depth pyrosequencing, discovered 73 CAZymes from 35 different families and also identified 18 multigenic clusters encoding complementary enzyme activities for fibre degradation.
Single cell genomics is an emerging technology in which single microbial cells are isolated from a sample, their DNA extracted and amplified and then shotgun sequenced [115]. The advantage of this approach is that genomic data can be placed in a phylogenetic context even where the function of a putative gene is unknown and information from rare or uncharacterized species can be obtained. This has the potential to complement metagenomics by aiding the functional assignment of metagenomic data.
Although metagenomics is a powerful tool for investigating the gut microbiota, it does have limitations. These have been discussed in detail by Wang et al. [111] but include the requirement for sufficient high-quality DNA, the impact of different DNA extraction methods and kits on results, and of particular relevance for functional metagenomics, the limitations in the size and quality of reference databases, which impedes the assignment of functions to the data obtained. Finally, the presence of a gene does not inform us about gene expression patterns. Metatranscriptomics, metaproteomics, and metabonomics enable the latter to be more effectively addressed.
Metatranscriptomics
Metatranscriptomics extracts and sequences mRNAs from a microbial ecosystem to determine the genes that may be expressed in that community. It usually involves reverse transcription to generate cDNA, which is then sequenced using similar methodologies as for metagenomics. Metatranscriptomics allows the identification of novel non-coding RNAs, including small RNAs thought to play important roles in biological processes such as quorum sensing and stress response [116]. The approach has mostly been applied to samples from water and soil environments and less frequently to the gut microbiota [117, 118] and the microbiota studies need to be interpreted with considerable caution, given the major limitation of the short half-life of bacterial mRNAs, although this is less of an issue for studies using ribosomal RNAs, which are more stable.
Gosalbes et al. [118] performed a metatranscriptomic analysis of faecal microbiota from 10 healthy subjects. Microbial cDNAs from each sample were sequenced by 454 methodology and analysis of the 16 S rRNA transcripts revealed that Firmicutes and Bacteroidetes were the sources of the greatest number of transcripts (49 and 31%, respectively) with smaller numbers from Proteobacteria (3.7%), Actinobacteria (0.4%), and Lentisphaerae (0.2%). The majority of the Firmicutes sequences fell into the Lachnospiraceae and Ruminococcaceae families, which contain pectin and cellulose degraders. In the Bacteroidetes phylum Bacteroidaceae, Prevotellaceae, and Rickenellaceae families were functionally the most important. Interestingly, the most active families were the same in all the volunteers.
The non-ribosomal transcripts from the faecal samples were searched by BLASTX against an established NCBI COG database to obtain a functional distribution for each sample. The pattern was very similar for all the samples with carbohydrate transport and metabolism, energy production and conversion, and synthesis of cellular components being the main activities. Other areas such as amino acid and lipid metabolism, cell motility, and secondary metabolite biosynthesis were underrepresented in the metatranscriptome. These results are consistent with an earlier, smaller study by Turnbaugh et al. [117] in monozygotic twins in which the genes with higher relative expression included those for carbohydrate metabolism, energy metabolism, nucleotide metabolism, and those associated with essential cell processes, e.g., RNA polymerase and glycolysis.
As with all the ‘-omics’ approaches, metatranscriptomics has its limitations and studies are challenging both technically and in terms of bioinformatics. The short half-life of mRNA leads to difficulty in the detection of short-term responses to environmental changes, consequentially extrapolating results obtained from transcriptional analysis of faecal samples to functions within the large intestine itself can present problems [115, 117].
Metaproteomics
Metaproteomics aims to characterize the complete profile of gene translation products and can yield additional information about post-translational modifications and localization over that provided by metatranscriptomics measurements [119]. One of the advantages of metaproteomics is that it is possible to link proteins to specific taxonomic groups, thus providing insight into the microbes at species and strain level involved in specific catalytic functions and pathways, i.e., genotype–phenotype linkages [120].
Methodologies for metaproteomics are in a state of development, but typically they involve heat treatment of the faecal sample and extensive bead beating to extract and denature the proteins, which are subsequently enzymatically digested to peptides. Peptide analysis is usually by nano-2D-LC-MS-MS and COG assignments are determined for each peptide sequence by BLAST against the NCBI COG database. Microbial community functions are analyzed by grouping proteins into COG categories.
Metaproteomic studies on the gut microbiota to date have been performed in small numbers of subjects (usually n = 1–3), which limits the conclusions that can be drawn, but the results have shown some consistencies. Verberkmoes et al. [121] conducted a faecal metaproteomic analysis of a pair of adult female monozygotic twins. Analysis was by nano-2D-LC-MS-MS and the proteins identified by database searches were classified into COG categories. In both subjects, the most abundant COG functions were energy production, amino-acid metabolism, nucleotide metabolism, carbohydrate metabolism, translation, and protein folding. The authors compared the metaproteomic profile with a previously published metagenomic profile of two individuals which revealed that in contrast to the most abundant functions identified in the metaproteome above, the metagenome was dominated by proteins involved in inorganic ion metabolism, cell wall and membrane biogenesis, cell division, and secondary metabolite biosynthesis.
Kolmeder et al. [122] investigated composition and temporal stability of the faecal metaproteome in samples collected at 2 time points from 3 healthy subjects over a period of 6–12 months. The results indicated that the faecal metaproteome is subject-specific and is stable over a 1-year period. A stable common core of about 1000 proteins was recognised in each of the subjects. The most abundant core protein was found to be glutamate dehydrogenase, and this enzyme showed high level of redundancy in the intestinal tract, since it was associated with a number of microbial families, Lachnospiraceae, Bacteroidaceae, Ruminococcaceae, and Bifidobacteriaceae. Other high abundance proteins included pyruvate-formate lyase, which converts pyruvate to acetyl-CoA and formate, and chaperone proteins involved in protein folding and Fe-S cluster formation. About 10% of the total proteome comprised proteins involved in carbohydrate transport and metabolism including ABC sugar transporters and glycolytic enzymes. When the COGs were mapped onto pathways, the main functional categories were metabolism of carbohydrates, nucleotides, energy, amino acids, and co-factors and vitamins (especially B12 and folic acid).
Metaproteomics has also been applied to faecal samples from a lean and an obese subject and to comparisons of Crohn’s Disease patients and healthy subjects (reviewed by Xiong et al. [119]). Young et al. [120] used shotgun proteomics to characterize the functional changes in the faecal microbiota 7–21 days after birth of a preterm infant. The results suggested that the developing microbial community initially focuses its resources on cell division, protein production, and lipid metabolism later switching to more complex metabolic functions, such as carbohydrate metabolism, and secreting and trafficking proteins. It is noteworthy that this functional distribution seen after 3 weeks was similar to that observed in the adult human gut [121].
Metaproteomics is a developing technology and has its limitations, in particular there is no reference protocol, so it can be difficult to compare studies and the bioinformatic systems for metaproteomics are less well developed than those for metagenomics. Kolmeder and de Vos [123] have discussed in detail published methodologies, highlighting the importance of sampling techniques and sample preparation and processing.
Metabolic profiling (metabonomics/metabolomics)
Metabolic profiling has emerged as a powerful systems biology approach simultaneously measuring the low-molecular weight compounds in a biological sample, capturing the metabolic profile or phenotype. In the host, these metabolic signatures contain thousands of molecular components that arise from endogenous and exogenous metabolic processes, environmental inputs, and metabolic interactions between the host and environment. The environmental inputs can include dietary components and products of gut microbial activity. A major strength of using metabonomics to study the gut microbiota is the ability to measure metabolites in host samples that derive directly from the microbiome, for example the SCFAs. This provides a direct read-out of gut microbial activity and variations due to diet. Furthermore, upon absorption from the gut, microbial products can enter host metabolic processes resulting in downstream metabolic perturbations and the generation of microbial-host co-metabolites, all of which can be captured by metabolic profiling.
Practically, metabolic profiling can be applied to a range of different sample types and experimental models. It can be used to characterize the metabolites in samples from in vitro experiments, including pure cultures and complex gut models, and various sample types collected from in vivo studies [13]. In vivo samples can include biofluids, such as urine, blood, faecal water, saliva, and cerebrospinal fluid, and various tissue samples such as those collected from the gut, liver and brain. To measure the metabolic profile of a sample, two analytical platforms are typically used, 1HNMR spectroscopy and mass spectroscopy (MS). Both techniques are capable of simultaneously capturing quantitative and structural information on a broad range of metabolites in an unbiased manner in a single measurement. Comprehensive reviews on these analytical techniques have been published [124–126]. 1H NMR spectroscopy measures protons (1H) on metabolites in a sample and MS measures the exact mass of molecular ions in a sample and how they fragment. This information can then be used to identify the metabolites present and their abundance. MS is usually preceded by a separation step to allow the analysis of complex mixtures, which includes liquid or gas chromatography, or in some cases capillary electrophoresis. Although a single analytical technique is routinely applied in metabonomic studies, these two techniques are complementary and their parallel application can provide wide metabolome coverage.
The metabolic phenotype acquired from these techniques is multivariate in nature containing hundreds to thousands of metabolites. To extract latent information associated with gut microbial function or their influence on host metabolism from this multi-dimensional data, a range of pattern recognition techniques are applied [127–129]. Standard multivariate statistical techniques used for metabolic profiling studies include the unsupervised approaches, principal components analysis (PCA), and hierarchical clustering analysis (HCA) and the supervised approach, projection to latent structures (PLS) analysis. Unsupervised methods are concerned with modelling variation within the data and have no a priori knowledge of sample classification. In contrast, supervised methods use known information of the samples (e.g., germ-free versus conventional status; placebo versus prebiotic intervention) to extract information in the metabolic data that are related to this information.
The utility of this approach for studying the microbiome has been demonstrated in animal models of altered microbial status, such as germ-free, gnotobiotic, antibiotic-treated animals, and also in human studies [82, 130–132]. These studies have shown the extensive reach of the gut microbiota throughout the metabolic system of the host and the diverse pathways modulated. This is not just restricted locally to the immediate environment of the gut but systemically to peripheral tissues such as the heart and brain. For example, dietary choline from sources such as red meat and eggs can be metabolized by the microbes in the gut to trimethylamine and dimethylamine. Trimethylamine is toxic and requires oxidation in the liver by the flavin-containing monooxygenase 3 (FMO 3) enzyme before being excreted as trimethylamine-N-oxide (TMAO). TMAO can be used as an electron acceptor by Escherichia coli, and, interestingly, has been implicated as a risk factor for CVD [133].
A vast amount of information is captured with metabolic profiling and various inherent (e.g., genetic constitution, age, and gender) and environmental (e.g., diet, alcohol intake, and drug therapy) factors can influence the metabolic phenotype. Unrelated variation in these metabolic signatures can often mask or obscure the variation resulting from gut microbial activity. As such, careful study design is essential when investigating the role of the gut microbiota on host metabolism to minimise this unrelated noise. Although this can be tightly controlled in animal studies, it represents a major challenge for human studies. One way to overcome this issue is through the use of statistical approaches. Orthogonal projections to latent structures (OPLS) is one such method applying an orthogonal signal correction (OSC) to remove metabolic variation unrelated to the variable being studied. This improves the interpretation of the data enabling the influence of the gut microbiota to be illuminated. Another limitation in metabolic profiling studies is the metabolite identification stage. Once significant metabolic associations are discovered assigning an identity, pathway and/or function to these features can represent a bottleneck in the metabonomic workflow. Advancements in databases, software platforms, and analytical approaches are helping to overcome these limitations and expedite this process.
Stable isotope probing (SIP)
The use of stable isotopes (e.g., 13C, 15N, and 18O) can help elucidate the fate of specific compounds within complex microbial systems such as the gut and can be particularly useful if combined with ‘-omics’ techniques [115]. Tannock et al. [134] used the technique to identify the main microbial users of inulin in the rat gut. Inulin labelled with 13C was fed to rats and RNA extracted from caecal contents by isopycnic buoyant density gradients was used to detect labelled RNA from cells that had metabolized the inulin. 16 S rRNA genes amplified from cDNA from the labelled fractions were sequenced and showed that Bacteroides uniformis, Blautia glucerasea, Clostridium indolis, and Bifidobacterium animalis were the main species utilizing inulin in these rats.
Use of mathematical modelling to mimic the gut ecosystem
Meta-omic analyses have resulted in a tremendous amount of data on the composition, encoded functionalities, and metabolic output of the human gut microbiota [135]. However, the inherent complexity of the gut ecosystem hinders the interpretation of this wealth of data [136]. A systems-level understanding of the microbiota has to include the underlying complex interactions, since the gut ecosystem as a whole is more than the sum of its parts [136]. A complete, integrated view of the human gut microbiota requires the use of mathematical models.
Identifying novel food ingredients which may have beneficial effects on the gut microbiota when provided as dietary supplements is often difficult due to the large number of variables that need to be compared in well-designed controlled human intervention studies. Experimental models (small animals and fermenter systems) have proved extremely useful, but even they prove time consuming and not all variables can be compared. Mathematical models offer an alternative to try and evaluate bacterial interactions and the impact of different dietary components on microbial composition and activity, at least with the aim of refining the choices to be used in the experimental situation.
One approach applied to simulate the behaviour of gut microbial communities is kinetic modelling. A kinetic model showed the role of bacterial cross-feeding in the conversion of lactate to butyrate by two distinct but abundant human gut bacteria, Eubacterium hallii and Anaerostipes coli [137]. Kettle et al. [138, 139] created a minimal model of the intestinal ecosystem, distilling the microbiota down into 10 bacterial functional groups, each comprising of a mixture of at least 10 bacteria. Pure and mixed culture data was used to provide assumptions of growth rates, substrate specificity and metabolic activities for each of the functional groups. The model successfully predicted the switch between high butyrate production at pH 5.5 and high propionate production at pH 6.5 that had occurred in a previous continuous flow fermenter experiment [139, 140]. The model was also used to estimate the effect of removing entire functional groups (or single strains within a functional group) on the community profile and activity, revealing changes in both SCFA concentrations and abundances of other groups [139]. Such models could be of huge benefit in assessing the consequence of bacterial species shown to be missing, or extra, in disease states on the development of the disease. They would also show whether simply adding back a ‘missing’ bacterium would be sufficient to potentially change the course of the development of the disease. Such models could also be used to illustrate the potential consequences to microbial composition and metabolite production during periods of starvation or substrate depletion or replacement.
Agent-based modelling
Agent-based modelling (ABM) is another approach frequently employed to study the dynamic behaviour of ecological systems in silico. In ABM, objects with well-defined properties (representing, e.g., bacterial cells), are allowed to interact with each other, resulting in a dynamic model that can depict real-time behaviour of a biological system [141]. A recent study used ABM to simulate the positive (e.g., mutualistic) and negative (e.g., competitive) interactions between two bacteria representing a typical Bacteroidetes and Firmicutes, respectively [141]. A simulation of exposure to antibiotics and subsequent recovery confirmed that feedback mechanisms between bacterial species enabled the restoration of system stability after antibiotic perturbation [141].
Topological analysis of metabolic networks
A genome-scale metabolic reconstruction summarizes known biochemical reactions of a target organism in a well-structured manner. Several studies have applied metabolic networks of the human gut microbiome, revealing topological properties of its global metabolic network and characterizing the microbiome’s metabolic potential (reviewed in Manor et al. and Heinken and Thiele [136, 142]). Recently, metabolic networks of gut microbes have been integrated with a Boolean dynamic model constructed from time series metagenomic data [143]. The model predicted that the commensal Barnesiella intestinihominis can inhibit the growth of Clostridium difficile, which was validated experimentally [143]. Another study integrated community-wide metabolic networks with metagenomic and metabolomic data and the community-wide metabolic turnover was subsequently predicted for the vaginal and the gut microbiome [144]. While correlation-based statistical analyses of metabolomic measurements are not mechanistic, this framework has the advantage of proposing mechanisms for the contributions of species to the turnover of particular metabolites [144].
Modelling emerging phenotypic properties
The constraint-based reconstruction and analysis (COBRA) approach uses genome-scale reconstructions (GENREs) that are constructed from the genome of a target organism and curated and validated against the available literature [145]. More than 20 GENREs have been built for microorganisms inhabiting the human body [142]. GENREs are converted into mathematical models that are tailored to specific conditions by enforcing constraints: physico-chemical (e.g., mass–charge balance), environmental (e.g., nutrient availability), and regulatory (e.g., gene expression) [145]. By defining an objective, e.g., biomass production, metabolic fluxes through the network that satisfy this objective are predicted [146]. For instance, by imposing constraints on nutrient availability, the growth requirements of a reconstructed organism can be predicted. This approach resulted in the prediction and subsequent experimental validation of a defined medium for Faecalibacterium prausnitzii [147]. Moreover, GENREs enable the evaluation of a species’ functional repertoire. In a large-scale study, metabolic networks were retrieved from the genomes of 301 representative human gut microbes and their metabolic capabilities were systematically compared in the context of phylogenetic distance between species [148]. The analysis revealed an exponential relationship between metabolic and phylogenetic distance, with closely related species being more metabolically diverse than could be expected in a linear relationship [148].
Of particular interest is the prediction of in silico interactions in microbial communities, and between the gut microbiota and the human host. Similar to the kinetic model described above, such a multi-species model can predict the effect of perturbing community composition (e.g., the removal of key species). Moreover, the effects of varying nutrient environments (e.g., different diets) on the community can be explored. In a first effort to model a host-gut microbe symbiosis, a metabolic model of the mouse was joined with Bacteroides thetaiotaomicron and their mutually beneficial cross-feeding was simulated on five dietary regimes [149]. Moreover, the rescue of lethal host gene defects by Bacteroides thetaiotaomicron was predicted [149]. The effect of a simplified model gut microbiota on human metabolites was modelled by joining the human reconstruction Recon2 with 11 published, manually curated GENREs of gut microbes from three phyla [150]. The combined contribution of secretion products by microbes and dietary input on the host’s metabolome was quantified on four simulated diets [150]. The underlying mechanisms were predicted for the microbial contribution to host secretion for the examples of glutathione, taurine, and leukotrienes [150].
Constraint-based modelling is commonly applied to the in silico prediction of microbe–microbe interactions (reviewed in Heinken and Thiele, Biggs et al., and Zomorrodi et al. [142, 151, 152]). Typically, two or more GENREs are joined to construct community models with well-defined species–species boundaries, thus allowing the prediction of metabolic cross-feeding between species [142]. To investigate the effect of varying metabolic environments on microbe–microbe interactions, 11 published gut microbe GENREs were joined in all pairwise combinations and the outcome (positive, neutral, negative interaction) was predicted [153]. The metabolic exchange and the distribution of resources between the pairs were simulated on four simulated gut microenvironments and three diets [153]. The model predicted that the need to regenerate reducing equivalents enforced mutualism in certain pairs under anoxic conditions [153]. In vitro screens of pairwise interactions between microbes are laborious and the described in silico framework constitutes an important first step for the prediction of candidate pairs of interest that would be subsequently validated experimentally.
GENREs also provide a useful framework for the contextualization of meta-omic data, with a variety of constraint-based methods for the integration of such measurements already available [154].
In summary, important advances have been made in the construction of mathematical models that capture key aspects of the gut microbiota and its interactions with its host, summarize the current knowledge on its metabolism, and propose hypotheses that can be experimentally validated. In future efforts, such models will result in the elucidation of previously unknown, non-intuitive relationships between the gut microbiota and host physiological states. Moreover, dietary and drug interventions to favourably manipulate the human gut microbiota may be predicted in silico prior to experimental validation. Knowledge of an individual’s microbial composition will ultimately enable models to be tailored to predict effects of specific diets or supplements at an individual level.
Conclusions
The wealth of metabolic functionality encoded within the gut microbiome extends the biochemical flexibility of the host to process a wide range of dietary substrates. Carbohydrate metabolism and transport is clearly a major catalytic function of the microbiota with important consequences for the host, and the metabolic pathways and end products have been well studied and are characterized by great flexibility in response to substrate availability. Similarly, pathways for metabolism of other dietary macromolecules, namely protein, have been elucidated as have those for vitamin synthesis. The microbiota also has extensive capacity to metabolize phytochemicals, particularly polyphenols, by diverse, well-characterized pathways. There is extensive evidence that inter-individual differences in metabolism of dietary polyphenols are largely a consequence of differences in gut microbiota composition and that these can have implications for the effects of some polyphenols on health. It should be noted that the uptake and utilization of microbial metabolites by other members of the microbiota and absorption by the host result in a highly dynamic system of metabolic fluxes and makes determination of changes in concentrations of metabolites with time very difficult using current single snapshot analyses in faecal samples. Application of stable isotope probes in combination with mathematical modelling may prove valuable in this area.
Importantly, there exists a large amount of functional redundancy within the microbiota with different bacteria performing the same or similar functions. BSHs and CAZymes are a good example of this redundancy, being encoded in the genomes of bacteria from several different phyla [62]. As such, compositional variation may not necessarily translate into a functional variation relevant to the host. Furthermore, extensive cross-feeding networks exist within this dynamic ecosystem resulting in a range of possible outcomes for the same substrate depending upon the species present and their proximity. Accordingly, there is a growing appreciation that measuring composition alone is no longer sufficient to gain meaningful insights into the functional status of the gut microbiota, its metabolic interaction with the host, and its potential to modulate host health and disease.
Gut microbiota-derived products can be absorbed from the gut and enter host endogenous and exogenous pathways to influence the overall metabolic phenotype of the host. In addition, metabolites generated by the host can be secreted into the gut via the enterohepatic circulation and serve as substrates for the resident microbes. Collectively, these processes result in a biochemical cross-talk between the host genome and microbiome with the microbiota able to exert a strong influence on the metabolic phenotype of the host. For example, gut microbial BSH enzyme expression in mice altered plasma bile acid signatures and concomitantly the transcription of genes involved in host lipid metabolism and metabolic signalling pathways and also influenced cholesterol metabolism and weight gain [155]. Recent evidence indicates that the SCFAs acetate, propionate and butyrate can play a significant role in central appetite regulation (via hypothalamic neuronal activation patterning and changes in the expression profiles of regulatory neuropeptides) and energy homeostasis via influencing intestinal gluconeogenesis. Further study of these types of interactions is essential to understand how the gut microbiota influence host health and disease.
Acknowledgements
This work was conducted by an expert group of the European branch of the International Life Sciences Institute, ILSI Europe. The expert group and this publication was coordinated by the Functional Foods and Prebiotics Task Forces. Industry members of this task force are listed on the ILSI Europe website at http://www.ilsi.eu. Experts are not paid for the time spent on this work; however, the non-industry members within the expert group were offered support for travel and accommodation costs to attend meetings to discuss the manuscript, and a small compensatory sum (honorarium) with the option to decline. The expert group carried out the work, i.e., collecting/analysing data/information and writing the scientific paper separate to other activities of the task forces. The research reported is the result of a scientific evaluation in line with ILSI Europe’s framework to provide a precompetitive setting for public–private partnership (PPP). The opinions expressed herein and the conclusions of this publication are those of the authors and do not necessarily represent the views of ILSI Europe nor those of its member companies. ILSI Europe facilitated scientific meetings and coordinated the overall project management and administrative tasks relating to the completion of this work. In particular, the authors would like to thank Dr. Tobias Recker, Dr. Estefanía Noriega, and Mr. Alex Rankin for their support. Furthermore, the authors would like to thank all participants in the ILSI Europe workshop The Gut Microbiome: Our Misunderstood Friend and Foe (3–4 December 2015, Brussels, Belgium) for their involvement in fruitful scientific discussions and their valuable insights which contributed to the refinement of the draft manuscript. For further information about ILSI Europe, please e-mail info@ilsieurope.be or call + 32 2 771 00 14.
Abbreviations
| AXOS | Arabinoxylan oligosaccharide |
| BSH | Bile salt hydrolase |
| CA | Cholic acid |
| cAMP | Cyclic adenosine monophosphate |
| CAzyme | Carbohydrate active enzyme |
| CDCA | Chenodeoxycholic acid |
| CE | Capillary electrophoresis |
| COG | Clustered orthologous group |
| CVD | Cardiovascular disease |
| DCA | Deoxycholic acid |
| FMO | Flavin-containing monooxygenase |
| FOS | Fructo-oligo-saccharide |
| FXR | Farnesoid X receptor |
| GC | Gas chromatography |
| GIT | Gastrointestinal tract |
| GPR | G protein-coupled receptor |
| HCA | Hierarchical clustering analysis |
| HGM | Human gut microbiota |
| IBD | Inflammatory bowel disease |
| IBS | Irritable bowel syndrome |
| LC | Liquid chromatography |
| LCA | Lithocholic acid |
| MS | Mass spectrometry |
| NMR | Nuclear magnetic resonance |
| PCA | Principal components analysis |
| qPCR | Quantitative polymerase chain reaction |
| PLS | Projection to latent structures |
| SCFA | Short chain fatty acid |
| SIP | Stable isotope probing |
| SRB | Sulphate reducing bacteria |
| TMAO | Trimethylamine-N-oxide |
| UDCA | Ursodeoxycholic acid |
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Ian Rowland, Phone: +32 (0)2 775 91 44, Email: eb.eporueisli@snoitacilbup, Email: ku.ca.gnidaer@dnalwor.i.
Glenn Gibson, Email: ku.ca.gnidaer@nosbig.r.g.
Almut Heinken, Email: ul.inu@neknieh.tumlA.
Karen Scott, Email: ku.ca.ndba@ttocS.K.
Jonathan Swann, Email: ku.ca.lairepmi@nnaws.j.
Ines Thiele, Email: ul.inu@eleiht.seni.
Kieran Tuohy, Email: ti.hcamf@yhout.nareik.
References
1. Rajilić-Stojanović M, de Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev. 2014;38:996–1047. doi: 10.1111/1574-6976.12075. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
2. Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
3. Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [PubMed] [CrossRef] [Google Scholar]
4. Greenblum S, Turnbaugh PJ, Borenstein E. Metagenomic systems biology of the human gut microbiome reveals topological shifts associated with obesity and inflammatory bowel disease. Proc Natl Acad Sci U S A. 2012;109:594–599. doi: 10.1073/pnas.1116053109. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
5. Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330–339. doi: 10.1136/gutjnl-2015-309990. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
6. Boyd SD, Liu Y, Wang C, et al. Human lymphocyte repertoires in ageing. Curr Opin Immunol. 2013;25:511–515. doi: 10.1016/j.coi.2013.07.007. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
7. Macfarlane GT, Gibson GR, Cummings JH. Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol. 1992;72:57–64. [PubMed] [Google Scholar]
8. Steliou K, Boosalis MS, Perrine SP, et al. Butyrate histone deacetylase inhibitors. Biores Open Access. 2012;1:192–198. doi: 10.1089/biores.2012.0223. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
9. De Vadder F, Kovatcheva-Datchary P, Goncalves D, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [PubMed] [CrossRef] [Google Scholar]
10. Brown AJ, Goldsworthy SM, Barnes AA, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [PubMed] [CrossRef] [Google Scholar]
11. Tazoe H, Otomo Y, Kaji I, et al. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J Physiol Pharmacol. 2008;59(Suppl 2):251–262. [PubMed] [Google Scholar]
12. Duncan SH, Holtrop G, Lobley GE, et al. Contribution of acetate to butyrate formation by human faecal bacteria. Br J Nutr. 2004;91:915–923. doi: 10.1079/BJN20041150. [PubMed] [CrossRef] [Google Scholar]
13. Frost G, Sleeth ML, Sahuri-Arisoylu M, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611. doi: 10.1038/ncomms4611. [PMC free article] [PubMed] [CrossRef] [Google Scholar]