Re: Caloric Restriction Mimetics against Age-Associated Disease - 꼭 읽어
작성자문형철작성시간22.10.29조회수135 목록 댓글 0
PERSPECTIVE
| VOLUME 29, ISSUE 3, P592-610, MARCH 05, 2019
Caloric Restriction Mimetics against Age-Associated Disease: Targets, Mechanisms, and Therapeutic Potential
The increase in life expectancy has boosted the incidence of age-related pathologies beyond social and economic sustainability. Consequently, there is an urgent need for interventions that revert or at least prevent the pathogenic age-associated deterioration. The permanent or periodic reduction of calorie intake without malnutrition (caloric restriction and fasting) is the only strategy that reliably extends healthspan in mammals including non-human primates. However, the strict and life-long compliance with these regimens is difficult, which has promoted the emergence of caloric restriction mimetics (CRMs). We define CRMs as compounds that ignite the protective pathways of caloric restriction by promoting autophagy, a cytoplasmic recycling mechanism, via a reduction in protein acetylation. Here, we describe the current knowledge on molecular, cellular, and organismal effects of known and putative CRMs in mice and humans. We anticipate that CRMs will become part of the pharmacological armamentarium against aging and age-related cardiovascular, neurodegenerative, and malignant diseases.Keywords
Caloric restriction (CR) consists of the chronic reduction of total calorie intake without malnutrition. Together with intermittent fasting (which can be regarded as a particular form of CR in which episodes of ad libitum feeding are alternated with episodes of up to zero caloric uptake), CR is the only known strategy to robustly improve health- and lifespan in most, if not all, living organisms. In Rhesus monkeys, two differently designed studies revealed contrasting results on lifespan (
) but similar health benefits and delayed onset of aging phenotypes. In humans, CR has been reported to counteract several age-associated alterations (Figure 1). In non-obese, healthy adults, 24 months of continuous CR (15%–25%) was safe (
), improved the quality of life (
), and caused 10%–13% weight loss (mostly, but not exclusively, reducing fat mass), which stabilized after 1 year (
). Fasting insulin levels, body temperature (a possible marker for metabolic rate), resting energy expenditure, oxidative stress, and thyroid axis activity were reduced under CR (
,
). “Metabolic adaptation,” a long-term effect of CR that reduces the metabolic rate below the expected value, occurs in humans and may support longevity (
,
). In healthy humans, CR also decreases the levels of circulating tumor necrosis factor-α and cardiometabolic risk factors (triglycerides, cholesterol, and blood pressure) (
,
). Upon CR and weight loss, insulin growth factor-1 (IGF1) levels and insulin resistance are reduced in obese patients (
). However, they are not improved in non-obese humans after the 1-year weight loss phase (
) (contrary to mouse studies) unless protein intake is also reduced (
). While CR inhibits inflammation, its effects on immunity need further clarification since different levels of CR may subvert and/or modulate immune defenses against bacterial (
) and viral infection (
). In obese humans, CR promotes significant weight loss and improves general health (
). Of note, the well-documented good health and high incidence of centenarians in the population of the Japanese Okinawa island have been attributed to nutritional cues including a mild and consistent CR (∼10%–15%) (
).
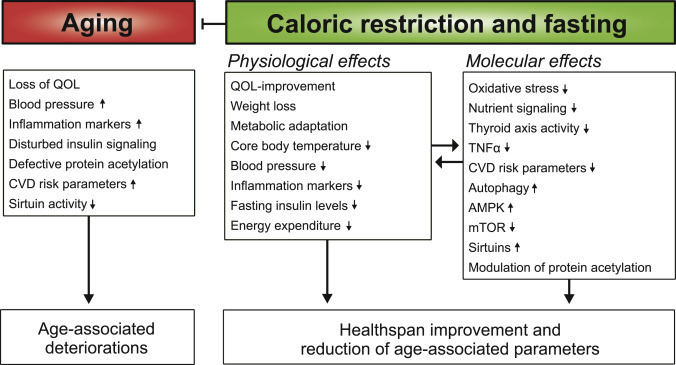
Figure 1Physiological and Molecular Effects of Caloric Restriction
Molecular Effects of CR and Fasting
Macroautophagy (hereafter referred to as autophagy) is a conserved cellular recycling program that eliminates dysfunctional organelles, proteins, and aggregates from the cytoplasm, hence protecting cellular functionality and integrity. Accordingly, impaired or dysregulated autophagy has been linked to advanced age, neurodegeneration, cardiovascular diseases (CVDs), and cancer. In turn, the activation of autophagy via genetic or pharmacological means extends lifespan and/or healthspan in numerous model organisms, including mice (
). As a catabolic process, autophagy is induced upon nutrient deprivation and plays an important role in the beneficial effects exerted by CR and fasting regimens. CR modulates several molecular key players involved in the regulation and execution of autophagy, nutrient signaling, and energy metabolism (Figure 1). For instance, CR activates AMP-activated protein kinase (AMPK) (
). AMPK is an energy sensor that inhibits the kinase activity of mechanistic target of rapamycin (mTOR), an autophagy repressor, under CR. Furthermore, CR directly and indirectly activates sirtuins (SIRTs), which are nicotine adenine dinucleotide (NAD+)-dependent lysine deacetylases (KDACs) and play central roles during aging and autophagy (
). SIRT1 and AMPK may engage in a positive feedforward loop to amplify the response to CR.
Protein acetylation is a major regulator of autophagy. The Nɛ-acetylation of lysines is a phylogenetically conserved, posttranslational protein modification that is catalyzed by lysine acetyltransferases (KATs) and reversed by KDACs. Nɛ-acetylation regulates multiple metabolic enzymes, facilitating the adaptation to nutrient availability. Of note, Nɛ-acetylation may occur in a non-enzymatic fashion in the presence of AcCoA, especially at an acidic pH (
). There are four ways to diminish Nɛ-acetylation of proteins: (1) by reducing the concentration of cytosolic AcCoA, the sole donor of acetyl groups used by KATs, e.g., via inhibition of its synthesis from glycolysis, β-oxidation of fatty acids, or the catabolism of branched amino acids, or via increase of its consumption, for instance by carnitine acetyltransferases that transfer AcCoA acetyl groups on carnitine; (2) by degrading S-acetyl glutathione by mitochondrial thioesterase glyoxalase 2, GLO2, or cytosolic GLO1, thus reducing intermediates for non-enzymatic Nɛ-acetylation; (3) by activating specific KDACs, mostly SIRTs; and (4) by inhibiting KATs such as E1A-binding protein p300 (EP300). Notably, SIRT1 activity is low in aged and obese mice. This correlates with the inhibitory hyperacetylation of SIRT3, and transgenic activation of SIRT3 may improve the hepatic consequences of obesity including glucose intolerance (
). Moreover, in mice, transgene-enforced overexpression of SIRT6 (
) or brain-specific expression of SIRT1 (
) is sufficient to extend lifespan. In an earlier study, however, whole-body overexpression of SIRT1 did not extend lifespan (
). Similarly, another report observed no lifespan extension upon overexpression of the SIRTs sir-2.1 and dSir2 in the nematode Caenorhabditis elegans and the fruit fly Drosophila melanogaster, respectively (
), thus contradicting previous results (
,
,
). While it seems clear that SIRTs exert important functions related to healthy aging, their specific role in promoting longevity remains to be clarified (
).
Interestingly, autophagy and protein acetylation are subjected to circadian fluctuations (
). This oscillation is lost with aging and has been proposed as a modulatory target of CR (
). The maintenance of rhythmic (de)acetylation by CR is hypothetically linked to increased NAD+ levels, coupled to SIRT1 activation and rhythmic changes in the inhibitory acetylation of acetyl-CoA-generating acyl-CoA synthase short-chain family member 1 (ACSS1) (
). In aged flies, protein acetylation is increased, a phenomenon that can be attenuated by reducing the AcCoA-generating enzyme ATP citrate lyase (ACLY) or by mutating the KAT Chameau, resulting in an extended lifespan (
). Similarly, the inhibitory hyperacetylation of the pro-autophagic transcriptional factor Foxo1 has been observed in aged mouse hearts (
). Moreover, CR deacetylates histones H3 and H4 in mouse fat pads (
) and reduces the levels of biotin, which acts as an endogenous inhibitor of SIRT1 (
). Both histone deacetylation and deacetylation of cytosolic proteins may affect the expression and activity, respectively, of autophagy-relevant proteins (
,
). Both in mice and in humans, acute starvation causes a reduction of the acetylation of cytoplasmic proteins in peripheral blood mononuclear cells (
). However, every-other-day fasting increases histone acetylation in the mouse retina (
), and acetylation is reduced in aged mouse livers, a phenomenon that is reversed by CR, which causes hepatic protein hyperacetylation (
). This is at odds with chronic alcohol abuse, which leads to NAD+ depletion and SIRT inhibition, resulting in hyperacetylation of multiple proteins in the liver (such as AMPK, β-catenin, histone H3, and the transcription factors SREBB2, PPARα, FOX01, NFκB, and NFAT) (
). Therefore, the impact of CR on acetylation might depend on tissue, cell type, and the precise protein species. Indeed, one study reports that CR causes hyperacetylation of mitochondrial proteins in the liver and reduces acetylation in brown adipose tissue, yet it fails to affect the acetylation of mitochondrial proteins from other tissues (
).
CR Mimetics
Despite the uncontestable health-promoting effects of CR, most individuals are unable to observe a CR lifestyle, likely explaining some failures in observational clinical studies (
). Although long-term compliance may be improved by periodic fasting regimens, pharmacological approaches that induce autophagy without the subjective discomfort linked to CR or periodic fasting are warranted. Indeed, several CR mimetics (CRMs) improve health parameters in rodents and humans (see below). We previously defined CRMs as compounds that activate autophagy by promoting the deacetylation of cellular proteins (
), by (1) depleting AcCoA, (2) inhibiting acetyltransferases, and/or (3) stimulating deacetylases (Figure 2).
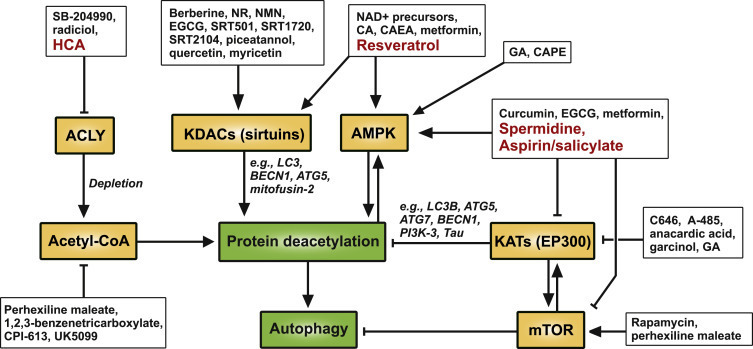
Figure 2Mechanistic Targets of Known and Potential CRMs
This definition reflects the fact that protein acetylation usually inhibits autophagy, while protein deacetylation favors autophagy. For instance, starvation is coupled to the inhibition of the acetyltransferase EP300 (due to the depletion of AcCoA), as well as to the activation of the deacetylase SIRT1 (due to the increase of the NAD+/NADH ratio and the activation of AMPK). This results in the deacetylation of hundreds of cellular proteins (
), reflecting multipronged regulatory effects on cell metabolism and the autophagic cascade. A systematic screen for KATs, the inhibition of which would induce autophagy, led to the identification of EP300 as a major negative regulator of autophagy that acts epistatic to starvation (
).
Interestingly, EP300 is subjected to activating phosphorylation by mTORC1 (
), while conversely, inhibition of EP300 generally results in mTORC1 inhibition (
), suggesting that both regulatory systems are intertwined. Similarly, protein deacetylation may be connected to the activation of AMPK, a potent autophagy inducer. Thus, deacetylation of liver kinase B1 (LKB1), for instance by SIRT2, favors the LKB1-mediated activation of AMPK (
). Likewise, EP300 inhibition results in AMPK activation (
). These examples illustrate how protein deacetylation may initiate autophagy, correlating with mTORC1 inhibition and AMPK activation. However, EP300 inhibition results in the induction of autophagy even in conditions in which AMPK is deleted, mTORC1 is artificially activated, or ULK1 is inhibited (
,
). This suggests that protein deacetylation can set off the autophagic cascade in a dominant fashion that is largely independent of other regulatory systems.
In accord with this interpretation, EP300 inhibition or SIRT activation may favor autophagy through deacetylation reactions that affect multiple autophagy-executory proteins (
). For instance, EP300 inhibition results in the deacetylation of phosphatidylinositol 3-kinase catalytic subunit type 3 (PI3K3) at K29 and K771, favoring its interaction with allosteric activators contained in the pro-autophagic Beclin 1 (BECN1) complex and its substrate phosphatidylinositol, respectively (
). BECN1 itself is also a substrate of EP300 and SIRT1 (at K430 and K437), and deacetylation of BECN1 favors the dissociation of its inhibitory interactor Rubicon (
). Of note, pro-autophagic derepression of BECN1 has been recently shown to promote longevity in mice (
). Furthermore, SIRT1 deacetylates nuclear microtubule-associated proteins 1A/1B light chain 3B (hereafter referred to as LC3) (at K49 and K51), stimulating its interaction with the nuclear protein DOR and its export to the cytoplasm, where it acts as a key initiator of autophagy (
). EP300 can also acetylate ATG5 and ATG7, both of which are involved in a conjugation system that promotes LC3 lipidation, which is required for autophagy induction. Of note, ATG5 is also deacetylated by SIRT2, supporting the notion that many autophagy regulators are substrates of both EP300 and SIRTs (
).
While the link between deacetylation of cytoplasmic proteins and autophagy seems rather unambiguous, it appears less straightforward with respect to nuclear proteins. On the one hand, the pro-autophagic transcriptional response has been linked, for example, to SIRT1- and spermidine-induced deacetylation of histones H4 and H3 (
), respectively. On the other hand, glucose deprivation stimulates AMPK activation with the final result that acetyl-CoA synthetase 2 (ACSS2) phosphorylated by AMPK translocates to the nucleus where it interacts with the transcription factor EB (TFEB) and binds to promoter regions of autophagy genes, locally producing acetyl-CoA and favoring pro-autophagic H3 hyperacetylation (
). These divergent outcomes may reflect feedback loops that impose a self-limitation on the autophagic process. For instance, rapamycin-induced autophagy is coupled to the hypoacetylation of H4K16 following the downregulation of lysine acetyltransferase 8 (KAT8), thereby reducing the transcription of pro-autophagic genes (
).
Besides autophagy-regulatory and executory proteins, deacetylation may also affect autophagic substrates. Depletion of general control of amino acid synthesis 5 (GCN5) like-1 (GCN5L1), a component of the mitochondrial acetyltransferase machinery, leads to mitochondrial protein deacetylation catalyzed by SIRT3, thus favoring mitophagy (
). SIRT1 deacetylates mitofusin-2, a protein tethered to the mitochondrial membrane, facilitating SIRT1-induced autophagy and mitophagy (
). As a further example, EP300 inhibition reduces the acetylation of Tau (a protein that forms pathogenic intraneuronal aggregates in Alzheimer's disease), which favors its clearance by autophagy (
).
Bona fide CRMs and Candidate CRMs
Several agents may be considered as CRMs since they cause protein deacetylation deriving in autophagy induction (Figure 2). We suggest that CRMs should also have the capacity to reproducibly extend lifespan and/or healthspan in model organisms, hence extending the functional definition of CRMs by another criterion. Here, we enumerate compounds that either fully comply with these stringent criteria (bona fide CRMs) or that do so at least partially according to the current state-of-the-art (potential CRMs) (Table 1).
Table 1Classification of Protective Substances as CRMs, Potential CRMs, and Other Compounds
GroupSubstance(Major) Molecular Target(s)(Nutritional) SourcesClinical Trialsa
| CRMs | aspirin (and salicylate) | AMPK, EP300, COX-1, COX-2, mTOR, NF-kB | willow bark, synthetic | several meta-analyses available, e.g., Cuzick et al., 2015 , Raju et al., 2016 |
| hydroxycitric acid | ACLY | diverse tropical plants, Garcinia cambogia, and Hibiscus sabdariffa | meta-analyses on weight loss through HCA in Onakpoya et al., 2011 ; NCT00699413 and NCT01238887 | |
| resveratrol | KDACs (SIRT1), AMPK, NF-κB | fruits, plants, and skin of grapes | reviewed in Berman et al., 2017 , e.g., NCT02621554 | |
| spermidine | KATs (EP300), mTOR, AMPK | wheat germs, soybeans, and nuts | safety evaluation in Schwarz et al., 2018 ; neuroprotection (Wirth et al., 2018 ); NCT02755246, NCT03378843, and NCT03094546 | |
| Potential CRMs | 1,2,3-benzenetricarboxylate | citrate transport protein | synthetic | – |
| acipimox | niacin receptor 1 | synthetic | several studies on obesity and diabetes, e.g., NCT00549614, NCT01488409, NCT00943059, and NCT01816165 | |
| berberine | SIRT1 | Berberis vulgaris and several other plants (roots and bark) | numerous phase 3 and 4 studies; reviewed in Imenshahidi and Hosseinzadeh, 2016 | |
| caffeic acid | AMPK and sirtuins | eucalyptus bark | 7 studies registered, e.g., NCT03070262; the clinical potential of CAPE reviewed in Murtaza et al., 2014 | |
| catechin | pleiotropic, exact mechanism unknown | cocoa, tea, and red wine | mainly green-tea combinations tested, few single compound studies; reviewed in Chacko et al., 2010 ; e.g., NCT03213340, NCT00233935, and NCT00448513 | |
| curcumin | AMPK, mTOR, and EP300 | Curcuma longa | numerous including phase 3 and 4 studies; reviewed in Gupta et al., 2013 ; e.g., NCT03085680, NCT01052025, NCT01975363, and NCT00099710 | |
| epicatechin | pleiotropic, exact mechanism unknown | cocoa, tea, and red wine | numerous studies using catechin-rich extracts, few single component trials, e.g., NCT01856868, NCT01880866, NCT02221791, NCT01691404, NCT02490527, and NCT02292342 | |
| EGCG | AMPK, mTOR, HATs, and KDACs | green tea | numerous phase 3 and 4 studies; reviewed and discussed in Mereles and Hunstein, 2011 | |
| gallic acid | AMPK and HATs | black tea and various plants | mainly polyphenolic combinations tested, e.g., NCT02800967, NCT02005939, and NCT03214276 | |
| metformin | AMPK, mTOR, HATs, and KDACs (sirtuins) | French lilac (Galega officinalis) | numerous phase 3 and 4 studies, reviewed in Nasri and Rafieian-Kopaei, 2014 ; e.g., NCT02432287 | |
| myricetin | SIRT1 | black tea, cole, parsley, garlic, curcuma, and fruits | reviewed in Li and Ding, 2012 | |
| NAD+ | KDACs (sirtuins), AMPK | various food | reviewed in Fang et al., 2017 ; many studies supplementing precursors, especially NR | |
| nicotinamide | KDACs (sirtuins) | various food | numerous, e.g., NCT02213094, NCT02416739, NCT03061474, and NCT01250990 | |
| nicotinamide mononucleotide | KDACs (sirtuins) | various food | NCT03151239 and UMIN000021309 (NIPH, Japan) | |
| nicotinamide riboside | KDACs (sirtuins) | various food | numerous studies, including phase 3 and 4; reviewed in Rolfe, 2014 , e.g., NCT03423342, NCT03423342, and NCT02921659 | |
| perhexiline maleate | carnitine O-palmitoyl transferase 1, mTOR | synthetic | numerous; reviewed in Chong et al., 2016 | |
| piceatannol | SIRT1 | passion fruit seeds | – | |
| quercetin | SIRT1 | black tea, onions, rocket, cole, curcuma, and fruits | reviewed in Miles et al., 2014 ; e.g., NCT00065676 and NCT01691404 | |
| rapamycin | mTOR | Streptomyces hygroscopicus | numerous; reviewed in Li et al., 2014 , e.g., NCT01649960 | |
| SRT1720 | SIRT1 | synthetic | – | |
| UK5099 | mitochondrial pyruvate carrier | synthetic | – | |
| Others | 4,4′-dimethoxychalcone | GATA transcription factors | Angelica keiskei | – |
| A-485 | EP300 | synthetic | – | |
| acarbose | α-glucosidase | bacterial (Streptomyces, Actinoplanes) | numerous including phase 3 and 4 studies; e.g., NCT02865499, NCT02953093, and NCT01490918 | |
| anacardic acid | EP300 | cashew nutshell, Anacardium occidentale | – | |
| C646 | EP300 | synthetic | – | |
| CAEA | AMPK, sirtuins | synthetic | – | |
| CAPE | AMPK | propolis | clinical potential reviewed in Murtaza et al., 2014 | |
| CPI-613 | pyruvate dehydrogenase | synthetic | several phase 2 studies; e.g., NCT01835041, NCT03370159, and NCT01902381 | |
| garcinol | EP300 | Garcinia indica | – | |
| glucosamine | hexokinase and mTOR | crustaceans, cartilage | numerous studies on arthritis, reviewed in Ogata et al., 2018 ; e.g., NCT02448199 | |
| radicicol | ACLY and HSP90 | Monosporium bonorden | – | |
| SRT501 | SIRT1 | see resveratrol | reviewed in Berman et al., 2017 | |
| SB-204990 | ACLY | synthetic | – | |
| SRT2104 | SIRT1 | synthetic | several phase 1 studies; three phase 2 studies registered: NCT01018017, NCT01154101, and NCT01018017 |
Classification was based on whether compounds are known (1) to induce protein deacetylation that is causal for protective autophagy and to exert health-promoting effects in higher models (CRMs), (2) to promote protective autophagy and have molecular targets involved in protein deacetylation (potential CRMs), and (3) to exert protective effects without evidence for either autophagy induction or protein deacetylation (others). ACLY, ATP citrate lyase; AMPK, AMP-activated protein kinase; CAEA, caffeic acid ethanolamide; CAPE, caffeic acid phenyl ester; COX, cyclooxygenase; EGCG, epigallocatechin-3-gallate; EP300, E1A-binding protein p300; HCA, hydroxycitric acid; HSP90, heat shock protein 90; KATs, lysine acetyltransferases; KDACs, lysine deacetylases; mTOR, mechanistic target of rapamycin; NF-kB, nuclear factor “kappa-light-chain-enhancer” of activated B-cells; SIRT1, sirtuin (silent mating type information regulation 2 homolog) 1.
a If applicable, reviews and meta-analyses, or examples of advanced clinical studies, are depicted, indicating the current average phase of trials; clinicaltrials.gov identifiers, if not stated otherwise
Resveratrol and Other SIRT1 Activators
Resveratrol is a polyphenolic phytoalexin that is particularly abundant in the skin of grapes and in red wine. It has been shown to promote longevity across species and to improve age-related parameters in mice. However, resveratrol seems to only prolong the lifespan of mice on a high-fat diet (HFD) (
), but not on regular chow. Still, resveratrol exerts a number of protective effects in mammalian models of metabolic syndrome, type 2 diabetes (an effect that is enhanced when resveratrol is combined with metformin), cancer, neurodegeneration, and CVD (
). However, contrary findings have been reported recently on its efficacy against metabolic syndrome (
). Interestingly, resveratrol can counteract the reduction of duodenal SIRT1 levels in rats fed an HFD, which is accompanied by improved insulin sensitivity (
). This indicates the potential of resveratrol as an agent to counteract obesity- and diabetes-induced insulin resistance as well as dysregulated glucose homeostasis. Moreover, resveratrol induces a CR-like transcriptional signature in mice and recapitulates metabolic changes of CR in humans (
).
Several studies have examined resveratrol on primates, also showing SIRT1 induction, NF-κB repression, improved insulin signaling, and attenuated inflammation in adipose tissue of high-fat, high-sugar (HFS)-fed animals (
), coupled to reduced CVD risk parameters induced by HFS (
). A large number of clinical trials assessing its effects on cancer, diabetes, obesity, non-alcoholic fatty liver (NAFL), neurological disease, and CVDs have been performed with mostly beneficial outcomes.
Resveratrol targets a number of stress-related cellular components, including AMPK (
), which might represent a major molecular target, and the NAD+-dependent deacetylase SIRT1. Both AMPK and SIRT1 have been shown to be required for resveratrol-induced health promotion (
,
). Resveratrol can stimulate SIRT1 (possibly indirectly), resulting in general protein deacetylation and autophagy induction (
,
,
).
Although a bona fide CRM, resveratrol is afflicted by rather low systemic availability and absorption. One strategy to improve this galenic problem consists in micronization to decreased particle size, yielding the proprietary formulation SRT501.
Other small-molecule activators of SIRT1 have been developed. For instance, SRT1720 has been demonstrated to extend lifespan and improve metabolic syndrome, insulin sensitivity, and endothelial dysfunction in mice (
). A related compound, SRT2104, which also extends murine lifespan, has undergone clinical phase I and II trials, revealing only minor adverse effects (
). Both SIRT1 activators have been shown to improve healthspan in mice, reducing inflammation and protecting from neurodegeneration (
). According to one clinical study, SRT2104 can reduce the serum levels of interleukin-6 and C-reactive protein induced by intravenous injection of lipopolysaccharide (
). Additional data on SRT2104 effects on human health will likely be reported in the near future.
Spermidine
Spermidine is a polyamine that induces autophagy in different model organisms, including mice (
,
,
), and this induction is causal for at least some of the observed beneficial effects. For instance, genetic ablation of autophagy abrogates spermidine-mediated lifespan extension in yeast, nematodes, and flies and attenuates cardioprotective effects (
) in mice. Spermidine inhibits the activity of several acetyltransferases (
), including EP300, and this suffices for autophagy induction (
). Intriguingly, these pro-autophagic deacetylation effects are synergistic with those of resveratrol (
), which instead promotes the deacetylase activity of SIRT1 (see above). Moreover, spermidine has been shown to inhibit mTORC1 and activate AMPK (
). It has also been speculated that spermidine might post-translationally hypusinate the translation factor eIF5A, which leads to the synthesis of the pro-autophagy transcription factor TFEB, at least in immune cells (
). Moreover, spermidine can promote mitophagy (a specialized form of autophagy that eliminates damaged or dysfunctional mitochondria) in cell culture (
) and mice (
). In human cells, this depends on ataxia-telangiectasia mutated protein kinase (ATM) and consequently on the phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1) (
), which has been linked to the promotion of mitophagy (
).
Spermidine is naturally produced in the body by cellular biosynthesis as well as by the intestinal microbiota. In addition, oral ingestion of spermidine contained in food items like wheat germs, soybeans, or nuts among others, results in a good bioavailability (
). Besides its role in general cell homeostasis (e.g., stabilization of DNA and RNA, cell growth, and translation regulation), dietary supplementation of spermidine has been associated to manifold health-promoting effects (
). Spermidine feeding promotes lifespan across species, including mice, suppresses tumorigenesis, enhances anticancer immune response, stimulates memory T cell formation, promotes cardioprotection, improves skeletal muscle regeneration, and mediates neuroprotection (
), thus qualifying this polyamine as a CRM. Accordingly, increased levels of whole-blood spermidine are linked to longevity in healthy nonagenarians and centenarians (
). Furthermore, epidemiological studies correlate elevated dietary polyamine uptake with diminished cardiovascular and cancer-related mortality (
). That said, increased polyamine levels have also been associated with various human pathologies (
), although this might represent a (non-causal) protective response.
Clinical trials are needed to explore possible contraindications of spermidine administration. A trial on supplementation of spermidine-rich plant extracts to elderly is currently ongoing (www.clinicaltrials.gov identifier: NCT02755246) and suggests good tolerability and safety of the compound (
). Moreover, a small pilot trial has already revealed the beneficial effects of spermidine supplementation in elderly people with subjective cognitive decline (
).
Hydroxycitric Acid and Other AcCoA-Depleting Agents
Hydroxycitric acid (HCA) acts as a competitive low-affinity inhibitor of ATP citrate lyase (ACLY), which generates cytosolic AcCoA and thus represents an AcCoA-depleting CRM. HCA is present in diverse tropical plants, including Garcinia cambogia and Hibiscus sabdariffa. HCA salts have been shown to reduce body weight, insulin resistance, and oxidative stress in obese Zucker rats (
). In a mouse model of multiple sclerosis, a garcinia extract containing 50% HCA exerted anti-inflammatory and anti-oxidative effects (
). Furthermore, in mice, HCA improves the antitumor efficacy of immunogenic chemotherapy, which required regulatory depletion of T cells (which dampen anticancer immunity) from the tumor bed (
,
) and tumors to be autophagy-competent (
). Indeed, HCA promotes autophagic flux in diverse organs, including the liver, the myocardium, and skeletal muscle, and this induction is required for body weight reduction in mice (
). HCA is an over-the-counter weight-loss drug, and clinical trials have shown its effectivity in obese patients, although only at high doses (≥3 g per day). Nevertheless, several rodent studies showing adverse effects on the male reproductive system upon administration of HCA preparations have incited health concerns.
Another ACLY inhibitor, the synthetic SB-204990, also stimulates autophagy in mice (
) and mediates cholesterol and triglyceride reduction as well as tumor growth suppression in rodents (
). Possibly, the Cullin3-KLHL25 (Kelch-like family member 25) ubiquitin ligase is responsible for the degradation of ACLY and subsequent inhibition of lipid synthesis and tumor progression (
). Future evaluation of the lifespan- and healthspan-promoting effects of SB-204990 must determine its potential as a CRM. The ACLY and HSP90 inhibitor radicicol (first isolated from the fungus Monosporium bonorden) exhibits diverse protective effects in rodents, e.g., against renal and myocardial ischemia-reperfusion damage, but its pro-autophagic potential remains elusive (
).
Further synthetic agents capable of depleting AcCoA have been proposed as CRMs. Perhexiline maleate reduces AcCoA levels via inhibition of carnitine O-palmitoyl transferase 1 and is able to reversibly inhibit mTORC1 signaling and to promote autophagy in vitro (
). It is a clinically approved anti-anginal agent that exhibits cardioprotective (
) and anti-tumor potential (
), although putative hepato- and/or neurotoxic effects need to be explored. Furthermore, UK5099 (a mitochondrial-pyruvate-carrier inhibitor) and 1,2,3-benzenetricarboxylate (an inhibitor of citrate transport) cause AcCoA depletion, protein deacetylation, and autophagy (
). However, their in vivo effects need further investigation. Similarly, the impact of the pyruvate dehydrogenase inhibitor CPI-613 (a synthetic lipoate analog) on autophagy requires further investigation. CPI-613 has been shown to be tolerable in humans and to exhibit potential anti-tumor and chemotherapy-potentiating activity at pre-clinical and clinical levels (
), whereas a recent phase II trial on small cell lung carcinoma patients failed to show beneficial effects.
NAD+ Intermediates
NAD+ concentrations decrease with age in rodents and humans at the systemic level, correlating with the development of age-associated pathologies (
). Importantly, the overexpression of the NAD+-generating enzymes CYB5R3 and NQO1 is sufficient to increase murine life- and healthspan (
). Interestingly, supplementation of NAD+ precursors, in particular nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN), also exerts anti-aging effects (
). For instance, in rodents, NMN and NR have been shown to cause hepato- and cardioprotective effects (
); alleviate vascular aging (
); improve learning, memory, and cognitive function in Alzheimer disease models; promote muscle function in models of muscular dystrophy (
); and ameliorate diabetic pathophysiology (
), among other effects. In fact, NR could extend the lifespan of mice even when administered late in life (
). NMN and NR are contained in a variety of daily natural food sources, including different vegetables, fruits, meat, and shrimp as well as in human milk.
Nicotinamide (NAM, also called vitamin B3), another NAD+ precursor, can prevent aging-associated glaucoma in a mouse model (
). Chronic feeding with NAM on a low-fat diet or HFD fails to improve longevity but promotes the healthspan of aged mice (
). Specifically, glucose homeostasis, body fat percentage (on a low-fat diet), and locomotor activity (on HFD) were improved, while steatosis and inflammation were reduced (on HFD) (
).
Besides direct supplementation of NAD+ metabolites and precursors, beneficial elevation of NAD+ levels may also be achieved by interacting with its intracellular generation. A recent report shows that the pharmacological inhibition of the enzyme α-amino-β-carboxymuconate-ɛ-semialdehyde decarboxylase (ACMSD), which is a limiting step in de novo NAD+ synthesis in the kidney and the liver, might be another possible mode of action for potential CRM-like agents (
). The authors also identified two specific inhibitors that could be delivered via chow supplementation (
).
At the molecular level, NAD+ is required for SIRT1 deacetylase activity and is thus instrumental for protein-deacetylation-mediated autophagy induction in general. The NAD+/SIRT1 route reportedly upregulates autophagy via deacetylation of Atg5, Atg7, and Atg8 in murine neurons (
) and stimulates mitophagy (
). In addition, NAD+ has been suggested to induce autophagy via AMPK (
). Preclinical safety assessments in rodents have suggested no adverse response upon short- or long-term treatment with NR or NMN (
). In the first controlled clinical trial of NR, good bioavailability and increased blood levels of NAD+ could be detected (
). NAM has been shown in a large phase III trial to reduce the incidence of non-melanoma skin cancers (
). Interestingly, treatment with the nicotinic acid analog acipimox (a medication against hyperlipidemia) improved skeletal muscle mitochondrial function in type 2 diabetes patients (
). A number of clinical trials are currently underway to assess the efficacy of NAD+ precursors in humans, especially of NR.
Aspirin
The non-steroidal anti-inflammatory drug acetylsalicylic acid, better known as aspirin, has been in extensive medical use since 1899. Before that, salicylates from willow bark were widely used in folk medicine. Aspirin, which quickly metabolizes to salicylate in vivo, has lifespan-increasing effects on model organisms, including mice (
), but seems to inhibit growth in yeast (
,
Carmona-Gutierrez et al., 2018
,
).
Salicylate inhibits EP300 by competing with acetyl-CoA, thus activating autophagy (
). Aspirin also reduces mTOR signaling and activates AMPK and autophagy in colorectal cancer (CRC) cells (
), which might explain its anti-cancer efficacy. However, contradictory results were reported on cardiac fibroblasts, in which it inhibited autophagy (
). AMPK activation was also found in vivo in mice (
) and might represent a major mechanism of aspirin-triggered CRM effects. Recently, aspirin has been suggested as an anti-cancer therapeutic (
), as continuous intake is associated with lower tumor occurrence and decreased metastasis of CRC and breast and prostate cancer in humans (
). Mortality-reducing effects were found in several human studies after the long-term use of low doses (3–10 years; circa 75–300 mg/day). Several meta-analyses have suggested aspirin as a primary and secondary prevention therapy of CVD, reducing both the risk for CVDs and mortality. In line, aspirin provided heart protection and improved glucose tolerance in rodents (
). Additionally, mitophagy was induced in cardiomyocytes (
) and fat-induced insulin resistance improved in mice (
), though opposite effects in humans have been reported (
).
The positive effects of long-term aspirin intake might outweigh the risk of negative ones, e.g., gastrointestinal bleeding, if it became possible to exclude patients at risk. Overall, aspirin seems a reasonable and cost-efficient CRM with a high therapeutic potential against multiple diseases but a generally low risk for human health.
(Poly)phenols
In general, phenolic compounds may represent an attractive source of (potential) CRMs. Epidemiological studies have linked an elevated intake of polyphenol-rich food (mostly fruits and vegetables) and drinks (including coffee, tea, and wine) to a reduced incidence of malignant, cardiovascular, and neurodegenerative diseases (
). Caffeic acid (CA; found in the eucalyptus bark) and gallic acid (GA; found in black tea and many plants), including derivatives thereof, for instance, reportedly induce autophagy (
), AMPK activation (
,
), protein deacetylation (
), extended longevity across species, and anti-diabetes effects (
). While GA was found to inhibit EP300 (
), CA might activate SIRTs, namely SIRT3 (
). Moreover, CA has been shown to induce autophagy and improve glucose and lipid metabolism as well as renal function in a diabetic rat model (
). Notably, CA phenyl ester (CAPE), an AMPK activator, has broad health-promoting properties and is a major bioactive component of propolis, a honeybee product commonly used in traditional medicine. CAPE has been reported to extend the lifespan of a mouse model of amyotrophic lateral sclerosis (ALS) (
). Additionally, an ethanolamide derivative (CAEA) has been shown to activate AMPK as well as SIRTs and ameliorate cardiac damage in a mouse model (
).
Another example is the stilbenoid piceatannol (found, e.g., in passion fruit seeds), an analog of resveratrol, which—along with its metabolite isorhapontigenin—was shown to stimulate SIRT1, activate autophagy (synergistically with resveratrol), deacetylate cytosolic proteins, improve parameters of metabolic syndrome in obese mice, promote murine astrocyte differentiation in vivo, and extend the lifespan of worms (
,
). However, to our knowledge, lifespan and healthspan data of piceatannol in higher models are elusive.
Curcumin is the major polyphenol in the rhizome of turmeric (Curcuma longa) and has a long tradition as a medical herb. Indeed, curcumin feeding extends the lifespan of non-rodent models and exerts cardioprotective, antineoplastic, and antidiabetic effects in rodent models (
). Dietary curcumin is readily metabolized to tetrahydrocurcumin (THC), which was shown to prolong the lifespan of middle-aged mice (
). Numerous clinical trials have aimed at assessing the health effects of curcumin on humans, with positive effects reported for multiple diseases including different types of cancer, metabolic syndrome, depression, and diabetes (
). Curcumin seems well tolerated and non-toxic. Its poor bioavailability can be significantly increased by several agents, including piperine (
) (a major component in black pepper). The mode of action of curcumin remains to be clarified and—as with other polyphenols—may involve antioxidant properties but also autophagy induction, at least in some pathological settings. Its pro-autophagic activity has been connected to AMPK activation (
) and mTOR signaling (
) as well as to inhibition of EP300 (
).
A number of polyphenols are known to act as EP300 inhibitors, associated with autophagy induction and health-promoting effects. These include anacardic acid (AC) (
) (from the nutshell of the cashew, Anacardium occidentale) and garcinol (
) (from the fruit of the Kokum tree, Garcinia indica). Similarly, the synthetic EP300 inhibitor C646 induces autophagy (
) and exerts protective effects, including immunostimulatory antitumor activity (
). However, histone acetyl transferase (HAT) selectivity might be compromised at higher concentrations (
). Interestingly, a novel EP300 inhibitor (A-485) shows higher potency as well as HAT selectivity and may suppress tumor growth (
).
Several flavonoids, a multifunctional and highly bioactive polyphenolic subclass, which comprises more than 5,000 plant-derived substances, also promote autophagy coupled to protein deacetylation (
). For instance, quercetin (inter alia found in black tea, onions, rocket, cole, curcuma, and fruits) and the nutritionally less abundant myricetin (sources: black tea, cole, parsley, garlic, curcuma, and fruits), which only differ in the position of a hydroxy group, were shown to induce autophagy and reduce protein acetylation to the same extent (
). Both agents activate SIRT1 (
,
) and extend lifespan in worms (DAF-16 dependent) (
,
). They also promote the survival of neurodegenerative fly models (
,
), while only quercetin was shown to increase the lifespan of wild-type Drosophila (
). Quercetin supplementation in mice did not cause beneficial effects on longevity (though this was only tested in combination with polyphenolic taxifolin and pycnogenol) (
). However, in combination with the chemotherapeutic dasatinib, quercetin acted senolytically, thus removing senescent cells in vivo, and improved cardiac function of aged mice while overall promoting the healthspan (
). Of note, genetic ablation of senescent cells by means of an inducible suicide gene can extend rodent lifespan up to 25% (
). However, the contribution of senescent cells to aging progression—although well explored—remains to be fully understood and incorporated into an applicable model (
). In fact, the concept of senolytic drugs has only recently been developed, and more extensive mechanistic studies are needed to strengthen and elaborate the idea. For instance, the above-mentioned studies suggesting quercetin (in combination with other agents) to be senolytic but to fail in extending lifespan (
,
) may reflect a greater contribution of senolytic activity to healthspan than to lifespan-extension. However, differences in the agents used for combinations, application, dosage, timing, and mouse strains among other factors in these two studies underline that further analyses are warranted to evaluate possible pro-longevity effects of quercetin.
Furthermore, in vivo anti-cancer and anti-inflammatory effects as well as improvements of insulin sensitivity and HFD-induced weight gain have been described for quercetin (
,
). Only slow progress has been made in translating these results to clinical trials: ambiguous results of quercetin supplementation on inflammation were reported. Quercetin improved blood pressure in hypertension, obesity, and type 2 diabetes patients (
), also reducing plasma levels of oxidized low-density lipoprotein (
). Although safe for human application, quercetin bioavailability is low and metabolization is high (
), two features that might reduce its clinical utility.
The flavonoids (+)−catechin and its cis-form (−)−epicatechin can be prominently found in cocoa, tea brews, and red wine. Several animal studies and human trials have shown cardiovascular protective properties of catechins (
). Both catechins reduce cytosolic protein acetylation and induce autophagy (
), likely via pleiotropic targets. Epicatechin was suggested to act in a SIRT1-independent fashion (
), and its dietary supplementation in wild-type flies and obese diabetic mice reduced mortality in both species and improved markers of systemic inflammation and diabetes-associated liver and aorta degeneration in the latter (
). This is of special interest since epicatechin has been suggested as an insulin receptor activator by in silico analyses (
). Catechin also improves stress resistance and extends the lifespan of nematodes, independently of antioxidative properties (
). The bioavailability of epicatechin appears favorable (
) and numerous clinical trials have proven basic safety in patients. Epicatechin was shown to improve insulin resistance (
) and alter gene expression profiles, slightly downregulating inflammation- and adipogenesis-associated genes (
). Interestingly, epicatechin supplementation improved CVD markers and reduced triglyceride levels in patients with hypertriglyceridemia (
Gutiérrez-Salmeán et al., 2016
). However, many studies use chemically non-defined catechin-enriched (tea) extracts, rendering their evaluation problematic.
Epigallocatechin-3-gallate (EGCG) is the major polyphenol in green tea. It has numerous biological effects and pleiotropic molecular targets. Notably, it strongly inhibits HATs, activates SIRT1 (
), and extends lifespan in worms (
), flies (
), and rats (
). It induces autophagy in cell culture in a reportedly Ca2+/calmodulin-dependent protein kinase kinase beta (CaMKKβ)-dependent fashion (
) and inhibits EP300/CBP (
). However, the exact role of protein deacetylation in EGCG-mediated pro-autophagic effects remains unclear. Additionally, reduced glucose metabolism and increased fitness of flies (
) were reported. In rodents, EGCG improved liver and kidney function and reduced NF-κB signaling (
). EGCG may stimulate AMPK, probably via the activation of the upstream kinase Ca2+/calmodulin-dependent protein kinase kinase (CaMKK) (
). Several studies found promising effects of EGCG on the reduction of obesity and high-calorie-associated effects in rodents (
). A 2013 study, however, did not find changes in metabolism, body weight, or liver function in nutritionally supplemented obese women (
). EGCG has also been suggested as a potential anti-cancer therapeutic (
) and improves insulin sensitivity, glucose metabolism, and endothelial function in mice (
). Prominently, EGCG was intensively studied in the context of neurodegeneration (including in clinical trials), as its anti-aggregation properties hold great promise (
). Of note, a green tea extract (31.7 % EGCG, 8.5 % epicatechin) was neuroprotective and improved learning capacities in a progeroid mouse model when fed lifelong or from adulthood (
). In humans, EGCG bioavailability is rather poor, challenging the utility of its clinical use (
). Moreover, it has been reported that continuous intake of more than 800 mg per day may cause liver toxicity in patients (
). Finally, the alkaloid polyphenol berberine activates autophagy via SIRT1 and reduces protein acetylation (
), protecting liver function (
), exerting neuroprotective effects (
), and reducing cardiac damage (
) in rodents. Berberine was also shown to extend the lifespan of Drosophila (
). Though oral bioavailability of berberine is poor (
), several clinical studies have been or are being performed with berberine supplementation. Noteworthy, berberine showed hypoglycemic effects and improved insulin parameters in type 2 diabetes patients (
).
In general, polyphenols represent a chemical group with a great potential to find pharmacological alternatives to CR. For instance, a recent study screening for pro-longevity drugs identified the flavonoid 4,4′-dimethoxychalcone as a pro-autophagic natural compound (present in the plant Angelica keiskei, also known as Ashitaba). 4,4′-Dimethoxychalcone can extend lifespan from yeast to flies and shows cardioprotective effects in mice, all in an autophagy-dependent manner (
Carmona-Gutierrez et al., 2019
).
It is important to note that most polyphenols possess antioxidant properties and have pleiotropic effects on several molecular targets, rendering it difficult to study their precise mode of action. Moreover, modifications such as glycosylation are likely to change their bioactive features in vivo. For instance, glycosylation greatly modulates the effects of quercetin on the worm lifespan and probably alters its bioavailability (
).
Metformin
Metformin (dimethylbiguanide hydrochloride) is a derivative of natural guanidines present in the French lilac (Galega officinalis), a plant that has been used in folk medicine for centuries. Originally described as a hypoglycemic and antimalarial drug, it is currently a widely prescribed agent in the treatment of type 2 diabetes. Interestingly, metformin administration extends the lifespan in different animal models, including mammals (
). In humans, metformin seems to be beneficial against a number of age-related diseases, including cancer and metabolic syndrome as well as cognitive and cardiovascular disorders (
,
). Indeed, a recent meta-analysis of diabetics on metformin use has revealed that this drug reduces all-cause mortality and age-associated diseases (
). This geroprotective potential paired with its little side effects has propelled the start of several clinical trials. Metformin recapitulates important metabolic effects of CR (
) and stimulates protective autophagy, for instance in mouse models of obesity and cardiac dysfunction (
,
).
Mechanistically, metformin has been associated with the activation of the master energy sensor AMPK (
) via the inhibition of the mitochondrial electron transport chain complex I (
), although it might activate AMPK through a lysosomal pathway, as well (
). In addition, metformin also inhibits mTORC1 independently of AMPK (
). Whether metformin effects rely on protein hypoacetylation remains to be systematically evaluated. In fact, metformin-mediated AMPK activation has been associated with both reduced EP300 and CREB-binding protein (
,
) and increased HAT (HAT1) activity (
). Similarly, metformin can inhibit class II HDACs (
) but can also stimulate class III HDAC SIRT1 activity, possibly downstream of AMPK activation (
). Metformin may also impact SIRT1 gene expression directly. Altogether the current data suggest that metformin could qualify as a bona fide CRM if causal effects on protein hypoacetylation were validated.
Rapamycin and Related Compounds (Rapalogs)
Rapamycin (sirolimus) is a macrolide compound produced by Streptomyces hygroscopicus. It was originally used as an anti-fungal drug and is an FDA-approved immunosuppressant that has been shown to extend lifespan in C. elegans, D. Melanogaster, and mice (
). Rapamycin has also been connected, for example, to cardioprotection (
), anti-neurodegenerative effects (
), and obesity prevention (
) in rodents. Numerous clinical trials have addressed the efficacy of rapamycin and rapamycin analogs (rapalogs) in treating diseases, including cancers. Rapamycin can inhibit mTORC1 by forming a complex with the protein FKBP12 (
). As a specific mTORC1 inhibitor, rapamycin promotes autophagy independently of SIRT1 (
) but links to possible autophagy-relevant deacetylation processes have been documented (
). Whether such deacetylation processes contribute to the beneficial effects of rapamycin will clarify if it fully qualifies as a CRM. However, its immunosuppressant properties compromise the broad clinical application. In addition, prolonged rapamycin treatment exacerbates insulin resistance and diabetes (
) and actually reduces the lifespan of diabetic mice (
). Whether intermittent administration of rapamycin might circumvent these adverse effects needs further investigation.
Rapamycin belongs to the so-called first generation mTOR inhibitors, which also comprise the rapalogs temsirolimus and everolimus, both of which also bind FKB12 but show improved pharmacokinetics (
). While the first generation rapalogs and rapamycin only block mTORC1, the second generation of mTOR inhibitors (NVP-BEZ235, PF-04691502, OSI-027, and others) acts by blocking the ATP site of the mTOR kinase, thus also affecting TORC2 (
). A series of clinical trials for specific medical applications have been conducted or registered for some of these drugs, but further evaluation is required to assess whether they also possess health- and/or lifespan-extending features. Finally, the third generation of mTOR inhibitors includes bivalent drugs that target multiple molecular targets in the TOR complexes (e.g., mTOR kinase, FRB domain, and FKBP12), providing enhanced effectivity against, for instance, tumorous cells (
,
). One recent example of this category is RapaLink-1, which is a specific TOR kinase inhibitor linked to rapamycin (
). This generation, however, has not surfaced to clinical studies yet.
Modulation of Glucose Metabolism
Blocking cellular energy utilization, specifically glycolysis, has been devised as a CRM strategy. Glucose deprivation alone is sufficient to induce autophagy via AMPK/mTOR (
). The hexokinase inhibitor glucosamine is a widely used agent to prevent and treat osteoarthritis and shows no relevant side effects. In fact, glucosamine medication has been associated with decreased mortality in humans (
). Autophagy activation by glucosamine was proposed to be both mTOR dependent (
) and independent (
), meaning that the exact pathway is still debated. Whether protein deacetylation is involved in glucosamine-mediated autophagy induction remains to be studied. Instead, 2-deoxyglucose (a synthetic hexokinase 2 inhibitor) increases nematode lifespan but seems to be cardiotoxic and to increase mortality in rats (
). In line with the latter results, 2-deoxyglucose treatment suppresses autophagy via activation of mTORC1 (
) and does not represent a CRM. Acarbose is an α-glucosidase inhibitor of bacterial origin (Streptomyces and Actinoplanes species) and widely used as an anti-diabetic medication, preventing the release of glucose from more complex carbohydrates (
). Although it might be connected to further lifespan-determining effects (
), additional studies that address its possible influence on autophagy and protein deacetylation are needed.
Conclusion
Ongoing and future clinical trials, as well as meta-analyses, will ultimately determine the actual beneficial impact of each CRM on human health (Figure 3). Further CRMs may be discovered and optimized versions of known CRMs obtained by medicinal chemistry that will need to be further evaluated. Besides, it will be important to tackle certain limitations (e.g., bioavailability) and unfold possibilities, including the prospect for combinatorial approaches. Many CRMs fail to extend lifespan to the same degree as CR or fasting does and some CRMs show sex-specific differences (Table 2), suggesting that CR might cumulatively ignite distinct pathways that are only partly targeted by single CRMs. This propels the idea of achieving additive effects by compound/treatment combinations. This applies to (1) combinations of distinct CRMs, (2) combinations of CRMs with other beneficial non-CRM compounds, and (3) combinations of CRMs with behavioral/nutritional approaches (e.g., fasting, CR, and exercise). With respect to (1), it can be expected that CRMs that act on distinct routes to achieve protein deacetylation (namely AcCoA depletion, acetyltransferase inhibition, or deacetylase activation) could interact in a synergistic fashion. For example, resveratrol (which promotes SIRT deacetylase activity) synergizes with spermidine (which is an acetyltransferase inhibitor) to promote autophagy in vitro (human cell culture) and in vivo (mice) (
). Moreover, rapamycin and metformin act synergistically on worm lifespan (
). These studies exemplify the substantial potential of such combinatorial approaches in the anti-aging field. Regarding (2), several health-promoting compounds including antioxidant and hormesis mimetics do not rely on the deacetylation-autophagy axis. It will be interesting to investigate whether they might be favorably combined with CRMs. Finally, (3) exercise, CR, fasting, and CRMs all promote autophagy, and additive or synergistic effects might be attained upon combining these interventions. For instance, several studies suggest that exercise might be combined with the CRM resveratrol (
).
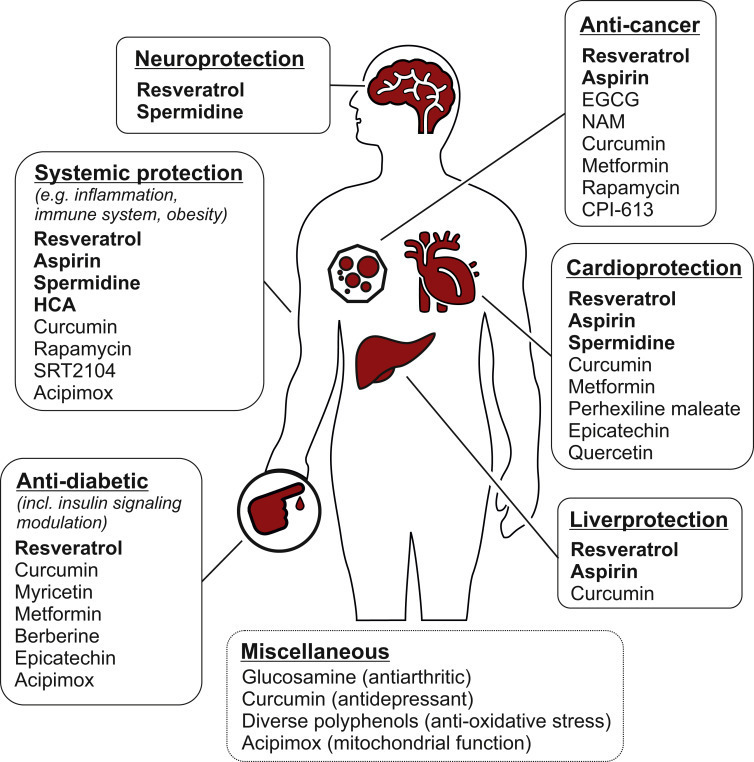
Figure 3Possible Physiological Effects of CRMs Seen in Clinical Studies
Table 2Effects of CRMs, Potential CRMs, and Other Compounds on Rodent Lifespan
GroupSubstanceOrganismLifespan ExtensionApplication SchemeReferences
| CRMs | aspirin (and salicylate) | mouse (male UM-HET3; failed in females) | ∼8% median lifespan increase | starting at 4 months; supplemented via Purina 5LG6 diet | Strong et al., 2008 |
| resveratrol | mouse (male C57BL/6NIA) | 31% reduced risk of death | starting at 12 months; supplemented via diet; on high-calorie diet | Baur et al., 2006 | |
| spermidine | mouse (male and female C57BL/6J) | ∼12% or ∼10% median lifespan increases for lifelong or late-in-life supplementation, respectively | starting at 4 or 18 months; supplemented via drinking water; standard diet | Eisenberg et al., 2016 | |
| Potential CRMs | curcumin (or tetrahydrocurcumin) | mouse (male C57BL/6) | 11.7% mean lifespan increase | starting at 13 months; supplemented via standard diet | Kitani et al., 2007 |
| epicatechin | mouse (db/db obese, diabetic model) | reduced mortality (8.4% in treated versus 50% in control group after 15 weeks of treatment) | starting at 5 weeks; supplemented via drinking water | Si et al., 2011 | |
| EGCG | rat (male Wistar) | ∼13.5% median lifespan increase | starting at 5 weeks; supplemented via drinking water | Niu et al., 2013 | |
| metformin | mouse (male C57BL/6 and B6C3F1) | 5.83% (C57BL/6) and 4.15% (B6C3F1) mean lifespan increase for low dose (0.1%); high dose (1%) led to 14.4% mean lifespan reduction | starting at 54 weeks; supplemented via standard diet | Martin-Montalvo et al., 2013 | |
| NR | mouse (C57BL/6J) | ∼5% mean lifespan increase | starting at 22–24 months; via diet; for 6 weeks; standard diet | Zhang et al., 2016c | |
| rapamycin | mouse (male and female C57BL/6, UM-HET3, 129/sv) | median (∼25%) and maximum lifespan increase (Miller et al., 2014 ); 10% median lifespan increase (Anisimov et al., 2011 ); median lifespan increase of 10% (males) and 18% (females) (Miller et al., 2011 ); mean lifespan increase of 9% (male) and 13% (female) (Harrison et al., 2009 ) | many different application methods and starting timepoints of intervention reported | reviewed in Ehninger et al., 2014 | |
| SRT1720 | mouse (male C57BL/6J) | 11% and 44% mean lifespan increase for low and high doses | starting at 12 months; supplemented via diet; on high-fat diet | Minor et al., 2011 | |
| Others | CAPE | mouse (male and female SOD1G93A ALS model with B6SJL background) | ∼7% lifespan increase | single daily oral dose after disease onset | Fontanilla et al., 2012 |
| SRT2104 | mouse (male C57BL/6J) | 9.7% mean lifespan increase | starting at 6 months; supplemented via diet; on standard diet | Mercken et al., 2014 |
Lifespan experiments that have been performed in rodents with compounds listed in Table 1. Animal specificities (including sex), quantitation of lifespan improvement, experimental design for compound administration, and corresponding references are noted. CAPE, caffeic acid phenyl ester; EGCG, epigallocatechin-3-gallate; NR, nicotinamide riboside.
Another important aspect is the timing of CRM application. First, the effective administration of CRMs in a middle-life stage (instead of lifelong application), before adverse age-associated symptoms manifest, would greatly enhance the therapeutic feasibility of CRMs for humans. Thus, exploring whether specific CRMs can be effective also upon administration late in life will help clarify the extended potential of these drugs. Second, the application of CRMs in a rhythmic fashion could reduce the drug load while promoting the same effects. This follows the idea that in humans, rhythmic variations of calorie intake (e.g., intermittent fasting) stimulate many of the favorable effects that constant CR does (
). In fact, in animal and human studies, the timing of meals regarding the circadian clock is of utmost importance (
). Third, the target availability for different CRMs might be optimized by timing the administration of a given CRM with the expression patterns of its corresponding target(s). In fact, if a specific cellular target is not expressed at relevant levels, a drug dose at this time point might be ineffective. Mechanistic data on cellular targets of specific CRMs are already available and solid. The experiments exploring “target expression-enhanced” administration might be especially interesting regarding variable expression profiles in different tissue types as well as with ongoing age and at a particular disease status.
Irrespective of the pending therapeutic validation of CRMs as a stand-alone and/or combinatory approach and other open questions, the available data substantiate the large potential of pharmacological autophagy induction as a feasible and effective strategy against multiple diseases.
Acknowledgments
F.M. is grateful to the Austrian Science Fund FWF (Austria) for grants P23490-B20 , P29262 , P24381 , P29203 P27893 , I1000 , and “ SFB Lipotox ” ( F3012 ), as well as to Bundesministerium für Wissenschaft, Forschung und Wirtschaft , and the Karl-Franzens University for grant “Unkonventionelle Forschung” and grant DKplus Metabolic and Cardiovascular Diseases ( W1226 ). We acknowledge support from NAWI Graz and the BioTechMed-Graz flagship project “EPIAge.” G.K. is supported by the Ligue contre le Cancer Comité de Charente-Maritime (équipe labelisée); Agence National de la Recherche (ANR)–Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France ; Institut National du Cancer (INCa); Institut Universitaire de France ; Fondation pour la Recherche Médicale (FRM); the European Commission (ArtForce); the European Research Council (ERC); the LeDucq Foundation ; the LabEx Immuno-Oncology ; the Recherche Hospitalo-Universitaire Torino Lumière , the Site de Recherche Intégrée sur le Cancer (SIRIC) Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI).
Author Contributions
F.M., D.C.-G., S.J.H., and G.K. contributed to the conceptualization, figure design, and writing of the manuscript.
Declaration of Interests
G.K. is inventor on a patent application (WO2015049365 A3) submitted by INSERM (Institut National de la Santé et de la Recherche Médicale), Assistance Publique-Hôpitaux De Paris (APHP), Université Paris Descartes, Université Pierre et Marie Curie (Paris 6), Université Paris Diderot–Paris 7, Université Paris–Sud, Institut Gustave Roussy, that covers the medical use of CRMs. F.M. and D.C.-G. have equity interests in TLL (The Longevity Labs), a company founded in 2016 that will develop natural food extracts. F.M., D.C.-G., and G.K. are the scientific co-founders of Samsara Therapeutics.
References
- Abbas S.
- Wink M.
- Admasu T.D.
- Batchu K.C.
- Barardo D.
- Ng L.F.
- Lam V.Y.M.
- Xiao L.
- Cazenave-Gassiot A.
- Wenk M.R.
- Tolwinski N.S.
- Gruber J.
- Dev. Cell. 2018; 47: 67-79.e5
- Alistar A.
- Morris B.B.
- Desnoyer R.
- Klepin H.D.
- Hosseinzadeh K.
- Clark C.
- Cameron A.
- Leyendecker J.
- D’Agostino R.
- Topaloglu U.
- et al.
- Lancet Oncol. 2017; 18: 770-778
- Anisimov V.N.
- Zabezhinski M.A.
- Popovich I.G.
- Piskunova T.S.
- Semenchenko A.V.
- Tyndyk M.L.
- Yurova M.N.
- Rosenfeld S.V.
- Blagosklonny M.V.
- Cell Cycle. 2011; 10: 4230-4236
- Aprotosoaie A.C.
- Miron A.
- Trifan A.
- Luca V.S.
- Costache I.I.
- Diseases. 2016; 4: 39
- Ara G.
- Afzal M.
- Jyoti S.
- Siddique Y.H.
- Bull. Fac. Pharm. Cairo Univ. 2017; 55: 259-262
- Ard J.D.
- Gower B.
- Hunter G.
- Ritchie C.S.
- Roth D.L.
- Goss A.
- Wingo B.C.
- Bodner E.V.
- Brown C.J.
- Bryan D.
- et al.
- J. Gerontol. A. Biol. Sci. Med. Sci. 2017; 73: 73-80
- Asghar M.
- Monjok E.
- Kouamou G.
- Ohia S.E.
- Bagchi D.
- Lokhandwala M.F.
- Mol. Cell. Biochem. 2007; 304: 93-99
- Baker D.J.
- Childs B.G.
- Durik M.
- Wijers M.E.
- Sieben C.J.
- Zhong J.
- Saltness R.A.
- Jeganathan K.B.
- Verzosa G.C.
- Pezeshki A.
- et al.
- Nature. 2016; 530: 184-189