
음식 알레르기(food allergy)
음식 과민증(food intolerance)
두가지는 반드시 구분되어야 한다.
참 어려운 영역의 탐구!!

https://www.youtube.com/watch?v=45qMEOAkpug
Nutrients. 2021 Jul; 13(7): 2228.
Published online 2021 Jun 29. doi: 10.3390/nu13072228
PMCID: PMC8308327
PMID: 34209583
Histamine Intolerance—The More We Know the Less We Know. A Review
Martin Hrubisko,1,2,† Radoslav Danis,3,*† Martin Huorka,4 and Martin Wawruch3
Mariluz Latorre-Moratalla, Academic Editor, Oriol Comas-Basté, Academic Editor, and M. Carmen Vidal-Carou, Academic Editor
Author information Article notes Copyright and License information PMC Disclaimer
Abstract
The intake of food may be an initiator of adverse reactions. Food intolerance is an abnormal non-immunological response of the organism to the ingestion of food or its components in a dosage normally tolerated. Despite the fact that food intolerance is spread throughout the world, its diagnosing is still difficult. Histamine intolerance (HIT) is the term for that type of food intolerance which includes a set of undesirable reactions as a result of accumulated or ingested histamine. Manifestations may be caused by various pathophysiological mechanisms or a combination of them.
The problem with a “diagnosis” of HIT is precisely the inconstancy and variety of the manifestations in the same individual following similar stimuli. The diagnosing of HIT therefore requires a complex time-demanding multidisciplinary approach, including the systematic elimination of disorders with a similar manifestation of symptoms. Among therapeutic approaches, the gold standard is a low-histamine diet. A good response to such a diet is considered to be confirmation of HIT. Alongside the dietary measures, DAO supplementation supporting the degradation of ingested histamine may be considered as subsidiary treatment for individuals with intestinal DAO deficiency. If antihistamines are indicated, the treatment should be conscious and time-limited, while 2nd or 3rd generation of H1 antihistamines should take precedence.
음식 섭취는
부작용을 유발할 수 있습니다.
식품 과민증(food intolerance)은 일반적으로
허용되는 용량의 식품 또는
그 성분 섭취에 대한 유기체의 비정상적인 비면역학적 반응 abnormal non-immunological response 입니다.
식품 과민증이
전 세계에 퍼져 있음에도 불구하고
진단은 여전히 어렵습니다.
히스타민 과민증(HIT)은
히스타민 축적 또는 섭취로 인한
일련의 바람직하지 않은 반응을 포함하는 이러한 유형의 식품 과민증을 일컫는 용어입니다.
증상은 다양한 병리 생리학적 메커니즘 또는 이들의 조합에 의해 발생할 수 있습니다. HIT의 '진단'의 문제점은 유사한 자극을 받은 후 같은 개인에게서 나타나는 증상의 불일치와 다양성입니다.
따라서
HIT를 진단하려면
유사한 증상을 보이는 장애를 체계적으로 제거하는 등
복잡하고 시간이 많이 걸리는 다학제적 접근 방식이 필요합니다.
치료 접근법 중 표준은
저히스타민 식이요법입니다.
이러한 식단에 대한 좋은 반응은 HIT의 확인으로 간주됩니다.
식이 요법과 함께
섭취한 히스타민의 분해를 돕는 DAO 보충제를
장내 DAO 결핍증 환자의 보조 치료로 고려할 수 있습니다.
https://cafe.daum.net/panicbird/S3Cq/372
항히스타민제 치료가 필요한 경우,
2세대 또는 3세대 H1 항히스타민제가 우선적으로 사용되어야 하며,
의식적이고 시간 제한적인 치료가 이루어져야 합니다.
Keywords: histamine intolerance, histamine, diamine oxidase, DAO, low-histamine diet, probiotics, food intolerance
1. Introduction
The intake of food may be an initiator of adverse reactions. We are referring to any kind of abnormal reaction related to the intake of foods. Adverse food reactions are nowadays rather accepted in practice but are however less frequently objectively examined [1]. In addition to specific and well-differentiated disorders, allergic reactions and food aversions, we also class food intolerance among them. Distinguishing food intolerance from an allergic reaction to food is possible on the basis of key pathophysiological differences through the use of relevant diagnostic approaches. Food allergy is an inadequate response of the immune system to an antigen (in the majority of cases of a protein nature) ingested in food, that is accompanied by IgE or non-IgE (cellular) immunological mechanisms. Its cumulative prevalence is 3–6%, appearing much more frequently in children [2]. Testing for foods IgG or IgA antibodies is not of fundamental clinical importance [3,4]. Levels of these antibodies may rather reflect an intestinal permeability disorder regardless of its origin, which is often post-infectious [5].
음식 섭취는
부작용을 유발할 수 있습니다.
음식 이상 반응이란
식품 섭취와 관련된 모든 종류의 비정상적인 반응을 말합니다.
오늘날에는 식품 이상 반응이 현실적으로 받아들여지고 있지만 객관적으로 조사되는 빈도는 낮습니다[1].
구체적이고 잘 구분되는 장애,
알레르기 반응 및
식품 혐오증 외에도
식품 과민증도 그중 하나로 분류합니다.
식품 과민증과
식품 알레르기 반응을 구별하는 것은
관련 진단 접근법을 사용하여
주요 병태생리학적 차이에 근거하여 가능합니다.
음식 알레르기는
음식에서 섭취한 항원(대부분의 경우 단백질 성분)에 대한 면역 체계의 부적절한 반응으로,
IgE 또는 비 IgE (세포) 면역학적 메커니즘 IgE or non-IgE (cellular) immunological mechanisms 을 수반합니다.
누적 유병률은 3~6%이며,
어린이에게 훨씬 더 자주 나타납니다[2].
식품 IgG 또는 IgA 항체 검사는 근본적으로 임상적으로 중요하지 않습니다[3,4].
이러한 항체의 수치는
오히려 그 기원에 관계없이 장 투과성 장애를 반영할 수 있으며,
이는 감염 후 발생하는 경우가 많습니다[5].

One specific form of an adverse food reaction with an immunopathological feature is celiac disease, in which genetic disposition and epigenetic influences lead to adverse immunity reactions to gluten. Food intolerance is an abnormal non-immunological response of the organism to the ingestion of food or its components in a dosage normally tolerated [6]. It is at the same time a simplified term for non-allergic food hypersensitivity according to the World Health Organization (WHO) [7]. Food hypersensitivity belongs among the most frequently occurring undesirable reactions to food. It affects from 15–20% of the population and can be the result of the pharmacological effects of food ingredients, non-celiac gluten sensitivity or malfunction of enzyme(s) or transport [8].
Despite the fact that food intolerance is spread throughout the world, its diagnosing is difficult and demanding. For example, in patients with suspected histamine intolerance (HIT) it is necessary to carefully consider other possible reasons for the manifestations of symptoms (Table 1) [1]. Food intolerance (and especially HIT) requires a comprehensive understanding of the symptoms, especially their diversity, severity and time of onset [6].
면역 병리학적인 특징을 가진 음식물 부작용의 한 가지 특정 형태는
유전적 성향과 후성유전학적 영향이
글루텐에 대한 면역 반응을 일으키는 셀리악병으로,
셀리악병은 유전적 성향과 후성유전학적 영향이
글루텐에 불리한 면역 반응을 유발합니다.
식품 불내성(식품 과민증, Food intolerance)은
일반적으로 허용되는 용량으로
음식이나 그 성분을 섭취하는 것에 대한
유기체의 비정상적인 비면역학적 반응 abnormal non-immunological response 입니다 [6].
동시에 세계보건기구(WHO)에 따르면
비알레르기성 식품 과민증에 대한 단순화된 용어이기도 합니다[7].
식품 과민증은
음식에 대해 가장 빈번하게 발생하는 바람직하지 않은 반응 중 하나입니다.
식품 과민증은
인구의 15~20%에서 발생하며
식품 성분의 약리학적인 영향,
nonceliac 글루텐 민감성 또는
효소 또는 수송 기능 장애의 결과일 수 있습니다 [8].
식품 불내증(food intolerance)이 전 세계에 퍼져 있음에도 불구하고
진단은 어렵고 까다롭습니다.
예를 들어,
히스타민 과민증(HIT)이 의심되는 환자의 경우
증상 발현에 대한 다른 가능한 이유를 신중하게 고려해야 합니다(표 1)[1].
음식 과민증(특히 HIT)은
증상, 특히 그 다양성, 심각성 및 발병 시기에 대한 포괄적인 이해가 필요합니다[6].
Table 1
Symptoms and differential diagnostics in patients with suspected adverse reactions to ingested histamine.
Adapted according to Reese et al. 2017 [1].
SymptomsDifferential Diagnosis
| Flushing | Neuroendocrine tumors |
| Itching | Urticaria, pruritus sine materia, prurigo |
| Nausea/vomiting/abdominal pain | Peptic ulcer disease, hiatal hernia, gastroesophageal reflux disease |
| Diarrhea and abdominal pain | Chronic inflammatory bowel disorders, disorders of carbohydrate metabolism |
| (lactose intolerance, fructose malabsorption), celiac disease | |
| Rhinitis | Allergic and non-allergic rhinitis of other origin |
| Dyspnea, dysphonia | Allergic and non-allergic asthma |
| Hypotension, vertigo, tachycardia | Anaphylaxis |
| Important differential diagnostic information is achieved by the analysis of symptoms with respect to their onset time. Adverse food reactions only considered if the symptoms manifested in less than 4 h from food intake. | |
In this review, we provide a critical overview on possible benefits of published diagnostic approaches. We present the current knowledge of the therapeutic options and suggest the management of HIT accordingly. Another strength of this review lies in its comprehensiveness of tables summarizing data of clinical importance.
1.1. Histamine Intolerance (HIT)
HIT is the term for that type of food intolerance which includes a set of undesirable reactions as a result of accumulated or ingested histamine. In the German guideline from 2017, German and Swiss specialists prefer the term “adverse reactions to ingested histamine” [1]. In older publications, this type of intolerance is designated by the expressions pseudoallergy, enteral histaminosis or histamine sensitivity. HIT is defined as a condition caused by an imbalance between the histamine released from food and the ability of the organism to degrade such an amount. It is accompanied by decreased activity of the DAO enzyme, leading to an increased concentration of histamine in plasma and the emergence of adverse reactions. In some publications, the state of decreased activity of DAO is referred to as a DAO deficiency. DAO deficiency predisposes a certain subgroup of the population to HIT. It can be of genetic, pathologic or pharmacological origin [9].
히스타민 과민증 HIT는
히스타민 축적 또는 섭취로 인한
일련의 바람직하지 않은 반응을 포함하는 이러한 유형의 식품 과민증을 일컫는 용어입니다.
2017년의 독일 가이드라인에서 독일과 스위스 전문가들은 "섭취한 히스타민에 대한 부작용"이라는 용어를 선호합니다[1]. 이전 간행물에서 이러한 유형의 과민증은 가성 알레르기, 장내 히스타민증 또는 히스타민 민감성이라는 표현으로 지정되었습니다. HIT는 음식에서 방출되는 히스타민과 그 양을 분해하는 유기체의 능력 사이의 불균형으로 인해 발생하는 상태로 정의됩니다.
이는
DAO 효소의 활성 감소를 동반하여
혈장 내 히스타민 농도를 증가시키고
부작용을 유발합니다.
일부 간행물에서는
DAO의 활동이 감소한 상태를 DAO 결핍이라고 합니다.
DAO 결핍은
인구의 특정 하위 그룹을 HIT에 취약하게 만듭니다.
유전적, 병리학적 또는 약리학적 기원 일 수 있습니다 [9].
It is necessary to distinguish HIT from histamine intoxication designated as scombroid syndrome, scombroidosis or histamine poisoning. The term originates from the name of the mackerel fish family (Scombridae), after the consumption of which the intoxication was most often observed. The Scombridae family includes tuna, herring and mackerel. Histamine poisoning is considered worldwide as one of the most frequent intoxications caused by the consuming of fish (Dalgaard, 2008 in [10]). According to Colombo et al., from 103 analysed samples which caused histamine poisoning, 101 showed fish or seafood sources, and only two contained cheese [10]. Manifestations of histamine intoxication may include rash, abdominal pain, vomiting, diarrhoea and shortness of breath, and the intoxication may also have a fatal outcome [11].
The term HIT is used in a similar manner as the concept of lactose intolerance (which occurs due to a lack of the lactase enzyme), since it is presumed that the HIT symptoms are related to a lack or diminished activity of the enzyme DAO. Ingested exogenous histamine is distributed into the blood stream and may trigger symptoms in the susceptible population. It should be stated that with HIT, the amount of histamine taken in is much lower than with histamine poisoning. HIT manifestations also have a milder course in comparison with intoxication [10].
스콤브로이드 증후군,
스콤브로이드증 또는
히스타민 중독으로 지정된 히스타민 중독과 HIT를 구별 할 필요가 있습니다. 이 용어는 중독이 가장 자주 관찰 된 소비 후 고등어 어류과 (Scombridae)의 이름에서 유래되었습니다.

Histamine fish poisoning, also known as scombroid poisoning, is the most common cause of ichythyotoxicosis worldwide and results from the ingestion of histamine-contaminated fish in the Scombroidae and Scomberesocidae families, including mackerel, bonito, albacore, and skipjack. This disease was first described in 1799 in Britain and re-emerged in the medical literature in the 1950s when outbreaks were reported in Japan. The symptoms associated with histamine fish poisoning are similar to that of an allergic reaction. In fact, such histamine-induced reactions are often misdiagnosed as IgE-mediated fish allergy. Indeed, histamine fish poisoning is still an underrecognized disease. In this review, we discuss the epidemiology, pathophysiology, evaluation, and treatment of scombroid disease. Because more than 80 % of fish consumed in the USA is now imported from other countries, the disease is intimately linked with the global fish trade (National Marine Fisheries Service, 2012). Preventing future scombroid outbreaks will require that fishermen, public health officials, restaurant workers, and medical professionals work together to devise international safety standards and increase awareness of the disease. The implications of scombroid poisoning go far beyond that of fish and have broader implications for the important issues of food safety.
히스타민 어류 중독은 스콤브로이드 중독으로도 알려져 있으며, 전 세계적으로 가장 흔한 아이키토독증의 원인으로 고등어, 가다랑어, 알바코어, 가다랑어 등 히스타민에 오염된 스콤브로이드과 및 스콤베레소키과 생선을 섭취할 때 발생합니다. 이 질병은 1799년 영국에서 처음 기술되었으며, 1950년대 일본에서 발병이 보고되면서 의학 문헌에 다시 등장했습니다. 히스타민 생선 중독과 관련된 증상은 알레르기 반응과 유사합니다. 실제로 이러한 히스타민 유발 반응은 종종 IgE 매개 생선 알레르기로 잘못 진단되기도 합니다. 실제로 히스타민 생선 중독은 여전히 잘 알려지지 않은 질병입니다. 이 리뷰에서는 스콤브로이드 질환의 역학, 병태생리, 평가 및 치료에 대해 설명합니다. 현재 미국에서 소비되는 어류의 80% 이상이 다른 나라에서 수입되기 때문에 이 질병은 전 세계 어류 무역과 밀접한 관련이 있습니다(국립해양수산청, 2012). 향후 스콤브로이드 발생을 예방하기 위해서는 어부, 공중 보건 관계자, 식당 종사자, 의료 전문가가 협력하여 국제 안전 기준을 마련하고 이 질병에 대한 인식을 높여야 합니다. 스쿠브로이드 중독의 영향은 어류의 문제를 훨씬 넘어 식품 안전이라는 중요한 문제에 더 광범위한 영향을 미칩니다.
고등어과에는
참치, 청어, 고등어가 포함됩니다.
히스타민 중독은
전 세계적으로 생선 섭취로 인한
가장 빈번한 중독 중 하나로 간주됩니다
(Dalgaard, 2008 년 [10]). 에 따르면
히스타민 중독을 일으킨 103개의 분석 샘플 중
101개가 생선 또는 해산물로 나타났으며,
단 2개만 치즈가 포함되어 있었습니다[10].
히스타민 중독의 증상으로는
발진, 복통, 구토, 설사, 호흡 곤란 등이 있으며,
중독으로 인해 치명적인 결과를 초래할 수도 있습니다 [11].
HIT라는 용어는
락타아제 효소 부족으로 인해 발생하는 유당 불내증의 개념과 유사한 방식으로 사용되는데,
이는 HIT 증상이
DAO 효소의 부족 또는 활동 감소와 관련이 있다고 추정되기 때문입니다.
섭취한 외인성 히스타민은
혈류로 분포되어 감수성이 있는 사람들에게
증상을 유발할 수 있습니다.
HIT의 경우
히스타민 중독보다
섭취되는 히스타민의 양이 훨씬 적다는 점을 명시해야 합니다.
HIT 증상은 또한 중독에 비해 경미한 경과를 보입니다 [10].
With intolerances, gender-specific variations generally apply; women are affected with intolerances more frequently than men, although this distinction is not satisfactorily explained [12]. Increased sensitivity to the intake of histamine was observed in women in the premenstrual phase [13]. Serum diamine oxidase (DAO) levels in premenopausal women appear to be associated with the menstrual cycle, with higher DAO activity measured during the luteal phase compared to the follicular phase [14]. Painful menstruation may be associated with increased sensitivity to histamine. Administration of H1 antihistamines on the first day of menstruation has had a preventive effect on dysmenorrhea. High levels of histamine metabolites in urine during the ovulatory phase could be related to the effect of oestrogens (especially oestradiol) [15].
과민증의 경우
일반적으로 성별에 따른 차이가 적용되며,
여성이 남성보다 과민증의 영향을 더 자주 받지만
이러한 구분이 만족스럽게 설명되지는 않습니다 [12].
월경 전 단계의 여성에서
히스타민 섭취에 대한 민감도 증가가 관찰되었습니다 [13].
폐경 전 여성의 혈청 디아민 산화효소(DAO) 수치는
월경 주기와 관련이 있는 것으로 보이며,
난포기에 비해
황체기에 더 높은 DAO 활성이 측정됩니다 [14].

월경통은
히스타민에 대한 민감도 증가와 관련이 있을 수 있습니다.
월경 첫날에
H1 항히스타민제를 투여하면
월경통에 예방 효과가 있습니다.
배란기 동안
소변에서 높은 수준의 히스타민 대사 산물은
에스트로겐(특히 에스트라디올)의 영향과 관련이 있을 수 있습니다[15].
참고) 섬유근막통 - dao 결핍과 연관되어 있다는 논문...
https://www.mdpi.com/2077-0383/12/20/6449


Manifestations of HIT
One of the reasons why the adverse reactions caused by the intake of histamine cannot be clearly defined and outlined, as against other sicknesses, is their heterogeneity. Due to the fact that histamine enters into the circulation and that histamine receptors occur ubiquitously in the human body, a typical clinical picture cannot be strictly defined. The adverse manifestations related to the intake of histamine are usually complex and may affect different organ systems. Paradoxically, if the set of manifestations appears in various ways, unexpectedly and randomly, and at the same time following the ingestion of food, the symptoms may have their origin in histamine intake.
히스타민 섭취로 인한 부작용을 다른 질병과 마찬가지로
명확하게 정의하고 설명할 수 없는 이유 중 하나는
그 이질성 때문입니다.
히스타민이 순환계에 유입되고
히스타민 수용체가
인체에 어디에나 존재한다는 사실 때문에
전형적인 임상상을 엄격하게 정의할 수 없습니다.
히스타민 섭취와 관련된 부작용은
일반적으로 복잡하며
다양한 장기 시스템에 영향을 미칠 수 있습니다.
역설적이게도
일련의 증상이 음식 섭취 후 예기치 않게
무작위로 다양한 방식으로 동시에 나타나는 경우 증상은
히스타민 섭취에서 비롯된 것일 수 있습니다.
As typical signs, we can observe skin manifestations—for example erythema in the facial area (flushing), pruritus, urticarial rash on the body. Gastrointestinal symptoms include diarrhoea (±vomiting) but also constipation and abdominal pain. Manifestations in the cardiovascular system, such as low blood pressure (counter-regulatory hypertension may subsequently occur) and tachycardia are less frequent [1], as are manifestations in the nervous and respiratory systems (Figure 1) [9].
전형적인 징후로는
얼굴 부위의 홍반(홍조),
가려움증,
두드러기 발진 등 피부 증상을 관찰할 수 있습니다.
위장 증상으로는
설사(±구토)뿐만 아니라
변비 및 복통도 포함됩니다.
저혈압(이후 역조절성 고혈압이 발생할 수 있음) 및
빈맥과 같은 심혈관계 증상은
신경계 및 호흡계 증상과 마찬가지로
덜 자주 나타납니다[1][9]입니다(그림 1).
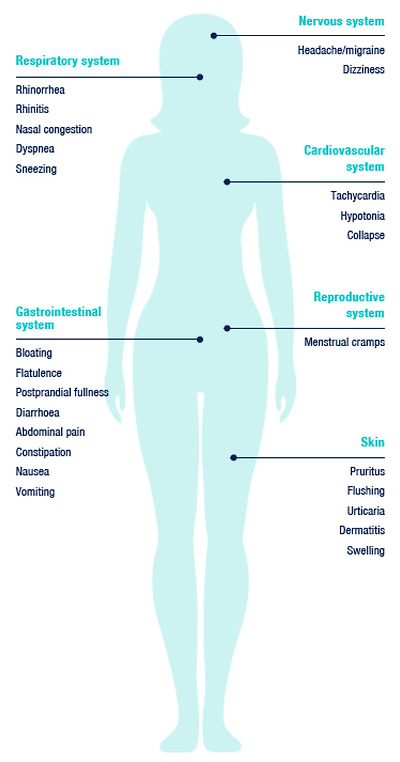
Typical manifestations related to the intake of exogenous histamine. Adapted according to [9].
The problem with a “diagnosis” of HIT is precisely the inconstancy and variety of the manifestations in the same individual following similar stimuli. In cross-over placebo-controlled trials in which symptoms were assessed, the subjects reacted randomly to the histamine provocation test. Although the total score of symptoms when histamine was administered was significantly higher as compared to the placebo, with many individuals no relationship between the ingestion of the histamine and the individual symptoms could be established [16].
HIT의 "진단"의 문제는
유사한 자극에 따라 동일한 개인에서 나타나는 증상의 불일치와 다양성입니다.
증상을 평가하는 교차 위약 대조 임상시험에서 피험자들은
히스타민 유발 테스트에 무작위로 반응했습니다.
히스타민을 투여했을 때
증상의 총 점수는 위약에 비해 유의하게 높았지만,
많은 개인에서 히스타민 섭취와
개별 증상 간의 관계를 확인할 수 없었습니다 [16].
New findings have been recorded just recently. A 45-year-old woman who experienced Nissen’s fundoplication for long-lasting laryngopharyngeal reflux developed episodes of throat clearing and coughing. Laryngopharyngeal reflux indicates the return flow of gastric contents to the laryngopharynx and upper aerodigestive space. It is a clinical unit different from the gastroesophageal reflux disease. In this case, consultations with nutrition specialists led to considerations of HIT. A low-histamine diet led to a significant improvement in the patient’s symptoms. For patients who do not respond according to expectations to typical laryngopharyngeal reflux treatment, a potential link to HIT should be taken into consideration [17], with a 3-month diet treatment prior to a possible operation.
최근에 새로운 연구 결과가 발표되었습니다.
후두인두 역류가 오래 지속되어
니센 내시경술을 경험한 45세 여성이
목이 쉬고 기침이 나는 증상을 겪었습니다.
후두인두 역류는
위 내용물이 후두인두와 상부 공기 소화 공간으로 역류하는 것을 말합니다.
위식도 역류 질환과는 다른 임상 단위입니다.
이 경우 영양 전문가와의 상담을 통해
HIT를 고려하게 되었습니다.
저히스타민 식단으로 환자의 증상이 크게 개선되었습니다.
일반적인 후두인두 역류 치료에
기대에 따라 반응하지 않는 환자의 경우,
수술 전에 3개월간의 식이 요법을 통해
HIT와의 잠재적 연관성을 고려해야 합니다[17].

In another study, 30 laryngopharyngeal reflux patients with chronic coughs underwent a histamine provocation test. Using a visual analogue scale, videolaryngostroboscopy findings and voice and throat symptoms were assessed directly before and after the exposure test. Moreover, the correlation between the relative changes in spirometry values in relation to changes in vocal fold oedema was also evaluated, along with redness and changes in the voice and throat symptoms reported by the patients during the histamine provocation test. The relative changes in inspiratory and expiratory air flow and voice and throat symptoms during the histamine challenge test correlated. Histamine induced oedema of the vocal folds, visible by videolaryngostroboscopic imaging, did not significantly influence spirometric air flow values [18].
또 다른 연구에서는
만성 기침을 하는 후두인두 역류 환자 30명을 대상으로
히스타민 유발 테스트를 실시했습니다.
시각적 아날로그 척도를 사용하여
노출 테스트 전후에 비디오 후두경 검사 결과와
음성 및 인후 증상을 직접 평가했습니다.
또한 성대 부종 변화와 관련된
폐활량 측정값의 상대적 변화와
히스타민 유발 테스트 중 환자가 보고한
발적 및 음성 및 인후 증상 변화 간의 상관관계도 평가했습니다.
히스타민 자극 검사 중
흡기 및 호기 공기 흐름의 상대적 변화와
음성 및 인후 증상은 상관관계가 있었습니다.
비디오 후두경 영상으로 볼 수 있는 히스타민 유발 성대 부종은 폐활량 기류 값에 큰 영향을 미치지 않았습니다[18].
1.2. Histamine
Histamine is a neuro-immuno-endocrine system mediator. In the human organism it influences the whole spectrum of physiologic functions of various tissues and cells, including immunity. From a chemical perspective, it is a ubiquitously occurring biogenic amine. In the organism, its synthesis is ensured by decarboxylation of the amino acid L-histidine by the L-histidine decarboxylase enzyme. In the human organism, histamine is primarily stored in the mast cells and basophils, but its presence has also been found in the enterochromaffin cells [19] and in the histaminergic neurons [20]. Histamine acts in the organism as an agonist of histamine H1, H2, H3 and H4 receptors. H1 and H2 receptors appear ubiquitously, with H2 mostly present in the digestive tract (stomach, duodenum, small intestine). The H3 receptors are abundant in the nervous system. The H4 receptors are present in certain tissues (skin, tonsils), but in a small amount [21]. Among other processes, histamine mediates inflammatory responses, vasodilation, gastric acid production in enterochromaffin cells, congestion and bronchospasm, and secretion in the respiratory system. Its pleiotropic effect was found in the nervous system, where it acts as a neuromediator and a neurohormone, influencing e.g., thermoregulation, alertness, appetite and cognitive and behavioural functions [22]. The microbiome can also be a source of histamine in the macroorganism [9]. Its production has been described in some species (see the Microbiome and HIT section). Food is the main exogenous source of histamine [23].
히스타민은
신경-면역-내분비계 매개 물질입니다.
인체에서는
면역을 포함한 다양한 조직과 세포의 생리학적 기능 전반에 영향을 미칩니다.
화학적 관점에서 볼 때,
이 물질은 어디에나 존재하는
생체 아민(biogenic amine)입니다.

참고) 생체 아민 : 아미노산에서 카르복실기가 제거된 물질. - 치즈발효, 와인발효, 고등어 단백질 변성 등의 과정에서
생체아민은 히스타민, 티라민, 카다베린(cadaverine), 푸트레신(putrecine) 등을 함
모노아민, 다이아민, 폴리아민으로 구성..



유기체에서는
L-히스티딘 탈카르복실화 효소에 의한
아미노산 L-히스티딘의 탈카르복실화에 의해 합성이 보장됩니다.
인간 유기체에서 히스타민은
주로 비만 세포와 호염기구에 저장되지만,
장 크로마핀 세포 [19]와 히스타민성 뉴런 [20]에서도 그 존재가 발견되었습니다.
mast cells and
basophils, but its presence has also been found in the
enterochromaffin cells [19] and in the
histaminergic neurons
히스타민은
유기체에서 히스타민 H1, H2, H3 및 H4 수용체의 작용제로 작용합니다.
H1 및 H2 수용체는 어디에나 존재하며,
H2는 주로 소화관(위, 십이지장, 소장)에 존재합니다.
H3 수용체는 신경계에 풍부합니다.
H4 수용체는 특정 조직(피부, 편도선)에 존재하지만 소량으로 존재합니다[21].
다른 과정 중에서도 히스타민은
염증 반응,
혈관 확장,
장 크로마핀 세포의 위산 생성,
혼잡 및 기관지 경련,
호흡기 분비를 매개합니다.
신경계에서는
신경 매개체 및 신경 호르몬으로 작용하여
체온 조절,
각성,
식욕,
인지 및 행동 기능에 영향을 미치는 등[22],
신경계에서 플레오트로픽 효과가 발견되었습니다.
마이크로바이옴은
또한 거대 유기체에서
히스타민의 공급원이 될 수 있습니다[9].
일부 종에서
히스타민의 생산이 설명되어 있습니다(마이크로바이옴과 HIT 섹션 참조).
음식은
히스타민의 주요 외인성 공급원입니다 [23].
https://my.clevelandclinic.org/health/articles/24854-histamine






Metabolism of Histamine
The quantity of endogenous histamine is controlled on a genetic level. In genes encoding the enzymes responsible for the synthesis and degradation of histamine, similarly as in histamine receptor-encoding genes, genetic polymorphisms have been identified [21]. Genetic polymorphisms for histamine receptors and for DAO are most likely associated with several specific symptoms and their combinations [24]. In certain polymorphisms of the gene encoding DAO (and similarly, the H3 receptor), diminished activity of this enzyme has been reported, which increases the risk of migraines. Reduced DAO activity however has also been recorded in healthy individuals. In addition to genetic predisposition, several factors (e.g., variability of histamine content in food etc.) appear to be responsible for the manifestation of symptoms; hence the functional and clinical significance of genetic polymorphisms remains elusive [21,24].
내인성 히스타민의 양은
유전적 수준에서 조절됩니다.
히스타민 수용체 코딩 유전자와 마찬가지로
히스타민의 합성과 분해를 담당하는 효소를 코딩하는 유전자에서도
유전적 다형성이 확인되었습니다 [21].
히스타민 수용체와
DAO에 대한 유전적 다형성은
몇 가지 특정 증상 및 그 조합과 관련이 있을 가능성이 높습니다 [24].
DAO(및 유사하게 H3 수용체)를 암호화하는 유전자의 특정 다형성에서
이 효소의 활동이 감소하여
편두통의 위험이 증가하는 것으로 보고되었습니다.
그러나
건강한 사람에서도
DAO 활성 감소가 관찰되고 있습니다.
유전적 소인 외에도
여러 요인(예: 음식 내 히스타민 함량의 변동성 등)이
증상 발현에 영향을 미치는 것으로 보이며,
따라서 유전적 다형성의 기능적 및
임상적 중요성은 아직 밝혀지지 않았습니다[21,24].
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10218803/

The half-life of histamine in plasma is relatively short, a few minutes [25]. Histamine is metabolized in several pathways in the organism. As clinically most significant is considered enzymatic degradation mediated by the DAO enzyme, with a second pathway represented by the histamine-N-methyl transferase enzyme (HNMT) [1].
The DAO enzyme is also identified in the literature according to the gene that encodes it, AOC1 Amine Oxidase Copper Containing 1 [26], formerly known as histaminase. The DAO molecule contains copper and is the essential enzyme responsible for the degradation of histamine from the extracellular space [27]. The product of oxidative deamination of histamine is imidazole-4-acetaldehyde (Figure 2).
혈장 내 히스타민의 반감기는
몇 분으로 비교적 짧습니다[25].
히스타민은
생체 내 여러 경로를 통해 대사됩니다.
임상적으로 가장 중요한 것은
DAO 효소에 의해 매개되는 효소 분해로 간주되며,
두 번째 경로는 히스타민-N-메틸 전이 효소(HNMT)로 대표되는 경로입니다[1].
histamine-N-methyl transferase enzyme (HNMT)
DAO 효소는
이전에는 히스타민 분해 효소로 알려진
AOC1 아민 산화 효소 구리 함유 1[26]을 암호화하는 유전자에 따라 문헌에서도 확인되었습니다.
DAO 분자는
구리를 포함하고 있으며
세포 외 공간에서 히스타민을 분해하는 데 필수적인 효소입니다[27].
히스타민의 산화 탈아민화 생성물은
이미다졸-4-아세트알데히드입니다(그림 2).
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8069563/

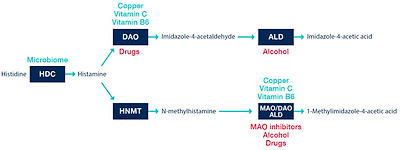
Histamine metabolism in vivo. HDC—histidine decarboxylase; DAO—diamine oxidase; ALD—aldehyde dehydrogenase; HNMT—histamine-N-methyl transferase; MAO—monoamine oxidase. The green are factors potentiating an endogenous capacity of enzymatic reaction.
The red are factors directly/indirectly inhibiting an enzymatic reaction. Adapted according to [9,13,21,27].
빨간색은
효소 반응을 직/간접적으로 억제하는 인자입니다. 9,13,21,27]에 따라 수정됨.
DAO is found in the epithelial cells of the (small) intestine, the placenta, the kidneys, the thymus and seminal plasma [28]. The physiological function of the DAO enzyme includes regulation of the inflammation processes, proliferation, allergic response and ischemia [29]. During digestion, the DAO enzyme is continuously synthesized in the mucosa of the small intestine. It is stored in vesicular structures on the basolateral membrane of the enterocytes and acts as a metabolic barrier against exogenous diamines, including histamine [30]. The accumulation of ingested histamine and its subsequent penetration into the circulation as a result of reduced or slowed catabolism by the DAO enzyme at the level of the small intestinal epithelium is considered as a possible reason for the HIT syndrome [6]. The activity and plasmatic level of the DAO may be dependent on the genetic variability of the relevant genes (AOC1 on the 7th chromosome) [21], or on the physiological state of the organism [13]. During pregnancy, greatly increased concentrations (up to 150-fold) of serum and plasma DAO were measured [27]—the placenta is a producer of this enzyme. This is regarded as the reason why during pregnancy, in women suffering from HIT manifestations, a lessening or complete regression of HIT symptoms is observed. DAO activity or histamine release may be influenced by a number of commonly used medicaments, such as N-acetylcysteine, ambroxol, verapamil, propafenone, amiloride, cefuroxime, clavulanic acid or non-steroidal anti-inflammatory drugs, metamizole, as well as radiological contrast agents (Figure 3) [13,31]. We summarize an extended list of substances possibly interfering with the activity of DAO in Table 2.
DAO는
(소장)의 상피 세포, 태반, 신장, 흉선 및 정액 혈장에서 발견됩니다 [28].
DAO 효소의 생리적 기능에는
염증 과정,
증식,
알레르기 반응 및 허혈의 조절이
포함됩니다 [29].
소화하는 동안
DAO 효소는
소장 점막에서 지속적으로 합성됩니다.
dao효소는 장 세포의 기저막에있는 소포 구조에 저장되며
히스타민을 포함한 외인성 디아민에 대한 대사 장벽으로 작용합니다 [30].
소장 상피 수준에서
DAO 효소에 의한 이화 작용의 감소 또는 둔화의 결과로
섭취 된 히스타민의 축적과
그 후 순환계로의 침투는 HIT 증후군의 가능한 원인으로 간주됩니다 [6].
DAO의 활성 및 원형질 수준은
관련 유전자 (7 번 염색체의 AOC1)의 유전 적 다양성 [21] 또는
유기체의 생리적 상태 [13]에 따라 달라질 수 있습니다.
임신 중에는
혈청 및 혈장 DAO의 농도가 크게 증가 (최대 150 배)한 것으로
측정되었습니다 [27]-
태반은
dao 효소의 생산자입니다.
이것이 임신 중에
HIT 증상으로 고통받는 여성에서
HIT 증상이 완화되거나 완전히 퇴행하는 이유로 간주됩니다.
참고) 임신 중 여성이 아토피가 감쪽같이 사라지기도 하는 이유...
악화되는 이유는?
DAO 활성 또는 히스타민 방출은
N-아세틸시스테인,
암브록솔,
베라파밀,
프로파페논,
아밀로라이드,
세푸록심,
클라불란산 또는
비스테로이드성 항염증제,
메타미졸 및 방사선 조영제와 같은 일반적으로 사용되는 여러 약물의 영향을 받을 수 있습니다 (그림 3) [13,31].
DAO의 활동을 방해할 가능성이 있는 물질의 확장된 목록은
표 2에 요약되어 있습니다.
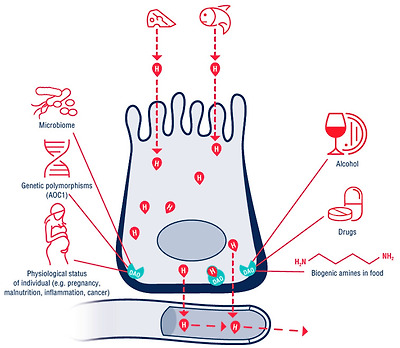
The function of diamine oxidase (DAO) in the enterocyte.
The red drop with H shows the histamine released from food. The histamine passes through the enterocyte into the circulation. The DAO enzyme on the basolateral membrane creates a barrier, and the histamine obtained from the food is metabolized (the red drop with H with a green contour). DAO activity is directly/indirectly dependent on internal and external factors such as the polymorphisms of the AOC1 gene, the physiological status of the organism, alcohol, other biogenic amines and medication intake.
AOC1—amine oxidase copper containing 1 gene.
장세포에서 디아민 산화효소(DAO)의 기능.
H가 있는 빨간색 방울은 음식에서 방출된 히스타민을 나타냅니다. 히스타민은 장 세포를 통해 순환계로 전달됩니다. 기저막의 DAO 효소가 장벽을 만들고 음식에서 얻은 히스타민이 대사됩니다(녹색 윤곽선이 있는 H가 있는 빨간색 방울). DAO 활성은 AOC1 유전자의 다형성, 유기체의 생리적 상태, 알코올, 기타 생물성 아민 및 약물 섭취와 같은 내부 및 외부 요인에 직/간접적으로 의존합니다.
1 개의 유전자를 포함하는 AOC1- 아민 산화 효소 구리.

https://www.mdpi.com/2218-273X/12/3/454

Table 2
Drugs and substances with possible effects on the metabolism and the distribution of histamine in the organism, which encompass decreasing DAO activity.
Adapted according to [13,31,32,33,34].
| DAO blocking foods | |
| Alcohol | wine and spirits especially |
| OTC drugs interfering with DAO activity (decreasing DAO activity) | |
| Expectorants, mucolytics | ambroxol, N-acetylcysteine |
| Nonsteroidal anti-inflammatory drugs | acetylsalicylic acid, ibuprofen |
| Rx drugs interfering with histamine metabolism or distribution | |
| Prokinetics | metoclopramide |
| Antiinfectives | clavulanic acid, isoniazid, cefuroxime, cefotiame, pentamidine, chloroquine, doxycycline, neomycin B, acriflavine, D-cycloserine |
| Bronchodilators | aminophylline, theophylline |
| Diuretics | amiloride, furosemide |
| Antidepressants | amitriptyline, monoaminooxidase 1 inhibitors |
| Anxiolytics | diazepam, barbiturates |
| Antipsychotics | haloperidol |
| Cytostatics | cyclophosphamide |
| Antihypertensives | verapamil, dihydrazine, alprenolol |
| Cardiotonics | dobutamine, dopamine |
| Opioids | pethidine, morphine, codeine |
| Analgesics | metamizole |
| Local anaesthetics | lidocaine, prilocaine, marcaine, procaine |
| General anaesthetics | thiopental |
| Muscle relaxants | pancuronium, alcuronium, D-tubocurarine |
| Antiarrhytmics | propafenone, verapamil, quinidine |
| Antihistamines decreasing DAO activity | |
| H1/H2 receptor blockers | cimetidine, promethazine |
| Other substances interfering with histamine metabolism | |
| Radiocontrast agents | iodine containing |
OTC-over the counter.
HNMT is a cytosolic enzyme whose role is to regulate intracellular histamine levels [6]. The inactivation of intracellular histamine is mediated by the methylation of the imidazole nucleus; this metabolite is subsequently oxidized [13]. Although it is also found in the gastrointestinal tract, it is unlikely to play a major role in the degradation of exogenous histamine or histamine produced by the gut microbiome [9].
HNMT는
세포 내 히스타민 수치를 조절하는 역할을 하는
세포질 효소입니다 [6].
세포 내 히스타민의 비활성화는
이미다졸 핵의 메틸화에 의해 매개되며,
이 대사 산물은 이후 산화됩니다 [13].
위장관에서도 발견되지만
장내 미생물에 의해 생성되는
외인성 히스타민 또는
히스타민의 분해에 중요한 역할을 하지는 않을 것으로 보입니다 [9].
1.3. Biogenic Amines in Food
Biogenic amines may be present in greater or lesser amounts in any food. Processing and storage are generally inevitable in cases where the ingredients spoil quickly and/or are rich in proteins. Storage raises the risk of accumulation of biogenic amines. It seems that their accumulation is totally dependent on the microorganisms that create histamine during food storage (especially in case of foods with a high L-histidine content) [35]. Overall, the fresher the food, the lower the probability of biogenic amine formation.
바이오제닉 아민은
모든 식품에 많거나 적은 양으로 존재할 수 있습니다.
일반적으로
재료가 빨리 상하거나
단백질이 풍부한 경우 가공 및 보관이 불가피합니다.
보관은
바이오제닉 아민의 축적 위험을 높입니다.
이들의 축적은
식품 보관 중 히스타민을 생성하는 미생물에 전적으로 의존하는 것으로 보입니다
(특히 L-히스티딘 함량이 높은 식품의 경우) [35].
전반적으로
식품이 신선할수록
생체 아민이 형성될 확률이 낮아집니다.
Amines are classified as monoamines, diamines and polyamines, depending on how many amine groups they contain. Among the most important biogenic amines found in food are monoamine tyramine, diamines histamine, putrescine and cadaverine, as well as the polyamines spermine and spermidine [36].
In the context of HIT syndrome, of clinical significance is tyramine, which may be present in excessive amounts in certain types of ripening cheeses. In sensitive people, this is related to increased blood pressure and the consequent occurrence of migraine pains [37].
아민은
함유된 아민기의 수에 따라
모노아민,
디아민,
폴리아민으로 분류됩니다.
식품에서 발견되는
가장 중요한 생체 아민 중에는
모노아민 티라민,
디아민 히스타민, 푸트레신 및 카데베린과
폴리아민 스페르민 및 스페르미딘이 있습니다 [36].
HIT 증후군의 맥락에서 임상적으로 중요한 것은
특정 유형의 숙성 치즈에
과도한 양으로 존재할 수 있는
티라민입니다.
민감한 사람들의 경우
이는 혈압 상승 및 그에 따른 편두통 발생과 관련이 있습니다 [37].
Biogenic amines may contribute to histamine toxicity by saturating enzymes responsible for the degradation of histamine in the mucosa (DAO, HNMT). The diamines putrescine and cadaverine are considered to be the amines with the greatest influence on the metabolism of histamine. This is due to the fact that the DAO enzyme breaks down them preferentially [13,23,36]. Foods with a high biogenic amine content are generally considered as risky and should be omitted from low-histamine diets [9,23].
생물성 아민은
점막에서 히스타민 분해를 담당하는 효소(DAO, HNMT)를 포화시켜
히스타민 독성에 기여할 수 있습니다.
디아민 푸트레신과 카데베린은
히스타민 대사에 가장 큰 영향을 미치는 아민으로 간주됩니다.
이는 DAO 효소가 이들을 우선적으로 분해하기 때문입니다[13,23,36].
생체 아민 함량이 높은 식품은
일반적으로 위험한 것으로 간주되며
저히스타민 식단에서 제외해야 합니다[9,23].
Values of Biogenic Amines and Histamine in Foods
A diet that ensures the complete elimination of histamine is unattainable [38]. The content of biogenic amines and histamine in foods differs in dependence on their source, freshness, types, pH, salt content, content of proteins (and L-histidine), processing and storage [13,23,39]. The wide range of content of histamine and/or other biogenic amines for individual foods makes these parameters inconclusive and so we do not regard the listing of specific value intervals per 100 g of food as authoritative. In Figure 4, we present a list of foods that are most often recommended to be excluded from diet in case of suspected HIT.
식품 내 바이오제닉 아민과 히스타민의 수치
히스타민의 완전한 제거를 보장하는 식단은
달성할 수 없습니다 [38].
식품 내 생체 아민과 히스타민의 함량은
식품의 공급원,
신선도,
유형,
pH,
염분 함량,
단백질(및 L-히스티딘) 함량,
가공 및 보관에 따라 다릅니다[13,23,39].
개별 식품에 대한 히스타민 및/또는 기타 생체 아민의 함량이
매우 다양하기 때문에
이러한 매개변수는 결정적이지 않으며,
따라서 식품 100g당 특정 값 간격의 목록은 권위 있는 것으로 간주하지 않습니다.
그림 4에는 HIT가 의심되는 경우 식단에서 제외할 것을 가장 자주 권장하는 식품 목록이 나와 있습니다.
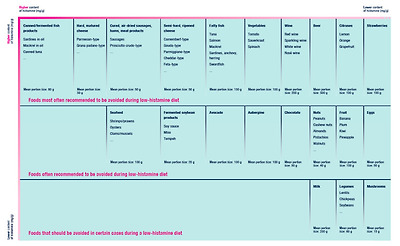
Foods that are most often recommended to be excluded from low-histamine diets. Adapted according to [9].
In Table 3, we list foods that in usual quantities are considered safe from triggering HIT symptoms.
Table 3
Foods that in usual quantities are considered safe from triggering HIT symptoms.
Adapted according to [40].
| Water, coffee, tea, homemade juices from allowed fruits and vegetables |
| Bread, pastry, potatoes, rice, pasta, cereals, millet, buckwheat, corn |
| Yoghurt, fresh soft cheese |
| Lettuce, cauliflower, broccoli, chicory, carrot, garlic, onion, cucumber, pumpkin, zucchini, pepper, radish, artichoke, rhubarb, asparagus |
| Apple, pear, cherry, amarelle, peach, apricot, watermelon, blueberries |
| Spices, herbs |
| Vegetable oil, vinegar |
| FRESH/IMMEDIATELY FROZEN meat: poultry, veal, beef, lamb, pork |
| FRESH/IMMEDIATELY FROZEN fish: cod/pollock, trout, zander, halibut |
| Ham (fresh, cooked and high-quality), eggs (cooked) |
| Jam made from allowed fruits, honey, butter, margarine |
In general, biogenic amines are thermostable. If they are already present in the food, heat treatment does not significantly degrade them [36]. However, boiling in water can reduce the biogenic amines content in certain types of vegetables, most likely by transferring them from the food to the water. Boiling spinach reduced the histamine level by 83% when compared to raw spinach, while analysis confirmed the transfer of the histamine from the spinach to the water (Latorre-Moratalla et al., 2015, in [23]). Heat treatment needs not always lead to a reduction of the biogenic amines contained in the food however. Heat treatment in the form of boiling and grilling showed an increase of the biogenic amines content in mg/100 g in aubergine, green and yellow beans [41,42]. In a work by Chung, the histamine content in mg/100 g in grilled seafood and meat increased, whereas boiling these foods reduced the histamine content in the meat. Boiling the vegetable had no influence on the content of histamine or reduced it only minimally [43].
일반적으로
바이오제닉 아민은
열에 안정적입니다.
이미 식품에 존재하는 경우 열처리해도 크게 분해되지 않습니다[36].
그러나
물에 끓이면
특정 유형의 야채에 있는 바이오제닉 아민 함량이
식품에서 물로 옮겨져 감소할 수 있습니다.
시금치를 삶으면
생 시금치에 비해 히스타민 수치가 83% 감소했으며,
분석 결과 히스타민이 시금치에서 물로 이동하는 것이 확인되었습니다(Latorre-Moratalla 외, 2015, [23]).
그러나
열처리가 항상 식품에 함유된 바이오제닉 아민의 감소로 이어지는 것은 아닙니다.
끓이거나 굽는 형태의 열처리는
가지, 녹두, 노란콩에서
바이오제닉 아민 함량을 100mg/g 증가시키는 것으로 나타났습니다 [41,42].
정 교수의 연구에서는
구운 해산물과
육류의 히스타민 함량이 100mg/100g 증가한 반면,
이러한 식품을 삶으면 육류의 히스타민 함량이 감소했습니다.
야채를 삶는 것은
히스타민 함량에 영향을 미치지 않거나
미미하게만 감소했습니다 [43].
1.4. Factors Contributing to Increased Sensitivity to Histamine
Among the factors increasing the sensitivity of individuals to the ingestion of histamine are classed other biogenic amines, alcohol (blocks the enzyme DAO, can release endogenous histamine), specific medications (with an inhibitory effect on DAO) and malnutrition, leading to an insufficiency of enzyme cofactors (vitamin C, copper, vitamin B6) [13].
히스타민 섭취에 대한 개인의 민감도를 높이는 요인 중에는
다른 생체 아민,
알코올 (효소 DAO 차단, 내인성 히스타민 방출 가능),
특정 약물 (DAO 억제 효과가 있는) 및
영양 실조로 분류되어 효소 보조 인자 (비타민 C, 구리, 비타민 B6) [13]의 부족으로 이어지는 영양 실조가 있습니다.
1.5. Microbiome and HIT
In 2018, Schink compared microbial patterns from 33 healthy individuals with 33 persons with suspected HIT, 8 of whom had decreased DAO enzyme activity in serum. In comparison with those patients suspected of HIT presence, the healthy people showed a greater abundance of the Bifidobacteriaceae family, with a median of 0.3% [44]. To this family belongs the Bifidobacterium genus, which confers health benefits to the host [45]. In persons having decreased DAO activity in serum, a greater abundance of the Proteobacteria genus was observed. The higher ratio in favour of Proteobacteria genus, which competes with strict anaerobes (including bacteria of the genus Bifidobacterium), may predict dysbiosis and/or impaired intestinal epithelial function [44]. If the bifidobacteria were used as a starting culture in the production of fermented sausages, the end products contained lower amounts of biogenic amines [46].
2018년에 Schink는
건강한 사람 33명과 HIT가 의심되는 사람 33명의 미생물 패턴을 비교했으며,
이 중 8명은 혈청에서 DAO 효소 활성이 감소했습니다.
HIT가 의심되는 환자들과 비교했을 때,
건강한 사람들은 비피도박테리아과가 중앙값 0.3%로 더 풍부한 것으로 나타났습니다 [44].
이 과에 속하는 비피도박테리움 속은
숙주에게 건강상의 이점을 제공합니다 [45].
혈청에서 DAO 활성이 감소한 사람의 경우
프로테오박테리아 속이 더 많이 관찰되었습니다.
엄격한 혐기성 박테리아(비피도박테리움 속 박테리아 포함)와 경쟁하는
프로테오박테리아 속의 비율이 높을수록
장 상피 기능의 이상 및/또는 손상을 예측할 수 있습니다 [44].
비피더스균을 발효
소시지 생산의 시작 배양으로 사용한 경우,
최종 제품에는 더 적은 양의 바이오제닉 아민이 함유되어 있었습니다[46].
Some bacterial strains also have an enzyme that ensures endogenous histamine synthesis in the human body. It should be emphasized that the presence of bacterial L-histidine decarboxylase is strain-, not species-, specific [47]. Accordingly, it is not possible to extrapolate this property from one strain to another, although within the same species. Hence, it is imperative to always assess independently the amount of produced histamine for the individual strains of bacterial species.
Certain strains which are considered potentially probiotic, for example Lactobacillus saerimneri 30a, produce a significant amount of histamine and other biogenic amines [48,49,50]. In the wide commercially used bacteria Limosilactobacillus reuteri DSM 17938, the presence of genes responsible for the synthesis of this enzyme was not proven [51]. From the 15 strains of the bacteria Lactobacillus acidophilus, Lacticaseibacillus casei, Lactobacillus delbrueckii ssp. bulgaricus, Lactobacillus lactis ssp. lactis, Lactococcus lactis ssp. lactis and Lactiplantibacillus plantarum, only two strains, L. casei TISTR 389 and L. bulgaricus TISTR 895, appeared to be potentially histamine-producing [47].
Certain strains of the following bacteria, yeasts and moulds genera and species have the capacity for histamine formation. In Table 4, we summarize microbial genera and species in which the presence of gene for the L-histidine decarboxylase was demonstrated.
일부 박테리아 균주에는
인체에서 내인성 히스타민 합성을 보장하는 효소도 있습니다.
박테리아 L- 히스티딘 데카르 복실 라제의 존재는
종이 아닌 균주 특이적이라는 점을 강조해야합니다 [47].
따라서 같은 종 내에서도
이 특성을 한 균주에서 다른 균주로 추정하는 것은 불가능합니다.
따라서
항상 박테리아 종의 개별 균주에 대해 생성되는
히스타민의 양을 독립적으로 평가하는 것이 필수적입니다.
예를 들어
락토바실러스 사에림네리 30a와 같이
잠재적 프로바이오틱스로 간주되는 특정 균주는
상당한 양의 히스타민과 기타 생체 아민을 생성합니다[48,49,50].
상업적으로 광범위하게 사용되는 박테리아
리모실락토바실러스 루테리 DSM 17938에서는
이 효소의 합성을 담당하는 유전자의 존재가 입증되지 않았습니다 [51].
락토바실러스 아시도필루스,
락티카제이바실러스 카세이,
락토바실러스 델브루에키 에스피시 불가리쿠스,
락토바실러스 락티스 에스피시 락티스,
락토코커스 락티스 에스피시.
락티스 및 락티플란티바실러스 플란타룸, L. 카세이 TISTR 389 및 L. 불가리쿠스 TISTR 895의 두 균주만이 잠재적으로 히스타민을 생성하는 것으로 나타났습니다 [47].
다음 박테리아, 효모, 곰팡이 속 및 종의 특정 균주는 히스타민 생성 능력이 있습니다.
표 4에는 L-히스티딘 탈카르복실효소 유전자의 존재가 입증된 미생물 속과 종을 요약한 표가 나와 있습니다.
Table 4
Microbial genera or species which may have the capacity for histamine formation. Adapted according to [39,50,52,53].
Bacteria
| Acinetobacter spp. | Chryseobacterium spp. | Pediococcus pentosaceus |
| Alcalingenes faecalis | Klebsiella oxytoca | Proteus spp. |
| Klebsiella pneumoniae | ||
| Arizona spp. | Providencia spp. | |
| Kluyvera spp. | Providencia heimbachae | |
| Cedecea spp. | ||
| Lacticaseibacillus casei | Pseudomonas putida | |
| Citrobacter freundii | Lacticaseibacillus paracasei | Pseudomonas lundensis |
| Citrobacter braakii | Lacticaseibacillus rhamnosus | Pseudomonas stutzeri |
| Lactiplantibacillus plantarum | ||
| Lactobacillus curvatus | ||
| Edwardsiella spp. | Lactobacillus delbrueckii | Psychrobacter spp. |
| Lactobacillus helveticus | ||
| Enterococcus casseliflavus | Lactococcus lactis ssp. lactis | Raoultella planticola |
| Enterococcus faecalis | Latilactobacillus spp. | Raoultella ornithinolytica |
| Enterococcus faecium | Lentilactobacillus buchneri | |
| Lentilactobacillus hilgardii | Salmonella enterica ssp. arizonae | |
| Enterobacter spp. | Lentilactobacillus parabuchneri | |
| Leuconostoc spp. | ||
| Escherichia coli | Levilactobacillus brevis | Serratia spp. |
| Escherichia fergusonii | Limosilactobacillus reuteri | |
| Limosilactobacillus vaginalis | Sphingobacterium spp. | |
| Hafnia alvei | ||
| Hafnia paralvei | Microbacterium foliorum | Streptococcus thermophilus |
| Halomonas spp. | Morganella morganii | Tetragenococcus halophilus |
| Yeasts and moulds | ||
| Debaryomyces hansenii | Geotrichum candidum | |
The presence of such bacteria in the gastrointestinal tract could for certain individuals increase sensitivity to ingested histamine. In a study from 2013, the effect of the histamine-producing strain of the Lacticaseibacillus rhamnosus species was studied in mice. Frei et al. came to the conclusion that alteration of the innate immune response (e.g., dendritic cells) may be mediated through the H2 receptor not only by endogenous histamine, but also by histamine produced by the microbiome [53]. The L. rhamnosus LGG and L. rhamnosus Lc705 strains suppressed the expression of the H4 receptor of mast cells and decreased mast cell activation and IgE response [54].
위장관에 이러한 박테리아가 존재하면
특정 개인의 경우
섭취한 히스타민에 대한 민감도가 높아질 수 있습니다.
2013년에 발표된 연구에서
락티카제이바실러스 람노수스 종의 히스타민 생성 균주의 영향이 생쥐를 대상으로 연구되었습니다.
Frei 등은
선천성 면역 반응(예: 수지상 세포)의 변화는
내인성 히스타민뿐만 아니라
마이크로바이옴에 의해 생성된 히스타민에 의해서도
H2 수용체를 통해 매개될 수 있다는 결론에 도달했습니다 [53].
L. 람노서스 LGG 및
L. 람노서스 Lc705 균주는
비만 세포의 H4 수용체 발현을 억제하고
비만 세포 활성화 및 IgE 반응을 감소시켰습니다 [54].
Since microorganisms play a crucial role in histamine formation; they have been studied for their ability to degrade biogenic amines in foods, particularly histamine and tyramine [55,56,57]. The Lactiplantibacillus plantarum D-103 strain was able to degrade histamine up to 100% in histamine MRS broth [57]. Although the microbial catabolic activities responsible for histamine degradation have yet to be completely elucidated, microbial copper-containing amine oxidases, such as histamine oxidase, are most likely involved [55,57]. The capacity of microorganisms to degrade biogenic amines is strain-specific [55]. In the future, eligible strains could be exploited to control the accumulation of biogenic amines in certain foods (e.g., cheese, wine, miso) [55,56,57]. This approach would require an excellent knowledge of the microbial metabolism, since some by-products of the enzymatic reactions (e.g., hydrogen peroxide) are not desirable [55]. Moreover, some strains could have properties for concomitant degradation and production of biogenic amines [56].
미생물은
히스타민 형성에 중요한 역할을 하기 때문에
식품의 생체 아민,
특히 히스타민과 티라민을 분해하는 능력에 대해 연구되어 왔습니다 [55,56,57].
락티플란티바실러스 플란타룸 D-103 균주는
히스타민 MRS 국물에서
히스타민을 최대 100%까지 분해할 수 있었습니다[57].
히스타민 분해를 담당하는 미생물 이화 작용은
아직 완전히 밝혀지지 않았지만,
히스타민 산화 효소와 같은 미생물 구리 함유 아민 산화 효소가 관여할 가능성이 가장 높습니다 [55,57].
미생물이 생체 아민을 분해하는 능력은
균주에 따라 다릅니다 [55].
향후에는
특정 식품(예: 치즈, 와인, 된장)에서 바이오제닉 아민의 축적을 제어하기 위해
적격 균주를 활용할 수 있습니다[55,56,57].
이 접근법은
효소 반응의 일부 부산물(예: 과산화수소)이 바람직하지 않기 때문에
미생물 대사에 대한 탁월한 지식이 필요합니다[55].
또한 일부 균주는
생체 아민의 분해 및 생산을 수반하는 특성을 가질 수 있습니다 [56].
2. Diagnostic Approaches
HIT is currently not a nosological unit. Direct HIT-specific diagnostic criteria or markers are lacking [9]. Generally, for intolerances and malabsorption disorders, several diagnostic strategies using various tests have been proposed. In distinction to some other disorders such as for example celiac disease, there is no valid diagnostic laboratory assay for HIT that has been was consensually accepted [1,6,9,24].
It is proposed that the manifestation of HIT symptoms has its origin in a reduced level/activity of DAO. Based on this, the measurement of concentration or activity of this enzyme should be useful for the diagnosing of HIT. However, the problem resides in the fact that a reference value for DAO levels in serum has not yet been established [24,27]. Moreover, a measured DAO value and/or activity in a serum may be incoherent with the current level/functional activity of DAO in the intestinal mucosa.
HIT는 현재 코의 단위가 아닙니다. 직접적인 HIT 관련 진단 기준이나 마커가 부족합니다[9]. 일반적으로 과민증과 흡수 장애의 경우 다양한 검사를 이용한 몇 가지 진단 전략이 제안되어 있습니다. 예를 들어 셀리악병과 같은 일부 다른 장애와 달리, 합의적으로 인정된 HIT에 대한 유효한 진단 실험실 분석법은 없습니다 [1,6,9,24].
HIT 증상의 발현은
DAO의 수치/활성도 감소에서 기인하는 것으로
추정됩니다.
이를 바탕으로
이 효소의 농도나 활성도를 측정하는 것은
HIT 진단에 유용할 것입니다.
그러나
문제는
혈청 내 DAO 수치에 대한 기준값이 아직 확립되지 않았다는 사실에 있습니다[24,27].
또한, 혈청에서 측정된 DAO 수치 및/또는 활동은
장 점막에서 현재 DAO의 수준/기능적 활동과 일치하지 않을 수 있습니다.
2.1. DAO Enzyme Activity in Serum
The most studied but still controversial laboratory diagnostic approach is the analysis of DAO enzyme activity in serum. The tests measure the amount of histamine that degrades over a specified time in a blood sample, using enzyme-linked immunosorbent assay (ELISA) or radioimmunoassay (RIA). The threshold for serum DAO enzyme activity by which the presence of HIT should be considered has been proposed at 10 U/mL [58,59]. A number of authors have pointed out the potential contribution of this diagnostic approach in the search for the causes of the symptoms in patients with suspected HIT [9,59,60,61]. The reason for the controversy was that DAO was not detected in the blood by monoclonal antibodies, at least not in relevant amounts [62]. Accordingly, the DAO enzyme activity in serum could not be considered as conclusive [1]. In 2017, however, Boehm et al. demonstrated that ELISA is able to reliably and accurately quantify human DAO in different biological fluids and concluded that the potential of DAO as a biomarker in various diseases can be evaluated [27]. Still, in some patients, the relation between DAO activity in blood serum and the clinical history of symptomatic patients suffering from symptoms typical for HIT could not be confirmed. This can be explained by the fact that the activity of the DAO enzyme may naturally vary (over the course of the day and/or over the month) in an individual [63]. Measuring the DAO enzyme activity in serum may be considered as a part of the complementary examinations in certain cases if the determination of DAO activity in the intestinal mucosa is not feasible.
가장 많이 연구되었지만
여전히 논란의 여지가 있는 실험실 진단 방법은
혈청 내 DAO 효소 활성도 분석입니다.
이 검사는
효소결합 면역 흡착 분석법(ELISA) 또는 방사선 면역 분석법(RIA)을 사용하여
혈액 샘플에서 지정된 시간 동안 분해되는 히스타민의 양을 측정합니다.
HIT의 존재를 고려해야 하는 혈청 DAO 효소 활성의 임계값은 10 U/mL로 제안되었습니다[58,59]. 많은 저자들은 HIT가 의심되는 환자에서 증상의 원인을 찾는 데 이 진단 접근법이 잠재적으로 기여할 수 있다고 지적했습니다[9,59,60,61]. 논란의 이유는 DAO가 단일 클론 항체에 의해 혈액에서 적어도 관련 양만큼 검출되지 않았기 때문입니다 [62]. 따라서 혈청 내 DAO 효소 활성은 결정적인 것으로 간주할 수 없었습니다 [1]. 그러나 2017년에 Boehm 등은 ELISA가 다양한 생체액에서 인간 DAO를 안정적이고 정확하게 정량화할 수 있음을 입증하고 다양한 질병에서 바이오마커로서 DAO의 잠재력을 평가할 수 있다는 결론을 내렸습니다 [27]. 그럼에도 불구하고 일부 환자에서는 혈청 내 DAO 활성과 HIT의 전형적인 증상을 앓고 있는 환자의 임상 이력 사이의 관계를 확인할 수 없었습니다. 이는 DAO 효소의 활동이 개인에 따라 자연적으로 (하루 및/또는 한 달에 걸쳐) 달라질 수 있다는 사실로 설명할 수 있습니다 [63]. 장 점막에서 DAO 활성도를 측정할 수 없는 경우 혈청에서 DAO 효소 활성도를 측정하는 것이 보완 검사의 일부로 고려될 수 있습니다.
2.2. DAO Activity in the Intestinal Mucosa
Determination of DAO activity in the intestinal mucosa could represent a reliable diagnostic method. The determination is conditioned by a biopsy of colon tissue during colonoscopy. There are only a few studies addressing this diagnostic approach. However, they showed reduced DAO catabolic activity in patients with urticaria [64] and a food allergy [65] with associated higher histamine levels; the importance of this approach however needs to be confirmed by more robust data [1,9].
장 점막의 DAO 활성도를 측정하는 것은
신뢰할 수 있는 진단 방법이 될 수 있습니다.
이 결정은
대장 내시경 검사 중 대장 조직 생검을 통해 이루어집니다.
이 진단 방법을 다룬 연구는 몇 개 되지 않습니다. 그러나 두드러기 환자[64]와 히스타민 수치가 높은 음식 알레르기[65] 환자에서 DAO 이화 작용이 감소하는 것으로 나타났으며, 이 접근법의 중요성은 보다 강력한 데이터를 통해 확인될 필요가 있습니다[1,9].
2.3. Faecal Histamine Levels
As was stated, the microbiota of the gastrointestinal tract can be a significant source of histamine [50]. The determination of faecal histamine levels is therefore not considered a reliable method [1].
앞서 언급했듯이
위장관의 미생물 총은
히스타민의 중요한 공급원이 될 수 있습니다 [50].
따라서
분변 히스타민 수치를 측정하는 것은 신뢰할 수 있는 방법으로 간주되지 않습니다 [1].
2.4. Skin Prick Test
In HIT diagnostics the skin prick test variant, whose results are read after a longer time (50 min) than in the standard prick test (20 min), has been applied. The resolution of the redness from the puncture occurs later in symptomatic patients in comparison with the controls, which may signal a reduced ability of the body to degrade intracutaneously administered histamine. The limitation of this test resides in the fact that it need not necessarily reflect the degradation of histamine in the small intestine. Moreover, it is difficult, on the basis of this test, to differentiate HIT from other, for example allergic, disorders [66].
HIT 진단에서는
표준 단자 검사(20분)보다 더 긴 시간(50분) 후에 결과가 판독되는
피부 단자 검사 변형이 적용되었습니다.
증상이 있는 환자의 경우
대조군에 비해 자침으로 인한 발적의 해결이 늦어지며,
이는 신체가 피내 투여된 히스타민을 분해하는 능력이 저하되었다는 신호일 수 있습니다.
이 검사의 한계는
소장에서의 히스타민 분해를 반드시 반영할 필요는 없다는 사실에 있습니다.
또한, 이 검사를 기반으로 HIT를 다른 질환(예: 알레르기 질환)과 구별하는 것은 어렵습니다[66].
2.5. Histamine Levels in Plasma
Fourteen patients with suspected HIT were given orally 75 mg of histamine or a placebo, and plasmatic levels of histamine were measured at 10 min. intervals over 1 h. These results were compared with 4 healthy individuals. In this small sample, no significant differences were found in plasma histamine levels between the experimental and placebo groups. After the administration of the placebo, 33% of the patients had an increased level of histamine in the plasma, while about 50% exhibited symptoms [67].
HIT가 의심되는 14명의 환자에게
75mg의 히스타민 또는 위약을 경구 투여하고
1시간 동안 10분 간격으로 혈장 히스타민 수치를 측정하여
그 결과를 건강한 4명과 비교했습니다.
이 소량의 샘플에서는
실험군과 위약군 간에 혈장 히스타민 수치에 유의미한 차이가 발견되지 않았습니다.
위약 투여 후 환자의 33%는
혈장 내 히스타민 수치가 증가했고,
약 50%는 증상이 나타났습니다[67].
2.6. The Histamine Challenge Test
The histamine challenge (provocation) test provides diagnostic output as well as a determination of the individual threshold dose of ingested histamine. The threshold dose of ingested histamine is the amount of histamine capable of triggering symptoms in an individual. On the other hand, it is difficult (or even impossible) to estimate the histamine content for each food because it varies depending on multiple factors [13,23,39]. The main disadvantage of this test is that it demands practically uninterrupted supervision by specialist personnel over a relatively long time-interval. In a number of the publications, the provocative dose was set at 75 mg of histamine [1], which should refer to a dose harmless to a healthy individual and a commonly occurring dietary dose [68]. However, a consensus on the critical value for histamine intoxication is lacking [10]. In a small placebo-controlled study, a dosage of 75 mg histamine immediately caused symptoms (tachycardia, sneezing, itchy nose, rhinorrhoea) in 5 of the 10 healthy patients tested [68]. An Austrian study from 2011 also questioned the decisiveness of the test. The patients were given 75 mg histamine, and those who reacted to it (n = 39), were divided into groups and given tea with/without a histamine and a capsule with a DAO enzyme, or a placebo. According to the study’s author, symptoms with patients suspected of HIT in individual organs manifested in a variety of situations. In some cases, the patients reacted unexpectedly, even randomly. For the diagnosing of HIT therefore, the reproducibility of the individual symptoms may not in itself be informative; a scoring system evaluating all symptoms could be more appropriate [16]. In addition, the histamine challenge (provocation) test itself carries the risk of serious side effects [9].
히스타민 챌린지(도발) 테스트는
진단 결과와 함께 섭취한 히스타민의 개별 역치 용량도 확인할 수 있습니다.
섭취한 히스타민의 역치 용량은
개인에게 증상을 유발할 수 있는 히스타민의 양입니다.
반면에 히스타민 함량은
여러 요인에 따라 달라지기 때문에
각 식품의 히스타민 함량을 추정하는 것은 어렵거나 불가능합니다[13,23,39].
이 검사의 가장 큰 단점은 비교적 긴 시간 동안 전문 인력이 실질적으로 중단 없이 감독해야 한다는 것입니다. 많은 논문에서 자극 용량은 히스타민 75mg으로 설정되었는데[1], 이는 건강한 개인에게 무해한 용량과 일반적으로 발생하는 식이 용량을 참조해야 합니다[68]. 그러나 히스타민 중독의 임계값에 대한 합의가 부족합니다[10]. 소규모 위약 대조 연구에서 75mg의 히스타민을 투여하면 10명의 건강한 환자 중 5명이 즉시 증상(빈맥, 재채기, 코 가려움증, 콧물)을 일으켰습니다 [68]. 2011년에 발표된 오스트리아의 한 연구에서도 이 테스트의 결정성에 의문을 제기했습니다. 환자들에게 75mg의 히스타민을 투여하고 이에 반응한 환자(n = 39)를 두 그룹으로 나누어 히스타민이 함유된 차, 히스타민이 함유되지 않은 차, DAO 효소가 함유된 캡슐 또는 위약을 투여했습니다. 이 연구의 저자에 따르면 개별 장기에 HIT가 의심되는 환자의 증상은 다양한 상황에서 나타났습니다. 어떤 경우에는 환자가 예기치 않게, 심지어 무작위로 반응하기도 했습니다. 따라서 HIT 진단을 위해 개별 증상의 재현성은 그 자체로는 정보가 될 수 없으며, 모든 증상을 평가하는 점수 시스템이 더 적절할 수 있습니다 [16]. 또한 히스타민 챌린지(도발) 검사 자체는 심각한 부작용의 위험을 수반합니다[9].
2.7. Single Nucleotide Polymorphisms (SNPs) of AOC1 Gene Evaluation
Currently, there are known 4 different SNPs of the AOC1 gene associated with a predisposition to HIT [21]. Genetic testing of AOC1 gene SNPs is a non-invasive procedure, where SNPs evaluated from blood or oral mucosa samples could be provided in ambulant care or at home. The results could be read in days. This simple test may play a part in the complementary testing of suspected individuals in order to support the results of other diagnostic outcomes.
현재 HIT의 소인과 관련된 AOC1 유전자의 SNP는 4가지로 알려져 있습니다[21]. AOC1 유전자 SNP의 유전자 검사는 비침습적 절차로, 혈액 또는 구강 점막 샘플에서 평가된 SNP는 외래 진료 또는 가정에서 제공될 수 있습니다. 결과는 며칠 내에 판독할 수 있습니다. 이 간단한 검사는 다른 진단 결과를 뒷받침하기 위해 의심되는 개인에 대한 보완 검사에서 일부 역할을 할 수 있습니다.
2.8. Determination of Histamine and Its Metabolite 1-Methylhistamine from Urine
In 2017, Comas-Basté proposed a new diagnostic approach. The principle of this non-invasive test is the determination of histamine and its metabolite 1-methylhistamine from urine by ultra-high performance liquid chromatography and fluorimetry [69]. This approach however is waiting for validation [1,9].
As there is currently no validated diagnostic method which would unequivocally identify the intake of exogenous histamine as a trigger of symptoms, we would recommend the approach described in Figure 5. In future it may be optimal to add the determination of histamine and its metabolites in urine among the diagnostic procedures carried out before the actual elimination of food histamine.
2017년에 Comas-Basté는 새로운 진단 접근법을 제안했습니다. 이 비침습적 검사의 원리는 초고성능 액체 크로마토그래피와 형광 분석법을 통해 소변에서 히스타민과 그 대사산물인 1-메틸히스타민을 측정하는 것입니다[69]. 그러나 이 방법은 검증을 기다리고 있습니다[1,9].
현재 증상을 유발하는 외인성 히스타민 섭취를 명확하게 식별할 수 있는 검증된 진단 방법은 없으므로 그림 5에 설명된 접근법을 권장합니다. 향후에는 실제 음식물 히스타민 제거 전에 수행되는 진단 절차에 소변 내 히스타민 및 그 대사산물의 측정을 추가하는 것이 최선일 수 있습니다.
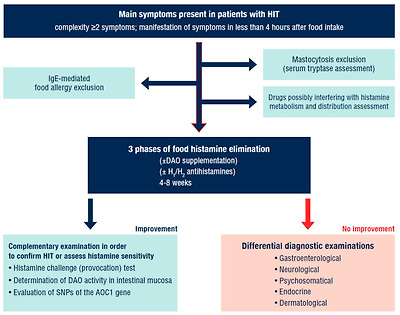
Diagnostic approach in a patient with suspected HIT.
IgE—immunoglobulin E; DAO—diamine oxidase; HIT—histamine intolerance; SNPs—single nucleotide polymorphisms; AOC1—amine oxidase copper containing 1. Adapted according to [1,6,9].
Concerns have been raised regarding fact that HIT suspected patients often receive questionnaires from non-scientific sources and after filling them out they come to the specialist already convinced of their diagnosis and accordingly refuse further examination. Questionnaires from non-scientific sources are not considered to be a reliable diagnostic approach [70].
2.9. Differential Diagnostic Exclusion of Other Diseases
Symptoms triggered by the ingestion of histamine are non-specific. Within diagnostic approaches, the exclusion of allergic disorders is recommended as the first step (IgE-mediated food allergy). The excessive formation of histamine may also be related to rare, relatively well-differentiated specific disorders. For the exclusion of mastocytosis in HIT suspected patients, their serum tryptase levels should be examined [6]. The repeated taking of samples is optimal—during the course of the symptoms and then after their diminution. Chronic disorders (Crohn’s disease, celiac disease) should be excluded, along with a potential infectious noxa (Helicobacter pylori, zoonoses). During these first examinations, it is recommended for the patient to compile a patient’s diary, where the food consumed and symptoms manifested will be recorded. A comprehensible smartphone application developed in cooperation with specialists could be the option.
After the differential diagnostic exclusion of other possible causes of the symptoms, there follows a low-histamine diet, which should be divided into 3 phases (Table 5). When the effect of the diet is elusive, histamine exposure could follow (e.g., the consumption of sauerkraut). The onset of symptoms shortly after exposure and their subsequent remission after dieting resumption may indicate HIT.
히스타민 섭취로 인해 유발되는 증상은
비특이적입니다.
진단 접근법에서는 알레르기 질환을 배제하는 것이 첫 번째 단계로 권장됩니다(IgE 매개 식품 알레르기). 히스타민의 과도한 형성은 희귀하고 비교적 잘 분화된 특정 질환과도 관련이 있을 수 있습니다. HIT 의심 환자의 비만 세포증을 배제하려면 혈청 트립타아제 수치를 검사해야 합니다[6]. 증상이 지속되는 동안과 증상이 감소한 후에 반복적으로 샘플을 채취하는 것이 가장 좋습니다.
만성 질환(크론병, 셀리악병)과
잠재적 감염성 녹사(헬리코박터 파일로리, 인수공통전염병)는 배제해야 합니다.
이러한 첫 번째 검사 동안 환자는 섭취한 음식과 나타난 증상을 기록하는 환자 일기를 작성하는 것이 좋습니다. 전문가와 협력하여 개발한 이해하기 쉬운 스마트폰 애플리케이션이 옵션이 될 수 있습니다.
증상의 다른 가능한 원인을 감별 진단으로 배제한 후 저 히스타민 식단을 따르며, 이는 3 단계로 나누어야합니다 (표 5). 식이요법의 효과를 확인하기 어려운 경우 히스타민 노출(예: 사우어크라우트 섭취)이 뒤따를 수 있습니다. 노출 직후 증상이 시작되고 식이 요법을 재개한 후 증상이 완화되는 것은 HIT를 나타낼 수 있습니다.
Table 5
Phases of dietary measures in patients with suspected HIT. Adapted according to [1].
PhaseObjectiveRecommendationDuration
| Phase 1: Elimination phase | Reduction in symptoms to a maximum possible level | • Change in diet composition - introduction of mixed diet measures with accent on fresh vegetables and reduction of biogenic amine intake, in particular histamine • Nutrient optimization | 10–14 days |
| Phase 2: Test phase | Reintroducing foods excluded in Phase 1, after taking into account individual risk factors (stress, menstruation, medication use etc.) | • Targeted gradual reintroduction of suspected foods taking into consideration patient’s individual dietary preferences • Assessment of individual sensitivity to ingested histamine | Up to 6 weeks |
| Phase 3: Long-term diet | Maintenance of high-quality of life Continual balanced diet | • Individual nutritional recommendations based on individual sensitivity to ingested histamine taking exogenous risk factors into consideration | _ |
If the response to a low-histamine diet is insufficient and no remission of symptoms has been observed, it is recommended to perform further professional examinations with specialists (gastroenterologist, neurologist, endocrinologist, dermatologist).
The HIT syndrome does not necessarily indicate one (transient) feature of the organism. Patients with inflammatory bowel diseases showed decreased serum DAO activity [13,71]. Similarly, in patients with ulcerative colitis, decreased DAO activity in the colonic mucosa has been shown [72]. The AOC1 gene polymorphism was related to the severity of the course of the ulcerative colitis [73]. The hypothesis that decreased DAO activity resulting from a gene polymorphism could act as a risk factor for the development of inflammatory bowel disease has not been proven [74]. It should be stated that with non-celiac gluten sensitivity have been reported intestinal and extra-intestinal symptoms which are very similar to those observed in HIT. Gluten-containing cereals are found in many foods, including bread, pasta, pizza, bulgur and couscous and in beverages such as beer. The majority of these foods and drinks however also contain histamine and/or are usually consumed with other products with a histamine content. Many bakery products containing gluten, and also beer, contain yeasts, thus live microorganisms which may contribute to the production of histamine are used in the process of their production. Bulgur, pasta and pizza are usually consumed with tomatoes and other vegetables which, due to their histamine content or ability to release endogenous histamine, could trigger symptoms in sensitive people [75].
In 18 of 20 patients with refractory celiac disease, Schnedl et al. diagnosed other intolerances/malabsorption and/or H. pylori infection. HIT syndrome was diagnosed in 11 patients. HIT would seem to play a significant role among patients with celiac disease who do not respond to therapeutic measures [76].
저 히스타민 식단에 대한 반응이 불충분하고
증상 완화가 관찰되지 않으면
전문의 (위장병 전문의, 신경과 전문의, 내분비 전문의, 피부과 전문의)와 함께 추가 전문 검사를 수행하는 것이 좋습니다.
HIT 증후군이
반드시 유기체의 한 가지 (일시적인) 특징을 나타내는 것은 아닙니다.
염증성 장 질환 환자는
혈청 DAO 활성이 감소한 것으로 나타났습니다 [13,71].
마찬가지로
궤양성 대장염 환자에서도
대장 점막의 DAO 활성도가 감소한 것으로 나타났습니다 [72].
AOC1 유전자 다형성은 궤양성 대장염의 진행 정도와 관련이 있었습니다 [73]. 유전자 다형성으로 인한 DAO 활성 감소가 염증성 장 질환 발병의 위험 요인으로 작용할 수 있다는 가설은 입증되지 않았습니다 [74]. 셀리악병이 아닌 글루텐 민감성에서도 HIT에서 관찰되는 것과 매우 유사한 장 및 장 외 증상이 보고된 바 있습니다. 글루텐 함유 시리얼은 빵, 파스타, 피자, 불구르, 쿠스쿠스 등 많은 음식과 맥주와 같은 음료에서 발견됩니다. 그러나 이러한 식품과 음료의 대부분은 히스타민을 함유하고 있거나 히스타민이 함유된 다른 제품과 함께 섭취하는 경우가 많습니다. 글루텐이 함유된 많은 베이커리 제품과 맥주에는 효모가 포함되어 있으므로 히스타민 생성에 기여할 수 있는 살아있는 미생물이 생산 과정에 사용됩니다. 불가르, 파스타 및 피자는 일반적으로 히스타민 함량 또는 내인성 히스타민 방출 능력으로 인해 민감한 사람들에게 증상을 유발할 수 있는 토마토 및 기타 야채와 함께 섭취합니다 [75].
불응성 셀리악병 환자 20명 중 18명에서 다른 과민증/흡수 장애 및/또는 H. 파일로리 감염을 진단했습니다. 11명의 환자에서 HIT 증후군이 진단되었습니다. HIT는 치료 조치에 반응하지 않는 셀리악병 환자들 사이에서 중요한 역할을 하는 것으로 보입니다 [76].
3. Therapeutic Approaches with HIT
Among therapeutic approaches, the gold standard is a low-histamine diet. A good response to such a diet is considered to be confirmation of HIT. Alongside the dietary measures, DAO supplementation supporting the degradation of ingested histamine is recommended as subsidiary treatment for individuals with intestinal DAO deficiency [9]. In acute and clinically more severe cases, or in cases where the ingested histamine cannot be completely removed by following the low-histamine diet [38], H1 (or possibly H2) antihistamines can be used (preferably short-term use).
치료 접근법 중 표준은
저히스타민 식단입니다.
이러한 식단에 대한 좋은 반응은
HIT의 확증으로 간주됩니다.
식이 요법과 함께
섭취한 히스타민의 분해를 돕는 DAO 보충제는
장내 DAO 결핍증 환자의 보조 치료법으로 권장됩니다[9].
급성 및 임상적으로 더 심한 경우 또는 저히스타민 식이요법[38]으로 섭취한 히스타민을 완전히 제거할 수 없는 경우에는 H1(또는 H2) 항히스타민제를 사용할 수 있습니다(단기간 사용하는 것이 바람직함).
3.1. Low-Histamine Diet
The principle of the low-histamine diet consists in a choice of foods where an excessive amount of histamine or biogenic amines respectively is not to be expected. In the first phase of the low-histamine diet, foods that typically contain a high amount of histamine (and other biogenic amines) should be completely excluded. There is a great variability between studies regarding type of foods that is recommended to avoid during elimination diet. Some of routinely excluded foods contain only low levels of biogenic amines and become designated as histamine liberators [77]. In Figure 4, we present examples of foods that are often recommended to exclude in low-histamine diet.
Foods that under normal circumstances do not contain high levels of biogenic amines should be consumed as fresh as possible (Table 3).
The low-histamine diet should be temporary and must not stress the patient. The patient should be aware that after certain period some eliminated foods may again be reintroduced to the menu.
Reese et al. proposed 3 phases during which the foods responsible for the symptoms are progressively eliminated and reintroduced respectively (Table 5). Adherence to a low-histamine diet has clearly led to improved gastrointestinal, cutaneous and neurological manifestations [23,78], and in some cases simultaneous raising of DAO serum levels was observed [79].
저히스타민 식단의 원칙은
과도한 양의 히스타민 또는
생체 아민이 함유되지 않은 식품을 선택하는 것입니다.
저히스타민 식단의 첫 단계에서는
일반적으로 히스타민(및 기타 생체 아민)이 다량 함유된 식품을 완전히 배제해야 합니다.
히스타민 제거 식단에서 피해야 하는 음식의 종류에 대해서는 연구마다 큰 차이가 있습니다. 일상적으로 제외되는 식품 중 일부는 낮은 수준의 생체 아민만을 함유하고 있으며 히스타민 해방제로 지정되기도 합니다 [77]. 그림 4에는 저히스타민 식단에서 제외할 것을 권장하는 식품의 예가 나와 있습니다.
정상적인 상황에서
높은 수준의 바이오제닉 아민을 함유하지 않은 식품은
가능한 한 신선하게 섭취해야 합니다(표 3).
저 히스타민 식단은
일시적이어야하며
환자에게 스트레스를 주지 않아야합니다. 환자는 일
정 기간이 지나면 일부 제거된 음식이 다시 메뉴에 다시 도입될 수 있음을 알고 있어야 합니다.
Reese 등은 증상을 유발하는 식품을 점진적으로 제거하고 다시 도입하는 3단계를 제안했습니다(표 5). 저히스타민 식단을 준수하면 위장, 피부 및 신경 증상이 개선되었으며 [23,78], 경우에 따라 DAO 혈청 수치가 동시에 상승하는 것이 관찰되었습니다 [79].
3.2. Exogenous Supply of DAO
Similarly as in lactose intolerance, lactase supplementation is used, the exogenous supply of the DAO enzyme has been proposed for HIT [9,16,32]. The European Food Safety Authority (EFSA) has authorized a porcine kidney extract containing 0.3 mg of DAO enzyme as a novel food. From 2002, it could be distributed on the market as a nutrition supplement and from 2013 as a food for special medical purposes. According to EFSA, the maximum daily dose of exogenously ingested enzyme is 3 × 0.3 mg, which corresponds to 0.9 mg of DAO. The dosage form must be gastro-resistant in order to deliver the enzyme undamaged to the place of presumed effect (small intestine) [80]. It has been demonstrated that DAO exogenous supplementation has improved the symptoms in clinical practice (Table 6).
유당 불내증에서
락타아제 보충제가 사용되는 것과 유사하게,
DAO 효소의 외인성 공급이 HIT에 대해 제안되었습니다 [9,16,32].
유럽 식품 안전청(EFSA)은
0.3mg의 DAO 효소를 함유한 돼지 신장 추출물을 새로운 식품으로 승인했습니다.
2002년부터는 영양 보충제로, 2013년부터는 특수 의료 목적의 식품으로 시중에 유통될 수 있었습니다. EFSA에 따르면 외인성 섭취 효소의 일일 최대 복용량은 3 × 0.3mg이며, 이는 0.9mg의 DAO에 해당합니다. 효소를 예상 효과 장소(소장)에 손상 없이 전달하기 위해서는 제형이 위장 내성이 있어야 합니다[80]. DAO 외인성 보충제가 임상에서 증상을 개선하는 것으로 입증되었습니다(표 6).
Table 6
Summary of clinical studies testing the effects of exogenous supplementation of diamine oxidase obtained from porcine kidneys. DAO—diamine oxidase; DBPC—double-blind placebo controlled; N/A—not available; 5-HT—5 hydroxytryptamine; UAS7—Urticaria Activity Score 7; NS—non-significant; C1—before treatment (consultation no. 1); C2—after treatment (consultation no. 2) [16,58,59,81,82].
StudyTrial DesignInterventionControlSample sizeDuration of InterventionReported Outcomes
| Komericki, 2011 | DBPC cross-over study | 0.5 mg DAO | Placebo | 39 | N/A | A significant improvement in symptoms after DAO administration as compared with placebo. |
| Manzotti, 2016 | Restrospective observational study | 2 × 0.3 mg DAO | N/A | 14 | 14 | 13 patients (93%) reported improvement in ≥ 1 of symptoms. |
| Yacoub, 2018 | DBPC cross-over study | 2 × 0.3 mg DAO | Placebo | 20 | 30 | A significant improvement in Urticaria Activity Score 7 (UAS7) in patients with urticaria unsatisfactorily controlled by antihistamines (p < 0.05). Mild significant reduction in antihistamines consumption (p < 0.05). |
| Izquierdo-Casas, 2019 | Randomized DBPC study | 3 × 0.6 mg DAO | Placebo | 82 | C1 without an intervention (30 days) + C2 with intervention (30 days) | A significant reduction in number (p < 0.001) and duration (p < 0.05) of migraine episodes in intervention group compared with baseline. A decrease in the percentage of patients using selective 5-HT receptor agonists (triptans). |
| Schnedl, 2019 | Open label interventional study | 3 × 0.3 mg DAO | N/A | 28 | 28 + 28 (follow-up) | Significant reduction in frequency and intensity of symptoms. 61% of patients showed mild increase in serum DAO levels (NS). During follow-up, without DAO supplementation, the symptoms sum score increased again and serum DAO levels slightly decreased. |
Despite the promising results, studies dealing with DAO exogenous supplementation are scarce. In addition, the studies conducted investigated only a small sample of patients. It is therefore necessary to confirm the clinical significance of exogenous supplementation of the DAO enzyme by more robust, well-designed clinical trials.
Other Potential Sources of DAO
DAO need not be necessarily of animal origin. In 2020, Comas-Basté carried out screening of the ability of the family of the Leguminosae (legumes) plant to break down histamine. The goal was to identify plants with DAO enzyme activity (in vitro) and then to consider their potential usage as an active component of enzyme supplements. Lyophilization of the sprouts kept their enzyme activity unchanged for at least 12 months. The results show that in the future certain edible legumes could be suitable for the manufacture of supplements with DAO content for the management of HIT [83].
DAO는
반드시 동물성 물질일 필요는 없습니다.
2020년에 코마스 바스테는
콩과 식물인 콩과 식물의 히스타민 분해 능력에 대한 스크리닝을 수행했습니다.
목표는 DAO 효소 활성을 가진 식물을 (시험관 내에서) 확인한 다음 효소 보충제의 활성 성분으로 잠재적 사용을 고려하는 것이었습니다. 새싹을 동결 건조하면 효소 활성이 최소 12개월 동안 변함없이 유지되었습니다. 이 결과는 향후 특정 식용 콩과 식물이 HIT 관리를위한 DAO 함량이있는 보충제 제조에 적합 할 수 있음을 보여줍니다 [83].
3.3. Antihistamines
The treatment of patients with antihistamines is empirical. There exist no randomized clinical trials that prove the contribution of this therapy in HIT. Therapeutic dosages and the choice of the generation and type of antihistamine (H1/H2) are in the competence of the clinician after the manifestation of the symptoms (gastrointestinal, neurological, dermatological) are taken into account; in consideration of effectiveness and safety however, 2nd or 3rd generation of H1 antihistamines should take precedence. H2 blockers could be used in patients with dominant gastrointestinal symptoms (hyperacidity and reflux as manifestations of HIT are also questions to discuss).
Treatment with antihistamines should be conscious and time-limited and should help create a picture of whether H1/H2 receptor blockade attenuates manifestations [1]. However, it may also be used as a therapeutic-diagnostic test.
항히스타민제 환자의 치료는 경험적입니다. HIT에서이 치료법의 기여를 증명하는 무작위 임상 시험은 존재하지 않습니다. 치료 용량과 항히스타민제의 세대 및 유형(H1/H2)의 선택은 증상(위장, 신경, 피부)의 발현을 고려한 후 임상의의 권한에 속하지만, 효과와 안전성을 고려할 때 2세대 또는 3세대 H1 항히스타민제를 우선적으로 사용해야 합니다. 위장관 증상이 우세한 환자에게는 H2 차단제를 사용할 수 있습니다(위산과다 및 역류도 HIT의 증상으로서 논의해야 할 문제입니다).
항히스타민제 치료는 의식적이고 시간 제한적으로 이루어져야 하며, H1/H2 수용체 차단이 증상을 약화시키는지 여부를 파악하는 데 도움이 되어야 합니다[1]. 그러나 치료 진단 검사로도 사용할 수 있습니다.
3.4. Complementary Strategies in HIT Management
Some authors regard supplementation of cofactors of the DAO enzyme as optional adjunctive therapy. Vitamin C, copper or vitamin B6 supplementation may be considered [32,33,40,84].
Supplementation with probiotic microorganisms could lead to such modulations of microbiome that would reduce the production of the microbial enzyme L-histidine decarboxylase. The precondition therefore is the administration of strains that do not produce L-histidine decarboxylase. In an ideal case, these would be strains capable at the same time of degrading histamine (or other biogenic amines). Clinical trials assessing the possible impact of probiotic administration in HIT are not found in literature up to the present.
Arising from experimental trials, it would seem at present that members of Bifidobacterium genus could be considered as candidates for appropriate supplementation, but studies confirming this assumption are warranted [44,46].
일부 저자는 DAO 효소의 보조 인자를 보충하는 것을 선택적 보조 요법으로 간주합니다. 비타민 C, 구리 또는 비타민 B6 보충제를 고려할 수 있습니다[32,33,40,84].
프로바이오틱 미생물을 보충하면 미생물 효소 L- 히스티딘 데카르 복실 라제의 생성을 감소시키는 미생물 군집의 조절로 이어질 수 있습니다. 따라서 전제 조건은 L-히스티딘 탈카르복실화 효소를 생산하지 않는 균주를 투여하는 것입니다. 이상적인 경우, 히스타민(또는 기타 생체 아민)을 동시에 분해할 수 있는 균주여야 합니다. HIT에서 프로바이오틱스 투여의 가능한 영향을 평가하는 임상 시험은 현재까지 문헌에서 찾을 수 없습니다.
실험적 실험을 통해 현재로서는 비피도박테리움 속의 구성원이 적절한 보충제 후보로 고려될 수 있는 것으로 보이지만, 이러한 가정을 확인하는 연구가 필요합니다 [44,46].
4. Conclusions
HIT represents a set of diverse symptoms that appear following the ingestion of food with a content of such amounts of histamine that usually do not provoke symptoms in a healthy person. Manifestations may be caused by various pathophysiological mechanisms, or a combination of them. It would seem that symptoms typical for HIT in an individual may potentiate the presence of other disorders such as, e.g., celiac disease or H. pylori infection. The diagnosing of HIT therefore requires a complex time-demanding multidisciplinary approach, including the systematic elimination of disorders with a similar manifestation of symptoms. A low-histamine diet is currently a suitable (not however sole) diagnostic and at the same time therapeutic measure. Recently, evidence has been growing regarding the therapeutic contribution of food for special medical purposes containing a DAO enzyme of animal origin. H1/H2 antihistamines may also be considered in management of HIT, with the daily dosage (usually higher than the standard) determined by a clinician on the basis of the patient’s clinical condition. Pharmacological treatment should have a limited time duration. The aim of the therapeutic approach should be to develop such dietary habits which will not in the future lead to the triggering of symptoms.
HIT는 일반적으로 건강한 사람에게 증상을 유발하지 않는 양의 히스타민이 함유된 음식을 섭취한 후 나타나는 일련의 다양한 증상을 나타냅니다. 증상은 다양한 병리 생리학적 메커니즘 또는 이들의 조합에 의해 발생할 수 있습니다. 한 개인에게 나타나는 전형적인 HIT 증상은 셀리악병이나 H. 파일로리 감염과 같은 다른 질환의 존재를 강화할 수 있는 것으로 보입니다. 따라서 HIT를 진단하려면 유사한 증상을 보이는 질환을 체계적으로 제거하는 등 복잡하고 시간이 많이 걸리는 다학제적 접근이 필요합니다. 저히스타민 식단은 현재 적절한(그러나 유일한 것은 아니지만) 진단 방법이자 동시에 치료 방법입니다. 최근에는 동물성 DAO 효소가 함유된 특수 의료용 식품의 치료적 기여에 대한 증거가 증가하고 있습니다. H1/H2 항히스타민제는 환자의 임상 상태에 따라 임상의가 일일 복용량(일반적으로 표준보다 높음)을 결정하여 HIT 관리에 고려할 수도 있습니다. 약물 치료는 기간이 제한되어 있어야 합니다. 치료적 접근의 목표는 향후 증상을 유발하지 않는 식습관을 개발하는 것이어야 합니다.
Acknowledgments
The authors would like to thank Kevin M. Slavin for his prompt and precise revision of the English text. The authors would like to thank also Miroslava Nikodemová and Mária Škultéty for help with visualization.
Author Contributions
Each author of the manuscript has contributed to conception of this review. M.H. (Martin Hrubisko) and R.D. wrote the draft of the manuscript. M.H. (Martin Huorka) made suggestions and corrections. M.W. made suggestions and critical supervision. All authors declare an approval of the final manuscript. Authors M.H. (Martin Hrubisko) and R.D. contributed equally to this article 35%. Author M.H. (Martin Huorka) contributed 10%. Author M.W. contributed 20%. All authors have read and agreed to the published version of the manuscript.
Funding
Publication of this manuscript was funded by the authors.
Conflicts of Interest
The authors declare no conflict of interest at the time of submission of the manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
1. Reese I., Ballmer-Weber B., Beyer K., Fuchs T., Kleine-Tebbe J., Klimek L., Lepp U., Niggemann B., Saloga J., Schäfer C., et al. German guideline for the management of adverse reactions to ingested histamine. Allergo J. Int. 2017;26:72–79. doi: 10.1007/s40629-017-0011-5. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
2. Sicherer S.H. Epidemiology of food allergy. J. Allergy Clin. Immunol. 2011;127:594–602. doi: 10.1016/j.jaci.2010.11.044. [PubMed] [CrossRef] [Google Scholar]
3. Stapel S.O., Asero R., Ballmer-Weber B.K., Knol E.F., Strobel S., Vieths S., Kleine-Tebbe J. Testing for IgG4 against foods is not recommended as a diagnostic tool: EAACI task force report. Allergy. 2008;63:793–796. doi: 10.1111/j.1398-9995.2008.01705.x. [PubMed] [CrossRef] [Google Scholar]
4. Burks A.W., Tang M., Sicherer S., Muraro A., Eigenmann P.A., Ebisawa M., Fiocchi A., Chiang W., Beyer K., Wood R., et al. ICON: Food allergy. J. Allergy Clin. Immunol. 2012;129:906–920. doi: 10.1016/j.jaci.2012.02.001. [PubMed] [CrossRef] [Google Scholar]
5. Matricon J., Meleine M., Gelot A., Piche T., Dapoigny M., Müller E., Ardid D. Associations between immune activation, intestinal permeability and the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2012;36:1009–1031. doi: 10.1111/apt.12080. [PubMed] [CrossRef] [Google Scholar]
6. Tuck C.J., Biesiekierski J.R., Schmid-Grendelmeier P., Pohl D. Food intolerances. Nutrients. 2019;11:1684. doi: 10.3390/nu11071684. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
7. Johansson S.G.O., Bieber T., Dahl R., Friedmann P.S., Lanier B.Q., Lockey R.F., Motala C. Revised nomenclature for allergy for global use: Report of the nomenclature review committee of the World Allergy Organization, October 2003. J. Allergy Clin. Immunol. 2004;113:832–836. doi: 10.1016/j.jaci.2003.12.591. [PubMed] [CrossRef] [Google Scholar]
8. Lomer M.C.E. The aetiology, diagnosis, mechanisms and clinical evidence for food intolerance. Aliment. Pharmacol. Ther. 2015;41:262–275. doi: 10.1111/apt.13041. [PubMed] [CrossRef] [Google Scholar]
9. Comas-Basté O., Sánchez-Pérez S., Veciana-Nogués M.T., Latorre-Moratalla M., del Carmen Vidal-Carou M. Histamine intolerance: The current state of the art. Biomolecules. 2020;10:1181. doi: 10.3390/biom10081181. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
10. Colombo F.M., Cattaneo P., Confalonieri E., Bernardi C. Histamine food poisonings: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2018;58:1131–1151. doi: 10.1080/10408398.2016.1242476. [PubMed] [CrossRef] [Google Scholar]
11. Yu Y., Wang P., Bian L., Hong S. Rare death via histamine poisoning following crab consumption: A case report. J. Forensic Sci. 2017;63:980–982. doi: 10.1111/1556-4029.13611. [PubMed] [CrossRef] [Google Scholar]
12. Afify S.M., Pali-Schöll I. Adverse reactions to food: The female dominance—A secondary publication and update. World Allergy Organ. J. 2017;10:43. doi: 10.1186/s40413-017-0174-z. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
13. Maintz L., Novak N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007;85:1185–1196. doi: 10.1093/ajcn/85.5.1185. [PubMed] [CrossRef] [Google Scholar]
14. Hamada Y., Shinohara Y., Yano M., Yamamoto M., Yoshio M., Satake K., Toda A., Hirai M., Usami M. Effect of the menstrual cycle on serum diamine oxidase levels in healthy women. Clin. Biochem. 2013;46:99–102. doi: 10.1016/j.clinbiochem.2012.10.013. [PubMed] [CrossRef] [Google Scholar]
15. Jarisch R. Histamine Intolerance. Springer; Berlin, Germany: 2014. Histamine intolerance in women; pp. 109–115. [Google Scholar]
16. Komericki P., Klein G., Reider N., Hawranek T., Strimitzer T., Lang R., Kranzelbinder B., Aberer W. Histamine intolerance: Lack of reproducibility of single symptoms by oral provocation with histamine: A randomised, double-blind, placebo-controlled cross-over study. Wien. Klin. Wochenschr. 2010;123:15–20. doi: 10.1007/s00508-010-1506-y. [PubMed] [CrossRef] [Google Scholar]
17. Alnouri G., Cha N., Sataloff R.T. Histamine sensitivity: An uncommon recognized cause of living laryngopharyngeal reflux symptoms and signs—A case report. Ear Nose Throat J. 2020 doi: 10.1177/0145561320951071. [PubMed] [CrossRef] [Google Scholar]
18. Ansaranta M., Kauppi P., Malmberg L.P., Vilkman E., Geneid A. Inspiratory and expiratory flow changes, voice symptoms and laryngeal findings during Histamine Challenge tests. Folia Phoniatr. Logop. 2019;72:29–35. doi: 10.1159/000495783. [PubMed] [CrossRef] [Google Scholar]
19. Waldum H.L., Sørdal Ø.F., Mjønes P.G. The enterochromaffin-like [ECL] cell—Central in gastric physiology and pathology. Int. J. Mol. Sci. 2019;20:2444. doi: 10.3390/ijms20102444. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
20. Yoshikawa T., Nakamura T., Yanai K. Histaminergic neurons in the tuberomammillary nucleus as a control centre for wakefulness. Br. J. Pharmacol. 2021;178:750–769. doi: 10.1111/bph.15220. [PubMed] [CrossRef] [Google Scholar]
21. Kucher A.N. Association of polymorphic variants of key histamine metabolism genes and histamine receptor genes with multifactorial diseases. Russ. J. Genet. 2019;55:794–814. doi: 10.1134/S102279541907010X. [CrossRef] [Google Scholar]
22. Scammell T.E., Jackson A.C., Franks N.P., Wisden W., Dauvilliers Y. Histamine: Neural circuits and new medications. Sleep. 2019;42:183. doi: 10.1093/sleep/zsy183. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
23. Sánchez-Pérez S., Comas-Basté O., Rabell-González J., Veciana-Nogués M.T., Latorre-Moratalla M.L., Vidal-Carou M.C. Biogenic amines in plant-origin foods: Are they frequently underestimated in low-histamine diets? Foods. 2018;7:205. doi: 10.3390/foods7120205. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
24. Schnedl W.J., Enko D. Considering histamine in functional gastrointestinal disorders. Crit. Rev. Food Sci. Nutr. 2020:1–8. doi: 10.1080/10408398.2020.1791049. [PubMed] [CrossRef] [Google Scholar]
25. Laroche D., Vergnaud M.-C., Sillard B., Soufarapis H., Bricard H. Biochemical markers of anaphylactoid reactions to drugs comparison of plasma histamine and tryptase. J. Am. Soc. Anesthesiol. 1991;75:945–949. doi: 10.1097/00000542-199112000-00004. [PubMed] [CrossRef] [Google Scholar]
26. Vakal S., Jalkanen S., Dahlström K.M., Salminen T.A. Human copper-containing amine oxidases in drug design and development. Molecules. 2020;25:1293. doi: 10.3390/molecules25061293. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
27. Boehm T., Pils S., Gludovacz E., Szoelloesi H., Petroczi K., Majdic O., Quaroni A., Borth N., Valent P., Jilma B. Quantification of human diamine oxidase. Clin. Biochem. 2017;50:444–451. doi: 10.1016/j.clinbiochem.2016.12.011. [PubMed] [CrossRef] [Google Scholar]
28. Schwelberger H.G., Feurle J., Ahrens F. Characterization of diamine oxidase from human seminal plasma. J. Neural Transm. 2013;120:983–986. doi: 10.1007/s00702-013-0983-3. [PubMed] [CrossRef] [Google Scholar]
29. McGrath A.P., Hilmer K.M., Collyer C.A., Shepard E.M., Elmore B.O., Brown D.E., Dooley D.M., Guss M. Structure and inhibition of human diamine oxidase. Biochemistry. 2009;48:9810–9822. doi: 10.1021/bi9014192. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
30. Ji Y., Sakata Y., Li X., Zhang C., Yang Q., Xu M., Wollin A., Langhans W., Tso P. Lymphatic diamine oxidase secretion stimulated by fat absorption is linked with histamine release. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;304:G732–G740. doi: 10.1152/ajpgi.00399.2012. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
31. Leitner R., Zoernpfenning E., Missbichler A. Evaluation of the inhibitory effect of various drugs/active ingredients on the activity of human diamine oxidase in vitro. Clin. Transl. Allergy. 2014;4:P23. doi: 10.1186/2045-7022-4-S3-P23. [CrossRef] [Google Scholar]
32. Kovacova-Hanuskova E., Buday T., Gavliakova S., Plevkova J. Histamine, histamine intoxication and intolerance. Allergol. Immunopathol. 2015;43:498–506. doi: 10.1016/j.aller.2015.05.001. [PubMed] [CrossRef] [Google Scholar]
33. Rosell-Camps A., Zibetti S., Pérez-Esteban G., Vila-Vidal M., Ferrés-Ramis L., García-Teresa-García E. Histamine intolerance as a cause of chronic digestive complaints in pediatric patients. Rev. Esp. Enferm. Dig. 2013;105:201–207. doi: 10.4321/S1130-01082013000400004. [PubMed] [CrossRef] [Google Scholar]
34. Kacik J., Wróblewska B., Lewicki S., Zdanowski R., Kalicki B. Advances in Experimental Medicine and Biology. Volume 1039. Springer; Cham, Switzerland: 2017. Serum diamine oxidase in pseudoallergy in the pediatric population; pp. 35–44. [PubMed] [Google Scholar]
35. Nei D. Evaluation of non-bacterial factors contributing to histamine accumulation in fish fillets. Food Control. 2014;35:142–145. doi: 10.1016/j.foodcont.2013.06.037. [CrossRef] [Google Scholar]
36. Verma N., Hooda V., Gahlaut A., Gothwal A., Hooda V. Enzymatic biosensors for the quantification of biogenic amines: A literature update. Crit. Rev. Biotechnol. 2019;40:1–14. doi: 10.1080/07388551.2019.1680600. [PubMed] [CrossRef] [Google Scholar]
37. D’Andrea G., D’Arrigo A., Carbonare M.D., Leon A. Pathogenesis of migraine: Role of neuromodulators. Headache. 2012;52:1155–1163. doi: 10.1111/j.1526-4610.2012.02168.x. [PubMed] [CrossRef] [Google Scholar]
38. Kohn J.B. Is there a diet for histamine intolerance? J. Acad. Nutr. Diet. 2014;114:1860. doi: 10.1016/j.jand.2014.09.009. [PubMed] [CrossRef] [Google Scholar]
39. Moniente M., García-Gonzalo D., Ontañón I., Pagán R., Botello-Morte L. Histamine accumulation in dairy products: Microbial causes, techniques for the detection of histamine-producing microbiota, and potential solutions. Compr. Rev. Food Sci. Food Saf. 2021;20:1481–1523. doi: 10.1111/1541-4337.12704. [PubMed] [CrossRef] [Google Scholar]
40. Martin I.S.M., Brachero S., Vilar E.G. Histamine intolerance and dietary management: A complete review. Allergol. Immunopathol. 2016;44:475–483. doi: 10.1016/j.aller.2016.04.015. [PubMed] [CrossRef] [Google Scholar]
41. Scalzo R.L., Fibiani M., Francese G., D’Alessandro A., Rotino G.L., Conte P., Mennella G. Cooking influence on physico-chemical fruit characteristics of eggplant (Solanum melongena L.) Food Chem. 2016;194:835–842. doi: 10.1016/j.foodchem.2015.08.063. [PubMed] [CrossRef] [Google Scholar]
42. Preti R., Rapa M., Vinci G. Effect of steaming and boiling on the antioxidant properties and biogenic amines content in green bean (Phaseolus vulgaris) varieties of different colours. J. Food Qual. 2017;2017:5329070. doi: 10.1155/2017/5329070. [CrossRef] [Google Scholar]
43. Chung B.Y., Park S.Y., Byun Y.S., Son J.H., Choi Y.W., Cho Y.S., Kim H.O., Park C.W. Effect of different cooking methods on histamine levels in selected foods. Ann. Dermatol. 2017;29:706–714. doi: 10.5021/ad.2017.29.6.706. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
44. Schink M., Konturek P.C., Tietz E., Dieterich W., Pinzer T.C., Wirtz S., Neurath M.F., Zopf Y. Microbial patterns in patients with histamine intolerance. J. Physiol. Pharmacol. 2018;69:579–593. [PubMed] [Google Scholar]
45. O’Callaghan A., van Sinderen D. Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 2016;7:925. doi: 10.3389/fmicb.2016.00925. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
46. Mokhtar S., Mostafa G., Taha R., Eldeep G.S.S. Effect of different starter cultures on the biogenic amines production as a critical control point in fresh fermented sausages. Eur. Food Res. Technol. 2012;235:527–535. doi: 10.1007/s00217-012-1777-9. [CrossRef] [Google Scholar]
47. Priyadarshani D., Mesthri W., Rakshit S.K. Screening selected strains of probiotic lactic acid bacteria for their ability to produce biogenic amines (histamine and tyramine) Int. J. Food Sci. Technol. 2011;46:2062–2069. doi: 10.1111/j.1365-2621.2011.02717.x. [CrossRef] [Google Scholar]
48. Coton M., Romano A., Spano G., Ziegler K., Vetrana C., Desmarais C., Lonvaud-Funel A., Lucas P., Coton E. Occurrence of biogenic amine-forming lactic acid bacteria in wine and cider. Food Microbiol. 2010;27:1078–1085. doi: 10.1016/j.fm.2010.07.012. [PubMed] [CrossRef] [Google Scholar]
49. Poveda J.M., Ruiz P., Sesena S., Palop M.L. Occurrence of biogenic amine-forming lactic acid bacteria during a craft brewing process. LWT Food Sci. Technol. 2017;85:129–136. doi: 10.1016/j.lwt.2017.07.003. [CrossRef] [Google Scholar]
50. Ferstl R., Frei R., Schiavi E., Konieczna P., Barcik W., Ziegler M., Lauener R.P., Chassard C., Lacroix C., Akdis C.A., et al. Histamine receptor 2 is a key influence in immune responses to intestinal histamine-secreting microbes. J. Allergy Clin. Immunol. 2014;134:744–746.e3. doi: 10.1016/j.jaci.2014.04.034. [PubMed] [CrossRef] [Google Scholar]
51. Spinler J.K., Sontakke A., Hollister E.B., Venable S.F., Oh P.L., Balderas M.A., Saulnier D.M., Mistretta T.-A., Devaraj S., Walter J., et al. From prediction to function using evolutionary genomics: Human-specific ecotypes of lactobacillus reuteri have diverse probiotic functions. Genome Biol. Evol. 2014;6:1772–1789. doi: 10.1093/gbe/evu137. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
52. Wechsler D., Irmler S., Berthoud H., Portmann R., Badertscher R., Bisig W., Schafroth K., Fröhlich-Wyder M.-T. Influence of the inoculum level of Lactobacillus parabuchneri in vat milk and of the cheese-making conditions on histamine formation during ripening. Int. Dairy J. 2021;113:104883. doi: 10.1016/j.idairyj.2020.104883. [CrossRef] [Google Scholar]
53. Frei R., Ferstl R., Konieczna P., Ziegler M., Simon T., Rugeles T.M., Mailand S., Watanabe T., Lauener R., Akdis C.A., et al. Histamine receptor 2 modifies dendritic cell responses to microbial ligands. J. Allergy Clin. Immunol. 2013;132:194–204. doi: 10.1016/j.jaci.2013.01.013. [PubMed] [CrossRef] [Google Scholar]
54. Oksaharju A., Kankainen M., Kekkonen R.A., Lindstedt K.A., Kovanen P.T., Korpela R., Miettinen M. Probiotic lactobacillus rhamnosus downregulates FCER1 and HRH4 expression in human mast cells. World J. Gastroenterol. 2011;17:750. doi: 10.3748/wjg.v17.i6.750. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
55. Alvarez M.A., Moreno-Arribas M.V. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci. Technol. 2014;39:146–155. doi: 10.1016/j.tifs.2014.07.007. [CrossRef] [Google Scholar]
56. Tittarelli F., Perpetuini G., Di Gianvito P., Tofalo R. Biogenic amines producing and degrading bacteria: A snapshot from raw ewes’ cheese. Lebensm. Wiss. Technol. 2019;101:1–9. doi: 10.1016/j.lwt.2018.11.030. [CrossRef] [Google Scholar]
57. Kung H.-F., Lee Y.-C., Huang Y.-L., Huang Y.R., Su Y.-C., Tsai Y.-H. Degradation of histamine by lactobacillus plantarum isolated from miso products. J. Food Prot. 2017;80:1682–1688. doi: 10.4315/0362-028X.JFP-17-135. [PubMed] [CrossRef] [Google Scholar]
58. Schnedl W.J., Schenk M., Lackner S., Enko D., Mangge H., Forster F. Diamine oxidase supplementation improves symptoms in patients with histamine intolerance. Food Sci. Biotechnol. 2019;28:1779–1784. doi: 10.1007/s10068-019-00627-3. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
59. Manzotti G., Breda D., Di Gioacchino M., Burastero S. Serum diamine oxidase activity in patients with histamine intolerance. Int. J. Immunopathol. Pharmacol. 2016;29:105–111. doi: 10.1177/0394632015617170. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
60. Mušič E., Korošec P., Šilar M., Adamič K., Košnik M., Rijavec M. Serum diamine oxidase activity as a diagnostic test for histamine intolerance. Wien. Klin. Wochenschr. 2013;125:239–243. doi: 10.1007/s00508-013-0354-y. [PubMed] [CrossRef] [Google Scholar]
61. Peralta T., Carla B., Beltran-Ortiz C., Magdalena D., Verónica R., Antonieta G.M., Pablo F. Histamine intolerance: Clinical characterization and determination of serum diamine oxidase (Dao) in a series of cases and controls. Res. Sq. 2020 doi: 10.21203/rs.3.rs-60226/v1. [CrossRef] [Google Scholar]
62. Schwelberger H.G., Feurle J., Houen G. New tools for studying old questions: Antibodies for human diamine oxidase. J. Neural Transm. 2012;120:1019–1026. doi: 10.1007/s00702-012-0936-2. [PubMed] [CrossRef] [Google Scholar]
63. Pinzer T.C., Tietz E., Waldmann E., Schink M., Neurath M.F., Zopf Y. Circadian profiling reveals higher histamine plasma levels and lower diamine oxidase serum activities in 24% of patients with suspected histamine intolerance compared to food allergy and controls. Allergy. 2017;73:949–957. doi: 10.1111/all.13361. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
64. Lessof M.H., Gant V., Hinuma K., Murphy G.M., Dowling R.H. Recurrent urticaria and reduced diamine oxidase activity. Clin. Exp. Allergy. 1990;20:373–376. doi: 10.1111/j.1365-2222.1990.tb02796.x. [PubMed] [CrossRef] [Google Scholar]
65. Schwelberger H.G., Weidenhiller M., Hahn E.G., Raithel M., Kuefner M.A. Both catabolic pathways of histamine via histamine-N-methyltransferase and diamine oxidase are diminished in the colonic mucosa of patients with food allergy. Inflamm. Res. 2004;53:S31–S32. doi: 10.1007/s00011-003-0314-5. [PubMed] [CrossRef] [Google Scholar]
66. Wagner A., Buczyłko K., Zielińska-Bliźniewska H., Wagner W. Impaired resolution of wheals in the skin prick test and low diamine oxidase blood level in allergic patients. Adv. Dermatol. Allergol. Postȩp. Dermatol. Alergol. 2019;36:538–543. doi: 10.5114/ada.2019.89504. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
67. Giera B., Straube S., Konturek P., Hahn E.G., Raithel M. Plasma histamine levels and symptoms in double blind placebo controlled histamine provocation. Inflamm. Res. 2008;57:73–74. doi: 10.1007/s00011-007-0636-9. [PubMed] [CrossRef] [Google Scholar]
68. Wöhrl S., Hemmer W., Focke M., Rappersberger K., Jarisch R. Histamine intolerance-like symptoms in healthy volunteers after oral provocation with liquid histamine. Allergy Asthma Proc. 2004;25:305–311. [PubMed] [Google Scholar]
69. Comas-Basté O., Latorre-Moratalla M., Bernacchia R., Veciana-Nogués M., Vidal-Carou M. New approach for the diagnosis of histamine intolerance based on the determination of histamine and methylhistamine in urine. J. Pharm. Biomed. Anal. 2017;145:379–385. doi: 10.1016/j.jpba.2017.06.029. [PubMed] [CrossRef] [Google Scholar]


