음식먹고 탈날 때
음식을 먹고 이유를 알수없는 불편함, 피부증상, 배아픈 증상, 두통, 혈압상승 등의 증상이 있을 때
히스타민 과민증
히스타민 중독증 - 스콤브로이드 증후군
글루텐 유당 과당 설탕 병원유발요인
이외 질병요인의 탐구... 꽤 많다는 사실...
Open AccessReview
Molecular Mechanisms of Scombroid Food Poisoning
by
Yury V. Zhernov
1,2,3,
add Show full affiliation list
*
Author to whom correspondence should be addressed.
Int. J. Mol. Sci. 2023, 24(1), 809; https://doi.org/10.3390/ijms24010809
Submission received: 15 October 2022 / Revised: 1 December 2022 / Accepted: 22 December 2022 / Published: 3 January 2023
Abstract
Scombroid food poisoning (SFP) is a foodborne disease that develops after consumption of fresh fish and, rarely, seafood that has fine organoleptic characteristics but contains a large amount of exogenous histamine. SFP, like other food pseudo-allergic reactions (FPA), is a disorder that is clinically identical to allergic reactions type I, but there are many differences in their pathogenesis. To date, SFP has been widespread throughout the world and is an urgent problem, although exact epidemiological data on incidence varies greatly. The need to distinguish SFP from true IgE-associated allergy to fish and seafood is one of the most difficult examples of the differential diagnosis of allergic conditions. The most important difference is the absence of an IgE response in SFP. The pathogenesis of SFP includes a complex system of interactions between the body and chemical triggers such as exogenous histamine, other biogenic amines, cis-urocanic acid, salicylates, and other histamine liberators.
Because of the wide range of molecular pathways involved in this process, it is critical to understand their differences. This may help predict and prevent poor outcomes in patients and contribute to the development of adequate hygienic rules and regulations for seafood product safety. Despite the vast and lengthy history of research on SFP mechanisms, there are still many blank spots in our understanding of this condition. The goals of this review are to differentiate various molecular mechanisms of SFP and describe methods of hygienic regulation of some biogenic amines that influence the concentration of histamine in the human body and play an important role in the mechanism of SFP.
스콤브로이드 식중독(SFP)은
신선한 생선 및
드물게는 미각적 특성은 좋지만
외인성 히스타민이 다량 함유된 해산물을 섭취한 후 발생하는 식품 매개성 질환입니다.
SFP는
다른 식품 유사 알레르기 반응(FPA)과 마찬가지로
임상적으로 알레르기 반응 1형과 동일하지만
발병 기전에 많은 차이가 있는 질환입니다.
현재까지 SFP는
전 세계적으로 널리 퍼져 있으며 시급한 문제이지만,
발병률에 대한 정확한 역학 데이터는 매우 다양합니다.
생선 및 해산물에 대한 진정한 IgE 관련 알레르기와
SFP를 구별해야 하는 것은
알레르기 질환의 감별 진단에서 가장 어려운 사례 중 하나입니다.
가장 중요한 차이점은
SFP에서 IgE 반응이 없다는 것입니다.
SFP의 발병 기전에는
외인성 히스타민,
기타 생체 아민,
시스-우로칸산,
살리실산염 및 기타 히스타민 해방제 histamine liberator 와 같은
신체와 화학 유발 물질 간의 복잡한 상호 작용 시스템이 포함됩니다.
이 과정에는
다양한 분자 경로가 관여하기 때문에
그 차이점을 이해하는 것이 중요합니다.
이는 환자의 좋지 않은 결과를 예측 및 예방하고
해산물 제품 안전을 위한
적절한 위생 규칙과 규정을 개발하는 데 도움이 될 수 있습니다.
SFP 메커니즘에 대한 방대하고 오랜 연구 역사에도 불구하고
이 질환에 대한 이해에는 여전히 많은 공백이 존재합니다.
이 리뷰의 목표는
SFP의 다양한 분자 메커니즘을 구분하고
인체 내 히스타민 농도에 영향을 미치고
SFP 메커니즘에 중요한 역할을 하는
일부 생체 아민의 위생적 조절 방법을 설명하는 것입니다.
Keywords:
food hypersensitivity; pseudo-allergy; mast cells; non-immunologic food intolerance; scombroid food poisoning; scombrotoxicosis
1. Introduction
Food pseudo-allergic reactions (FPA), also known as false food allergies (FFA), are one type of food hypersensitivity under which a pathological process is clinically similar to food allergic reaction type I, but has no immunological stage [1,2]. Currently, non-immunological adverse reactions to food, including FPA, are prevalent in 15–20% of the population, which is four times as much as true food allergies [3]. A special type of FPA is scombroid food poisoning (SFP), also known as scombrotoxicosis, scombrotoxism, scombroid ichthyotoxicosis, or Mahi-Mahi flush [4]. To date, SFP has been widespread throughout the world and is an urgent problem, although exact epidemiological data on incidence varies greatly. SFP is considered to be responsible for approximately 40% of all food intoxications in the EU and the US [5]. The incidence ranges from 2–5 cases per million people in New Zealand, France, and Denmark to 31 cases per million people in Hawaii [5]. Despite the prevalence of SFP, healthcare professionals’ awareness of this disease is low. Thus, according to a prospective descriptive cross-sectional study in Tanzania, the awareness of healthcare professionals about SFP was 60%, of which only 6.3% had good knowledge [6].
The clinical presentation of SFP is similar to that of an IgE-associated food allergy reaction. It may include hives, angioneurotic oedema, gastrointestinal symptoms, bronchospasm, skin redness, headache, oedema, hypotension, and shock [7,8]. Cases are known when such an FPA reaction as SFP leads to severe intoxication and death [9,10,11,12,13]. Therefore, for correct SFP diagnosis, it is important to evaluate the patient’s previous allergic anamnesis and indirect epidemiologic signs, and to perform a molecular allergology examination, such as ImmunoCAP (Immuno Solid-phase Allergy Chip) ISAC assay, MeDALL allergen-chip assay, or highly sensitive ELISA-based assay, which will help rule out a true IgE-associated allergy to fish and seafood associated with sensitization to parvalbumins [14,15,16,17].
There are no antigen-specific immune complexes in the SFP molecular mechanism, but there is histamine release, complement activation, atypical eicosanoids synthesis, and inhibition of bradykinin decomposition. The basis of SFP is the non-specific release of the inflammatory mediators from the target cells when mast cells are affected directly by the food antigens and indirectly when the complement, kinine, and other biological systems induce the clinical symptoms of allergy [18,19,20]. However, the molecular mechanisms of this disease are still poorly understood.
This review aims to provide a modern understanding of the molecular mechanisms of FPA reactions as well as a discussion of recent discoveries in the mechanism of formation of SFP, which can be considered on the one hand as one of the variants of food hypersensitivity with a predominance of pseudo-allergic reactions, and on the other hand as a typical food intoxication. Understanding the molecular mechanisms of food pseudo-allergic reactions, such as SFP, is important for differential diagnosis and personalized treatment of such conditions, as well as improving the quality of life of patients.
가짜 식품 알레르기(FFA false food allergy )라고도 알려진
식품 유사 알레르기 반응(FPA)은
병리학적인 과정이 식품 알레르기 반응 유형 I과 임상적으로 유사하지만
면역학적 단계가 없는 식품 과민증의 한 유형입니다[1,2].
현재
FPA를 포함한 식품에 대한
비면역학적 이상 반응은
인구의 15~20%에서 발생하며,
이는 실제 식품 알레르기의 4배에 달하는 수치입니다[3].
특별한 유형의 FPA는
스콤브로독증, 스콤브로독증, 스콤브로독증 또는 마히-마히 홍조[4]로도 알려진
스콤브로이드 식중독(SFP)입니다.
현재까지 SFP는
전 세계적으로 널리 퍼져 있으며 시급한 문제이지만,
발생률에 대한 정확한 역학 데이터는 매우 다양합니다.
SFP는 EU와 미국에서
전체 식중독의 약 40%를 차지하는 것으로 알려져 있습니다[5].
뉴질랜드, 프랑스, 덴마크에서는
인구 백만 명당 2~5건에서
하와이에서는 인구 백만 명당 31건까지 발생하고 있습니다[5].
SFP의 유병률에도 불구하고
이 질병에 대한 의료 전문가의 인식은 낮습니다.
탄자니아에서 실시한 전향적 서술적 횡단면 연구에 따르면
의료 전문가들의 SFP에 대한 인지도는 60%였으며,
이 중 6.3%만이 제대로 알고 있는 것으로 나타났습니다[6].
SFP의 임상 증상은
IgE 관련 식품 알레르기 반응과 유사합니다.
두드러기,
혈관 신경성 부종,
위장 증상,
기관지 경련,
피부 발적,
두통,
부종,
저혈압 및 쇼크가 포함될 수 있습니다 [7,8].
SFP와 같은 FPA 반응이 심각한 중독과 사망으로 이어지는 경우도 알려져 있습니다[9,10,11,12,13].
따라서 정확한 SFP 진단을 위해서는
환자의 이전 알레르기 기억상실증과
간접적인 역학 징후를 평가하고,
파발부민 감작과 관련된 생선 및 해산물에 대한
진정한 IgE 관련 알레르기를 배제하는 데 도움이 되는
ImmunoCAP(면역 고상 알레르기 칩) ISAC 분석,
MeDALL 알레르겐 칩 분석 또는
고감도 ELISA 기반 분석과 같은 분자 알레르기 검사를 수행하는 것이 중요합니다 [14,15,16,17].
SFP 분자 메커니즘에는
항원 특이 면역 복합체는 없지만
히스타민 방출,
보체 활성화,
비정형 에이코사노이드 합성 및
브라디키닌 분해 억제가 있습니다.
histamine release,
complement activation,
atypical eicosanoids synthesis, and
inhibition of bradykinin decomposition

SFP의 기본은
비만 세포가
식품 항원에 의해 직접적으로 영향을 받고
보체,
키닌 및
기타 생물학적 시스템이
알레르기의 임상 증상을 유발할 때
간접적으로 표적 세포에서 염증 매개체가
비특이적으로 방출되는 것입니다 [18,19,20].
그러나
이 질환의 분자적 메커니즘은
아직 잘 알려져 있지 않습니다.
이 리뷰는 한편으로는
의사 알레르기 반응이 우세한 식품 과민증의 변종 중 하나로,
다른 한편으로는 전형적인 식품 중독으로 간주 될 수있는
SFP의 형성 메커니즘에 대한 최근 발견에 대한 논의뿐만 아니라
FPA 반응의 분자 메커니즘에 대한 현대적인 이해를 제공하는 것을 목표로합니다.
SFP와 같은 식
품 유사 알레르기 반응의 분자 메커니즘을 이해하는 것은
이러한 질환의 감별 진단과 개인 맞춤형 치료는
물론 환자의 삶의 질을 개선하는 데 중요합니다.
2. Contributing Factors of Scombroid Food Poisoning
SFP is a foodborne disease that develops after consumption of fresh fish and, rarely, seafood that has fine organoleptic characteristics but contains a large amount of exogenous histamine. According to statistics, exogenous histamine concentration in the blood of 1–2 ng/mL leads to increased gastric secretion and heart rate; 3–5 ng/mL causes headache, itching, and tachycardia; 6–8 ng/mL lowers blood pressure; 7–12 ng/mL induces bronchospasm, and concentration over 100 ng/mL leads to heart arrest [21]. To evaluate the mean histamine concentration in fish that leads to poisoning in 2017, Columbo et al. conducted a review with an analysis of the cases of histamine poisoning and came to the conclusion that SFP occurs with a mean concentration of 1100 mg/kg and that it varies from 423 to 2900 mg/kg [22]. Obviously, such significant dispersion may be caused by a large number of mechanisms, from exogenous histamine intoxication and histamine liberators’ development to the individual activity of DAO of different patients.
The SFP mechanism can be influenced by a combination of three external contributing factors (fish species, saprophytic microorganism species that convert histidine to histamine, and environmental conditions), as well as internal individual factors such as exogenous histamine consumed, inactivation speed, and individual sensitivity [23]. The totality of this can be called the SFP formula (Figure 1).
SFP는
신선한 생선이나
드물게는 미각적 특성은 좋지만
외인성 히스타민이 다량 함유된 해산물을 섭취한 후
발생하는 식품 매개성 질환입니다.
통계에 따르면
혈중 외인성 히스타민 농도가
1~2ng/mL이면 위 분비 및 심박수 증가,
3~5ng/mL이면 두통, 가려움증, 빈맥,
6~8ng/mL이면 혈압 저하,
7~12ng/mL이면 기관지 경련 유발,
100ng/mL 이상이면 심장 마비를 일으킨다[21].
2017년에
중독을 유발하는 어류의 평균 히스타민 농도를 평가하기 위해
콜롬보 등은 히스타민 중독 사례를 분석하여 검토한 결과,
SFP는
평균 1100 mg/kg의 농도로 발생하며
423~2900 mg/kg까지 다양하다는 결론에 도달했습니다[22].
분명히 이러한 현저한 분산은
외인성 히스타민 중독 및
히스타민 해방제의 개발에서
다른 환자의 DAO의 개별 활동에 이르기까지 많은 메커니즘에 의해 발생할 수 있습니다.
SFP 메커니즘은
세 가지 외부 기여 요인(어종, 히스티딘을 히스타민으로 전환하는 부생 미생물 종, 환경 조건)과
소비된 외인성 히스타민,
비활성화 속도,
개별 민감도와 같은 내부 개별 요인의 조합에 의해 영향을 받을 수 있습니다 [23].
이를 모두 합쳐서 SFP 공식이라고 부를 수 있습니다(그림 1).
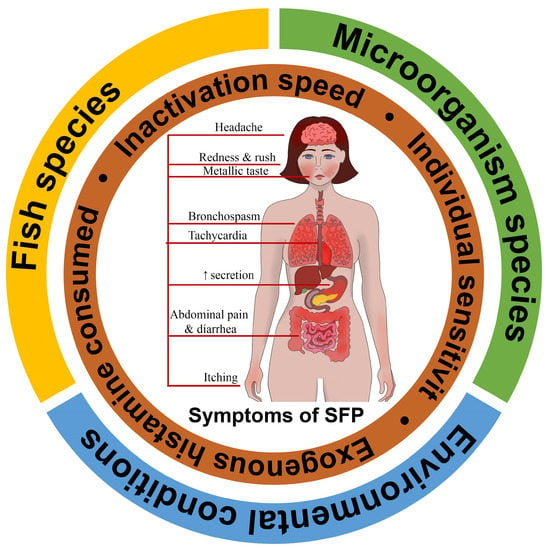
Figure 1. Scombroid food poisoning formula.
External contributing factors: fish species, saprophytic microorganism species that convert histidine to histamine, and environmental conditions; Internal individual factors: exogenous histamine consumed, inactivation speed, and individual sensitivity, etc.
2.1. Fish Species
The Codex Alimentarius and the European Commission designate Clupeidae (herring, sardines, shad), Coryphaenidae (mahi-mahi), Engraulidae (anchovies), Pomatomidae (bluefish), Scombridae (mackerel, tuna, scomber, skipjack, bonito), and Scomberesocidae (saury) as potentially dangerous sources of SFP [24,25]. There had been a lengthy discussion on whether Salmonidae are scombrotoxic families until the Food and Agriculture Organization of the United Nations (FAO) 2018 review concluded that Salmonidae do not present such a danger as the families listed above, but they may participate in the development of SFP if consumed after the expiry date [26]. The most common sources of SFP among Scombridae are tuna, mackerel, and scomber [27]. Istiophorus sp. (sailfish) and Seriola lalandi (yellowtail kingfish) are among other potentially scombrotoxic species [28,29].
Exogenous histamine is a product of muscle tissue histidine decarboxylation that occurs in these fish. Because high levels of histamine may form in fish with fine organoleptic characteristics, fresh fish may contain histamine as well as spoiled fish [30]. However, histamine intoxication most often develops after ingestion of spoiled fish, especially if it is from the Scombridae family. It occurs as a result of bacteria contaminating fish fermenting for 6 h at temperatures greater than 25 °C or longer if the temperature is lower [31]. These microorganisms are found in various products either as normal or contaminating flora.
The sources of exogenous histidine in the tissue can be not only fish but also crabs. There have already been several cases of SFP due to the consumption of crabs, such as mangrove crab Scylla serrata [12]. Cases of SFP resulting from consumption of dolphins (Delphinidae) have also been described [32].
국제식품규격위원회와 유럽연합 집행위원회는
Clupeidae(청어, 정어리, 정어리), C
oryphaenidae(마히마히),
Engraulidae(멸치),
Pomatomidae(등푸른 생선),
Scombridae(고등어, 참치, 가다랑어, 꽁치),
Scomberesocidae(꽁치)를
잠재적으로 위험한 SFP 공급원으로 지정하고 있습니다[24,25].
2018년 유엔 식량농업기구(FAO)의 검토에서
살모니과가 스콤브로독성 어류에 해당하는지에 대한 오랜 논의가 있었으나,
살모니과가 위에 열거된 어류만큼 위험하지는 않지만
유통기한이 지난 후 섭취할 경우 SFP 발병에 관여할 수 있다는 결론을 내렸습니다[26].
참치, 고등어, 가다랑어과에서
가장 흔한 SFP의 공급원은
참치, 고등어, 가다랑어입니다 [27].
이스티오포루스(Istiophorus sp.)(돛새치)와 세리올라 랄란디(Seriola lalandi)(황새치)도 잠재적인 스쿠브로독성 종입니다[28,29].
외인성 히스타민은
이러한 어류에서 발생하는
근육 조직 히스티딘 탈카르복실화의 산물입니다.
미각적 특성이 좋은 생선에는
높은 수준의 히스타민이 형성될 수 있기 때문에
신선한 생선뿐만 아니라
부패한 생선에도 히스타민이 포함될 수 있습니다 [30].
그러나
히스타민 중독은
상한 생선을 섭취한 후,
특히 스콤브리대과의 생선을 섭취한 후에 가장 자주 발생합니다.
이는 25°C 이상의 온도에서
6시간 동안 발효된 생선을 오염시키는 박테리아의 결과로 발생하며,
온도가 더 낮을 경우 더 오래 발효됩니다[31].
이러한 미생물은 다양한 제품에서 정상적으로 또는 오염된 식물상으로 발견됩니다.
조직 내 외인성 히스티딘의 공급원은
생선뿐만 아니라
게도 될 수 있습니다.
맹그로브 게인 실라 세라타와 같은 게의 섭취로 인한 SFP 사례는 이미 여러 차례 보고된 바 있습니다[12].
돌고래(돌고래과)의 섭취로 인한 SFP 사례도 기술되어 있습니다 [32].
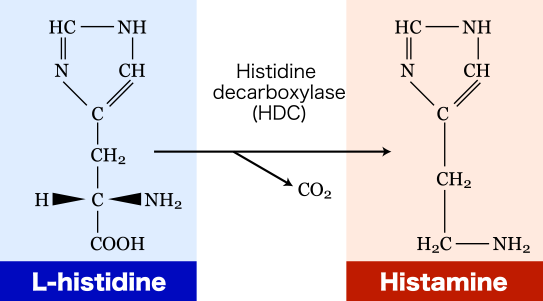
2.2. Bacteria Species
Histidine from different fish species can be found in food products initially or may be naturally released as a result of proteolysis induced by the processing of food or storage conditions [33]. The L-histidine decarboxylase found in E. coli, Proteus, Klebsiella, and Morganella morganii [34] turns the histidine of fish tissues into histamine [35]. All Morganella morganii strains produce histamine in quantities of more than 5000 ppm. It was shown that one of these strains produced 5253 ppm in tuna that was stored at 25 °C and 2769 ppm at 15 °C [30]. It was also reported that Enterobacter aerogenes and Raoultella planticola participate in SFP [36]. Significant histamine production was found in five species of photobacteria: Photobacterium angustum, Photobacterium aquimaris, Photobacterium kishitanii, Photobacterium damselae and Photobacterium phosphoreum. Of all of them, only Photobacterium phosphoreum was associated with the SFP outbreak. However, the classification of this microorganism has been changed and now it is identified either as Photobacterium kishitanii or Photobacterium aquimaris. Because of that, researchers cannot clearly tell whether these bacteria are capable of histamine production or not, but Bjornsdottir-Butler et al. have found out that, although these microorganisms have decarboxylase and they produce histamine, their decarboxylase genes are different from the ones of E. coli [34]. Photobacterium phosphoreum was capable of synthesizing histamine at a temperature lower than 10 °C [30].
다양한 어종의 히스티딘은
처음에 식품에서 발견되거나
식품 가공 또는 보관 조건에 의해 유도된 단백질 분해의 결과로
자연적으로 방출될 수 있습니다 [33].
대장균,
프로테우스,
클렙시엘라,
모가넬라 모르가니[34]에서 발견되는
L-히스티딘 탈카르복실효소는
어류 조직의 히스티딘을 히스타민으로 전환합니다[35].
모든
모르가넬라 모가니 균주는
5000ppm 이상의 히스타민을 생성합니다.
이 균주 중 하나는
25°C에서 보관한 참치에서
5253ppm,
15°C에서
2769ppm의 히스타민을 생성하는 것으로 나타났습니다[30].
또한
엔테로박터 에어로제네스와
라울텔라 플란티콜라가
SFP에 관여하는 것으로 보고되었습니다 [36].
5종의 광박테리아에서
상당한 히스타민 생산이 발견되었습니다:
포토박테리움 앙구스툼, 포토박테리움 아키마리스, 포토박테리움 키시타니이, 포토박테리움 담셀래, 포토박테리움 포스포룸입니다. 이 중 포토박테리움 포스포룸만이 SFP 발병과 관련이 있었습니다. 그러나 이 미생물의 분류가 변경되어 현재는 포토박테리움 키시타니 또는 포토박테리움 아퀴마리스로 확인되고 있습니다. 이 때문에 연구자들은 이 박테리아가 히스타민을 생산할 수 있는지 여부를 명확하게 알 수 없지만, 비욘스도티르-버틀러(Bjornsdottir-Butler) 등은 이 미생물이 탈카르복실효소를 가지고 있고 히스타민을 생산하지만 탈카르복실효소 유전자가 대장균의 것과 다르다는 사실을 발견했습니다[34]. 포토박테리움 포스포룸은 10°C보다 낮은 온도에서 히스타민을 합성할 수 있었습니다[30].
Table 1 shows the bacteria species and food storage conditions that lead to the accumulation of exogenous histamine in fish and, as a result, make them dangerous to eat.
Table 1. Bacteria species that can produce histamine and their characteristics.
2.3. Environmental Conditions
In 2020, the FDA determined that a histamine concentration of 500 mg/kg is sufficient to cause SFP. However, evaluating the exogenous histamine concentration in a shipment of fish is difficult because the distribution of histamine in a fish is not homogenous and often depends on environmental conditions. Therefore, the FDA assumes that if any part of one fish or of the whole shipment contains more than 500 mg/kg of histamine, then it is most likely that other parts may contain concentrations higher than 500 mg/kg [45]. In the European Union, fish species with high levels of histidine are monitored in a special way. The maximum allowed level of histamine in fish of the “risk group” is 100 mg/kg [46].
Fish storage and cooking temperatures are important environmental factors in the pathogenesis of SFP. Keeping products at a cold temperature prevents the growth of the bacteria and the synthesis of their histidine decarboxylase [47]. Despite the fact that the Food and Drug Administration USA (FDA) introduced regulations on the rapid cooling of caught fish to ensure the safety of seafood, SFP remains one of the most prevalent diseases in the world associated with fish [48]. Histamine formation may occur in fish that have been stored or transported incorrectly [30,49]. For example, keeping fish above 4.4 °C for more than 4 h after initial chilling will result in the accumulation of free histidine in the fish [50]. Chung B.Y. et al. found that cooking (grilling and frying) causes the inactivation of histamine-producing bacteria, but histamine can remain in cooked foods. Increasing the cooking temperature can increase histidine decarboxylase activity and, therefore, histamine production. When heated above 50 °C, histidine decarboxylase is inactivated, but remaining exogenous histamine can still lead to SFP [51].
Another factor influencing the formation of endogenous histamine is sodium chloride, which is used in cooking in the form of sea salt and table salt. Histamine production may also be stopped by salt, which is a histidine decarboxylase inhibitor. Research shows that when salting fish, there are no signs of microbiological growth or elevation in the concentration of the amines [47]. Still, some bacteria can produce biogenic amines even in a 12 percent salted environment. For example, an increased concentration of biogenic amines was noticed in salted sardines. Histamine concentrations are also elevated in marinated fish, even if it is stored properly. Biogenic amine levels do not decrease even after sterilization; studies show that up to 90% of amines remain in the products even after this procedure [52].
Humidity is also an environmental factor that can influence the formation of exogenous histamine, so the concentration of endogenous histamine in fish, in terms of the total weight of the product, can increase its drying or humidity loss during cooking [51]. Maintaining anoxic conditions, such as when vacuum-packing fish, is not an effective method of preventing the formation of histamine [50].
Therefore, the quality of fresh fish is determined by the proper timing of cooling, whereas the quality of canned fish is determined by multiple factors, such as the freshness of the primary products, fish species that are canned, the method of processing of the fish, and conditions of storage and transportation.
2020년 FDA는
히스타민 농도가 500mg/kg이면
SFP를 유발하기에 충분하다고 판단했습니다.
그러나
어류의 히스타민 분포가 균일하지 않고
환경 조건에 따라 달라지기 때문에
선적된 어류의 외인성 히스타민 농도를 평가하는 것은 어렵습니다.
따라서
FDA는 한 어류 또는
전체 선적물의 일부에 500 mg/kg 이상의 히스타민이 포함되어 있으면
다른 부분에도 500 mg/kg보다 높은 농도가 포함되어 있을 가능성이 높다고 가정합니다[45].
유럽연합에서는
히스티딘 함량이 높은 어종을 특별한 방식으로 모니터링하고 있습니다.
"위험 그룹"의 생선에서 허용되는
최대 히스타민 수치는 100mg/kg입니다 [46].
생선 보관 및 조리 온도는
SFP의 발병에 중요한 환경 요인입니다.
제품을 저온에 보관하면
박테리아의 성장과
히스티딘 데카르 복실 라제의 합성을 방지 할 수 있습니다 [47].
미국 식품의약국(FDA)이 해산물의 안전을 보장하기 위해
어획된 생선의 급속 냉각에 대한 규정을 도입했음에도 불구하고
SFP는 여전히 전 세계에서 가장 널리 퍼진 어류 관련 질병 중 하나입니다 [48].
히스타민 형성은
잘못 보관되거나 운송된 생선에서 발생할 수 있습니다 [30,49].
예를 들어,
최초 냉장 후 4.4°C 이상에서 4시간 이상 보관하면
생선에 유리 히스티딘이 축적될 수 있습니다 [50].
정 비와이 등은
조리(굽기 및 튀김)는
히스타민 생성 박테리아의 비활성화를 유발하지만
히스타민은 조리된 음식에 남아있을 수 있음을 발견했습니다.
조리 온도를 높이면
히스티딘 탈카르복실효소 활성이 증가하여
히스타민 생성이 증가할 수 있습니다.
50°C 이상으로 가열하면
히스티딘 탈카르복실효소는 비활성화되지만,
남아있는 외인성 히스타민은 여전히 SFP를 유발할 수 있습니다[51].
내인성 히스타민의 형성에 영향을 미치는 또 다른 요인은
바다 소금과 식탁용 소금의 형태로
요리에 사용되는 염화나트륨입니다.
히스타민 생성은
히스티딘 탈 카르 복실 효소 억제제 인
소금에 의해서도 중단 될 수 있습니다.
연구에 따르면
생선을 소금에 절일 때
미생물학적 성장이나 아민 농도 상승의 징후는 없습니다 [47].
하지만 일부 박테리아는
12%의 소금에 절인 환경에서도 생체 아민을 생성할 수 있습니다.
예를 들어,
소금에 절인 정어리에서
생물성 아민의 농도가 증가하는 것이 발견되었습니다.
히스타민 농도는
제대로 보관하더라도 절인 생선에서도 높아집니다.
살균 후에도 생물성 아민 수치는 감소하지 않으며, 연
구에 따르면 이 절차 후에도 최대 90%의 아민이 제품에 남아 있습니다 [52].
습도는 또한
외인성 히스타민의 형성에 영향을 미칠 수 있는 환경적 요인이므로
제품의 총 중량 측면에서 생선의 내인성 히스타민 농도는
조리 중 건조 또는 습도 손실을 증가시킬 수 있습니다 [51].
생선을 진공 포장할 때와 같이 무산소 상태를 유지하는 것은 히스타민 형성을 방지하는 효과적인 방법이 아닙니다 [50].
따라서
신선 생선의 품질은
적절한 냉각 시기에 따라 결정되는 반면,
통조림 생선의 품질은 1차 제품의 신선도,
통조림에 사용되는 어종,
생선의 가공 방법,
보관 및 운송 조건 등 여러 요인에 의해 결정됩니다.
3. Molecular Mechanisms of Scombroid Food Poisoning
Since SFP is an FPA, exogenous histamine intoxication is strictly dose dependent. Increased intoxication with exogenous histamine leads to an increase in symptoms and a deterioration in the human condition. The need to distinguish SFP from true IgE-associated allergies to fish and seafood is one of the most difficult examples of the differential diagnosis of allergic conditions. There are several key differences in the mechanisms between SFP and true IgE-associated allergies to fish and seafood (Table 2).
SFP는
FPA이므로 외인성 히스타민 중독은
엄격하게 용량에 따라 달라집니다.
외인성 히스타민에 의한 중독이 증가하면
증상이 증가하고 인간의 상태가 악화됩니다.
생선 및 해산물에 대한 진정한 IgE 관련 알레르기와
SFP를 구별해야 하는 것은
알레르기 질환의 감별 진단에서 가장 어려운 예 중 하나입니다.
SFP와 생선 및
해산물에 대한 진정한 IgE 관련 알레르기의 메커니즘에는
몇 가지 주요 차이점이 있습니다(표 2).
Table 2. The distinctions in mechanisms between true IgE-associated allergies to fish and seafood and SFP.
True fish allergy is usually associated with sensitization to parvalbumins, which are physically and chemically resistant. Parvalbumins are found in various fish species and their cross-activity is often observed, so it is not enough to avoid only certain fish species to prevent the allergic reaction [53]. It is important to note that parvalbumins may be inhaled, as well as food allergens. That is why allergic reactions to fish have severe clinical courses not only when fish is ingested but also when the smell of fish is inhaled. The reaction in this case is reproducible and may develop even after the shortest contact with fish [54]. The main factors in the pathogenesis of true allergy to fish and seafood involve not only immune complexes but also the stage of silent sensibilization to the allergen. After that, the allergic reaction may be triggered by the smallest amount of allergen and develop more slowly than in SFP. The target cells are activated directly by IgE [55].
Meanwhile, SFP is induced by the presence of saprophytic bacteria and an increased amount of exogenous histamine and histamine-like chemicals in fish caused by improper storage [56]. The intensity of the SFP reaction is directly proportional to the amount of exogenous activator consumed, and the reaction itself develops rapidly.
Below, we consider some of the known molecular mechanisms of SFP, the role of which has been proven to varying degrees.
진정한 생선 알레르기는
일반적으로 물리적 및 화학적 내성이 있는
파르발부민에 대한 감작과 관련이 있습니다.
파르발부민은
다양한 어종에서 발견되며 교차 작용이 종종 관찰되므로
알레르기 반응을 예방하기 위해
특정 어종만 피하는 것만으로는 충분하지 않습니다 [53].
파르발부민은
음식 알레르겐뿐만 아니라
흡입 될 수 있다는 점에 유의하는 것이 중요합니다.
그렇기 때문에
생선에 대한 알레르기 반응은
생선을 섭취 할 때뿐만 아니라
생선 냄새를 흡입 할 때에도 심각한 임상 과정을 겪습니다.
이 경우 반응은
재현 가능하며 생선과 가장 짧은 접촉 후에도 발생할 수 있습니다 [54].
생선과 해산물에 대한 진정한 알레르기의 발병 기전의 주요 요인은
면역 복합체뿐만 아니라
알레르겐에 대한 조용한 감작 단계도 포함합니다.
그 후
알레르기 반응은
가장 적은 양의 알레르겐에 의해 촉발되고
SFP보다 더 느리게 진행될 수 있습니다.
표적 세포는 IgE에 의해 직접 활성화됩니다 [55].
한편,
SFP는
부적절한 보관으로 인한
부생 박테리아의 존재와
어류의 외인성 히스타민 및
히스타민 유사 화학 물질의 증가에 의해 유발됩니다 [56].
SFP 반응의 강도는
소비되는 외인성 활성화제의 양에 정비례하며,
반응 자체는 빠르게 진행됩니다.
3.1. The Role of Exogenous Histamine and Its Metabolic Pathways
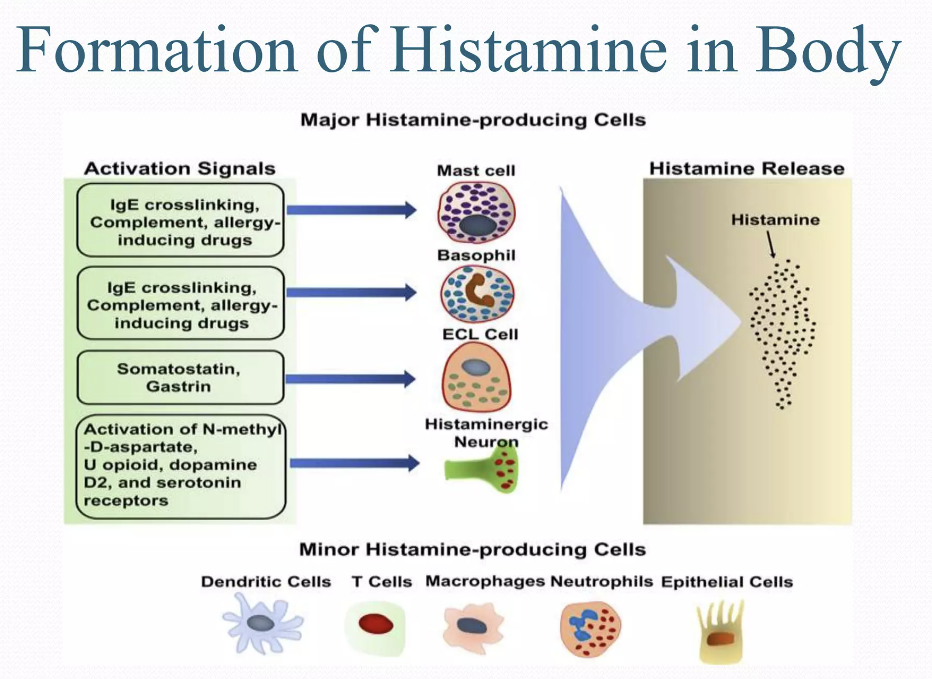
Endogenous histamine, contained in the mast cells and basophiles, is one of the most important mediators of non-IgE-mediated clinical reactions, as well as IgE-mediated ones. There are two known ways of histamine metabolism in the human body: methylation by histamine-N-methyltransferase (HNMT) and oxidative degradation by diamine oxidase (DAO) [57]. Besides the endogenous histamine synthesized in the human body, this substance can enter exogenously via the alimentary route and cause SFP in high doses. Large doses of exogenous histamine can cause poisoning: 100 mg and up can cause light intoxication, while 1000 mg and up can cause severe intoxication [58].
The source of exogenous histamine is often fish, cheese, and dairy products contaminated by saprophytic bacteria. According to the FDA, high quality fish must contain histamine at a concentration of less than 10 parts per million, and the level of defect action must be 50 parts per million [59]. While histamine levels of ≥50 parts per million in fish tissues are considered to be a sign of decay, levels of ≥500 parts per million indicate a potential threat to human health and regulatory measures are taken regarding these products [34]. In concentrations greater than 500 parts per million, generalized reactions were observed [60]. Another food product besides fish in which exogenous histamine can be formed under the action of saprophytic microflora is cheese and dairy products. It is known that Lactobacillus buchneri, which possesses histidine decarboxylase and plays an important role in the dairy industry, is capable of converting histidine into histamine. Potentially, it may be responsible for outbreaks of histamine poisoning with different sorts of cheese. The toxicity of this histamine may probably double because of other biogenic amines that are produced by the bacteria in cheese. Some of these amines inhibit histamine-metabolizing enzymes and elevate the concentration of exogenous histamine, significantly slowing its decomposition [61].
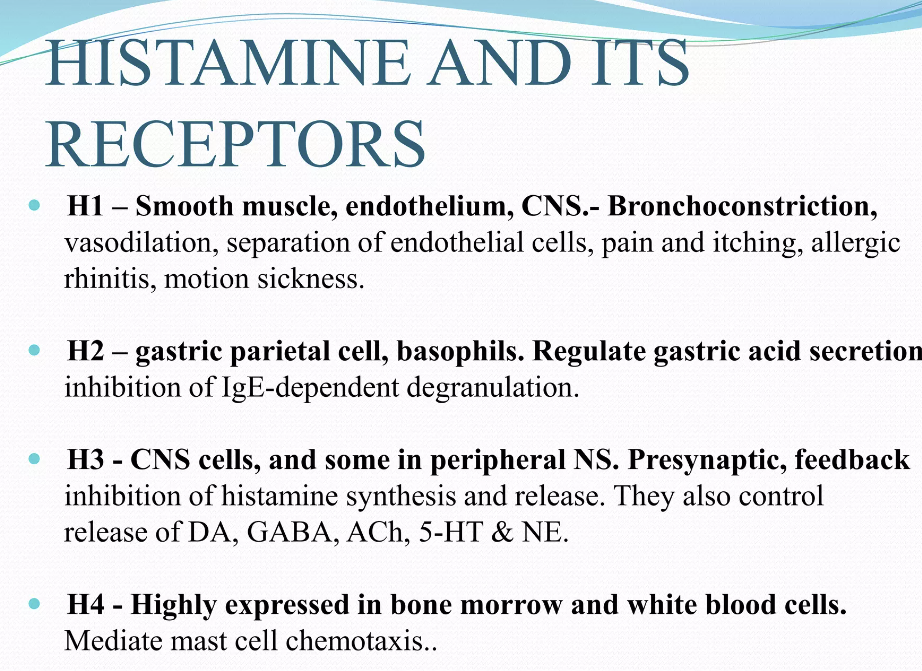
Many of the toxic effects of exogenous histamine in SFP are realized through endogenous histamine receptors. These receptors are H1R, H2R, H3R, and H4R, and they all belong to the family of G-protein-coupled receptors [57]. The sarcolemma of vascular smooth myocytes contains H2 histamine receptors that are associated with GS protein. Upon reacting with the H2 receptors, exogenous histamine triggers an intracellular biochemical cascade, as follows in Figure 2.
비만 세포와 호염기구에 포함된
내인성 히스타민은
IgE 매개 임상 반응뿐만 아니라
비-IgE 매개 임상 반응의 가장 중요한 매개체 중 하나입니다.
인체에서
히스타민 대사에는
histamine-N-methyltransferase 히스타민-N-메틸전달효소(HNMT)에 의한 메틸화와
디아민 산화효소(DAO)에 의한 산화적 분해의 두 가지 방법이 알려져 있습니다[57].
인체에서 합성되는 내인성 히스타민 외에도
이 물질은 소화 경로를 통해 외인성으로 유입되어
고용량으로 SFP를 유발할 수 있습니다.
다량의 외인성 히스타민은 중독을 유발할 수 있습니다:
100mg 이상은 가벼운 중독을 유발할 수 있고,
1000mg 이상은 심각한 중독을 유발할 수 있습니다 [58].
외인성 히스타민의 공급원은
종종 부생 박테리아에 의해 오염된 생선, 치즈 및 유제품입니다.
https://www.sciencedirect.com/science/article/abs/pii/S0308814623006647


FDA에 따르면 고품질 생선은
히스타민 농도가 10ppm 미만이어야 하며,
결함 작용 수준은 50ppm이어야 합니다[59].
어류 조직에서 히스타민 수치가 50ppm 이상이면
부패의 징후로 간주되지만,
500ppm 이상이면 인체 건강에 잠재적인 위협이 될 수 있으며
이러한 제품에 대한 규제 조치가 취해집니다 [34].
500ppm 이상의 농도에서는 일반화된 반응이 관찰되었습니다 [60].
부생 미생물의 작용으로
외인성 히스타민이 형성 될 수있는 생선 이외의 또 다른 식품은
치즈와 유제품입니다.
히스티딘 데카르 복실 라제를 보유하고
유제품 산업에서 중요한 역할을하는
락토 바실러스 부흐 네리는
히스티딘을
히스타민으로 전환 할 수있는 것으로 알려져 있습니다.
잠재적으로
다양한 종류의 치즈에서
히스타민 중독이 발생할 수 있습니다.
이 히스타민의 독성은
치즈 속 박테리아에 의해 생성되는
다른 생체 아민으로 인해 두 배가 될 수 있습니다.
이러한
아민 중 일부는
히스타민 대사 효소를 억제하고
외인성 히스타민의 농도를 높여 분해를 상당히 느리게 합니다[61].
SFP에서
외인성 히스타민의 독성 효과 중 상당수는
내인성 히스타민 수용체를 통해 실현됩니다.
이러한 수용체는
H1R, H2R, H3R 및 H4R이며,
모두 G 단백질 결합 수용체 계열에 속합니다 [57].
혈관 평활근 세포의 육종에는
GS 단백질과 관련된 H2 히스타민 수용체가 포함되어 있습니다.
외인성 히스타민은
H2 수용체와 반응하면
그림 2와 같이 세포 내 생화학적 캐스케이드를 촉발합니다.
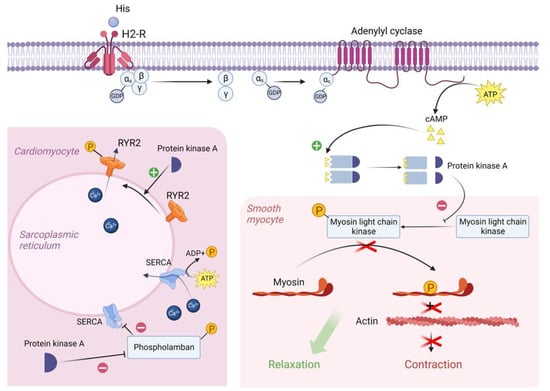
Figure 2. Molecular mechanism of H2 receptor activation by exogenous histamine in SFP. Exogenous histamine binds to the H2-R and leads to the adenylyl cyclase system activation. In smooth muscle cells, PKA phosphorylates MLCK, leading to the smooth muscle cell relaxation. In cardiomyocytes, PKA phosphorylates Phospholamban and RYR2, resulting in an increased heart rate. PKA—proteinkinase A, GTP—guanosine triphosphate, MLCK—myosin light chain kinase, GDP—guanosine diphosphate, cAMP—cyclic adenosine monophosphate, RYR2—ryanodine receptor 2, SR—sarcoplasmic reticulum, SERCA—sarco/endoplasmic reticulum Ca—ATPase.
In the resting state, a GDP molecule is bound to the αs subunit of the Gs protein, keeping it in an inactive conformation. However, the interaction of histamine with the receptor contributes to a change in the conformation of the as subunit, which leads to a decrease in its affinity for GDP and an increase in its affinity for GTP (i.e., GDP is detached from the αs subunit and GTP is attached in its place). The point is that the binding of the αs subunit to GDP was important to keep this subunit in the complex with the β and γ subunits, but now that it is bound to GTP, the αS subunit changes its conformation a second time and disconnects from the β and γ subunits. Then, the αs subunit uses lateral diffusion (lateral movement along the inner surface of the cell plasma membrane) to the transmembrane enzyme adenylyl cyclase. Once attached to adenylyl cyclase, the αs subunit activates it. Once activated, adenylyl cyclase begins to convert an ATP molecule into cAMP. In turn, four molecules of cAMP bind to the active centers of two regulatory subunits of type 1 PKA enzyme located in the sarcoplasm of smooth myocytes. As a result, type 1 PKA changes its conformation and its two catalytic subunits are activated and begin phosphorylation of serine and threonine OH-groups of proteins [57,62].
In this case, the target of type 1 PKA is MLCK (myosin light chain kinase), which it inactivates by phosphorylation. The fact is that in the resting state, MLCK phosphorylates myosin regulatory light chains and thereby stimulates the activation of the ATP-ase domain of myosin heads, leading to ATP hydrolysis and, as a consequence, contraction of smooth myocytes. But as we mentioned earlier, the cascade of reactions triggered by histamine results in inactivation of MLCK and, as a consequence, relaxation of vascular smooth myocytes, which in turn leads to vasodilation [63].
H2 receptors are also located on the sarcolemma of typical and atypical cardiomyocytes. When activated, PKA begins to phosphorylate L-type Ca channels, promoting their opening and, as a consequence, the entry of large amounts of Ca from the extracellular space into the intracellular on a concentration gradient. In addition, PKA also phosphorylates RYR2 (Ryanadine receptor 2) located on the membrane of the sarcoplasmic reticulum, promoting their opening, which will lead to the release of Ca from the SR lumen into the cell cytosol. All this will lead to an increase in Ca concentration in the sarcoplasm of atypical cardiomyocytes and, as a consequence, to an increase in HR [62,64].
It is worth noting that in the sarcoplasm of typical cardiomyocytes there is a phospholamban protein that binds to SERCA (Sarco/endoplasmic reticulum Ca—ATPase) at rest and reduces its affinity for Ca, thus preventing Ca entry back from the cardiomyocyte sarcoplasm into the lumen of the sarcoplasmic reticulum. However, PKA phosphorylates phospholamban by the OH groups of serine and threonine and thereby promotes its uncoupling from SERCA. This creates an opportunity for Ca to leave the sarcoplasm and allows the muscle to relax for even more accelerated contraction [62,65,66].
Vascular epithelial cells have H1 histamine receptors that are associated with the Gq protein. Upon reacting with H1 receptors, histamine triggers an intracellular biochemical cascade as follows in Figure 3.
휴식 상태에서 GDP 분자는 Gs 단백질의 αs 서브유닛에 결합하여 비활성 상태를 유지합니다. 그러나 히스타민과 수용체의 상호 작용은 as 서브유닛의 형태를 변화시켜 GDP에 대한 친화력은 감소하고 GTP에 대한 친화력은 증가합니다(즉, αs 서브유닛에서 GDP가 분리되고 그 자리에 GTP가 부착됨). 요점은 αs 서브유닛이 β 및 γ 서브유닛과 복합체 내에 유지되기 위해서는 αs 서브유닛이 GDP에 결합하는 것이 중요했지만, 이제 αs 서브유닛이 GTP에 결합하게 되면 αS 서브유닛은 한 번 더 형태를 바꾸고 β 및 γ 서브유닛과 분리된다는 것입니다. 그런 다음 αs 서브유닛은 측면 확산(세포질 막의 내부 표면을 따라 측면으로 이동)을 통해 막 통과 효소인 아데닐릴 사이클레이즈에 부착됩니다. 아데닐릴 시클라제에 부착되면 αs 서브유닛이 아데닐릴 시클라제를 활성화합니다. 활성화되면 아데닐릴 시클라제는 ATP 분자를 cAMP로 전환하기 시작합니다. 차례로, 4개의 cAMP 분자가 평활근 세포의 세포질에 위치한 1형 PKA 효소의 두 조절 서브유닛의 활성 중심부에 결합합니다. 결과적으로 1형 PKA는 형태를 변경하고 두 개의 촉매 서브유닛이 활성화되어 단백질의 세린 및 트레오닌 OH 그룹의 인산화를 시작합니다[57,62].
이 경우, 1형 PKA의 표적은 인산화에 의해 비활성화되는 MLCK(미오신 경쇄 키나아제)입니다. 사실 MLCK는 휴식 상태에서 미오신 조절 경쇄를 인산화하여 미오신 헤드의 ATP- 효소 도메인의 활성화를 자극하여 ATP 가수 분해를 유도하고 결과적으로 평활근 세포의 수축을 유발합니다. 그러나 앞서 언급했듯이 히스타민에 의해 촉발된 일련의 반응은 MLCK의 비활성화를 초래하고 결과적으로 혈관 평활근 세포의 이완을 초래하여 혈관 확장으로 이어집니다 [63].
H2 수용체는 또한 전형적인 심근 세포와 비정형 심근 세포의 육종에 위치합니다. 활성화되면 PKA는 L 형 Ca 채널을 인산화하기 시작하여 개방을 촉진하고 결과적으로 농도 구배에 따라 세포 외 공간에서 세포 내로 많은 양의 Ca가 유입됩니다. 또한 PKA는 소포체 막에 위치한 RYR2(라이아나딘 수용체 2)를 인산화하여 개방을 촉진하여 SR 루멘에서 세포질로 Ca가 방출되도록 유도합니다. 이 모든 것이 비정형 심근 세포의 실질에서 Ca 농도를 증가시키고 결과적으로 HR [62,64]의 증가로 이어질 것입니다.
전형적인 심근 세포의 실질에는 휴식시 SERCA (Sarco / 소포체 Ca-ATPase)에 결합하여 Ca에 대한 친 화성을 감소시켜 심근 세포 실질에서 실질 내강으로 Ca가 다시 들어가는 것을 방지하는 포스 포람 반 단백질이 있다는 점에 주목할 가치가 있습니다. 그러나 PKA는 세린과 트레오닌의 OH 그룹에 의해 포스포람반을 인산화하여 SERCA로부터의 결합 해제를 촉진합니다. 이렇게 하면 Ca가 세포질을 떠날 수 있는 기회가 생기고 근육이 이완되어 수축이 더욱 가속화됩니다[62,65,66].
혈관 상피 세포에는 Gq 단백질과 관련된 H1 히스타민 수용체가 있습니다. 히스타민은 H1 수용체와 반응하면 그림 3과 같이 세포 내 생화학적 캐스케이드를 촉발합니다.
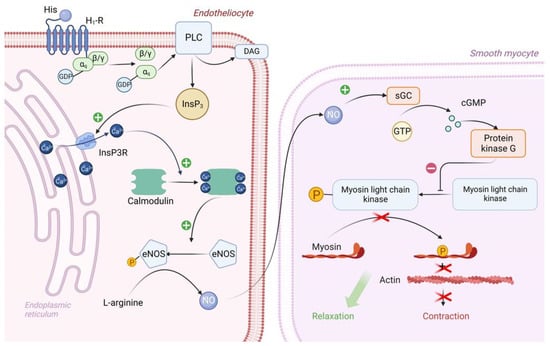
Figure 3. Molecular mechanism of H1 receptor activation by exogenous histamine in SFP. Exogenous histamine binds to the endothelial H1-R and leads to the inositol phosphate pathway activation. InsP3 stimulates Ca release, thereby leading to the Ca/Calmodulin complex formation. Ca/Calmodulin complex activates eNOS, which catalases NO formation. NO enters the sarcoplasm of smooth muscle cells and eventually activates PKG. PKG phosphorylates MLCK, leading to smooth muscle cell relaxation. PLC—Phospholipase C, PIP2—phosphotidylinositol diphosphate, InsP3—inositol triphosphate, DAG—diacylglycerol, PKC—proteinkinase C, eNOS—endothelial NO synthase, sGC—soluble guanylate cyclase, GTP—guanosine diphosphate, cGMP—cyclic guanosine monophosphate, PKG—proteinkinase G, MLCK—myosin light chain kinase.
The αq subunit of the Gq protein undergoes similar changes as the αs subunit of the Gs protein. In this case, the αq subunit activates the enzyme PLC (Phospholipase C), which is located on the inner surface of the endotheliocyte membrane. PLC begins to hydrolyze PIP2 (phosphotidylinositol diphosphate), which is a typical representative of cell membrane phospholipids, to inositol triphosphate (phospholipid head) and diacylglycerol (phospholipid tail). DAG remains in the cell membrane and takes part in PKC activation. IP3 binds to IP3 receptors located on the ER membrane and stimulates them. It is important to note that IP3 receptors are Ca channels and that after interaction with IP3 they open, promoting the release of large amounts of Ca ions from the ER into the cell cytosol along a concentration gradient. Ca will be involved in PKC activation, but most importantly, four Ca ions bind to calmodulin located in the cytosol of endotheliocyte, forming a calmodulin-4Ca complex [62,67].
The endothelial cell cytosol contains the enzyme eNOS (endothelial NO synthase), which is normally in the inactive conformation. Calmodulin-4Ca complex binds to eNOS and converts it to the active conformation. Once activated, eNOS catalyzes the synthesis of NO from the amino acid L-arginine. Next, NO synthesized by endothelial cells enters the sarcoplasm of vascular smooth myocytes by simple diffusion, where it activates sGC (soluble guanylate cyclase)—its feature is that it is located in the sarcoplasm of myocytes, not on the sarcolemma. When activated, sGC catalyzes the conversion of GTP to cGMP. In turn, two molecules of cGMP bind to PKG and activate it. PKG begins to phosphorylate MLCK by serine/threonine OH-group, inactivating it, which leads to relaxation of vascular smooth myocytes and, consequently, to vasodilation [68,69].
In addition, Ca, which enters the cytosol of endotheliocytes in large quantities under the action of IP3, stimulates actomyosin interaction in the cytoskeleton, which in turn leads to the contraction of endothelial cells and the formation of interendothelial gaps between them. By this mechanism, there is an increase in capillary permeability in SFP [70].
Gq 단백질의 αq 서브유닛은 Gs 단백질의 αs 서브유닛과 유사한 변화를 겪습니다. 이 경우 αq 서브유닛은 내피세포막의 안쪽 표면에 있는 효소 PLC(포스포리파제 C)를 활성화합니다. PLC는 세포막 인지질의 대표적인 물질인 PIP2(포스포티딜이노시톨 이인산염)를 가수분해하여 이노시톨 삼인산염(인지질 머리)과 디아실글리세롤(인지질 꼬리)로 분해하기 시작합니다. DAG는 세포막에 남아 PKC 활성화에 참여합니다. IP3는 ER 막에 위치한 IP3 수용체에 결합하여 이를 자극합니다. IP3 수용체는 Ca 채널이며 IP3와 상호 작용한 후 개방되어 농도 구배를 따라 ER에서 세포질로 다량의 Ca 이온이 방출되도록 촉진한다는 점에 유의하는 것이 중요합니다. Ca는 PKC 활성화에 관여하지만, 가장 중요한 것은 내피세포의 세포질에 위치한 칼모둘린에 4개의 Ca 이온이 결합하여 칼모둘린-4Ca 복합체를 형성한다는 것입니다[62,67].
내피 세포 세포질에는 일반적으로 비활성 상태인 효소 eNOS(내피 NO 합성 효소)가 포함되어 있습니다. 칼모둘린-4Ca 복합체는 eNOS에 결합하여 활성 형태로 전환합니다. 활성화되면 eNOS는 아미노산 L-아르기닌에서 NO 합성을 촉매합니다. 다음으로 내피 세포에서 합성된 NO는 단순 확산을 통해 혈관 평활근 세포의 실질로 들어가 sGC(용해성 구아닐레이트 사이클라제)를 활성화하는데, 이 sGC는 근세포의 실질에 위치하는 것이 아니라 sarcoplasm에 위치한다는 특징이 있습니다. sGC가 활성화되면 GTP가 cGMP로 전환되는 것을 촉매합니다. 그러면 두 개의 cGMP 분자가 PKG에 결합하여 활성화됩니다. PKG는 세린/트레오닌 OH 그룹에 의해 MLCK를 인산화하기 시작하여 이를 비활성화함으로써 혈관 평활근 세포의 이완을 유도하고 결과적으로 혈관 확장을 일으킵니다[68,69].
또한 IP3의 작용으로 내피 세포의 세포질에 다량으로 들어가는 Ca는 세포 골격에서 액토 마이신 상호 작용을 자극하여 내피 세포의 수축과 내피 세포 사이의 간극 형성을 유도합니다. 이 메커니즘에 의해 SFP에서 모세혈관 투과성이 증가합니다 [70].
3.2. The Role of Other Biogenic Amines and Their Metabolic Pathways
In studies regarding the pathological mechanism of SFP, researchers’ attention was focused on the reactions caused by the products’ high histamine content. However, it should be taken into account that other biogenic amines, known as putrefactive (cadaverine, tryptamine, tyramine, serotonin, and others) and/or polyamines (putrescine, spermine, and others), are also capable of inducing similar reactions by influencing the metabolism of histamine in the human body [71].
Tyramine was the first discovered substrate of monoamine oxidase (MAO) and was derived from cheese. Tyramine oxidase is found in a high concentration in the mucosa of the intestines. As a result of bacterial decarboxylation of amino acids, vasopressor amines are formed [72]. Besides its indirect influence on histamine, tyramine also plays a role in adverse reactions of MAO inhibitors. These reactions include headaches and hypertensive crises [73]. Hypertensive crises caused by consuming products containing tyramine and other biogenic amines have also been reported after consuming wine, beer, some fruit and vegetables (sauerkraut, banana peels, avocado, beans) [74,75], and fish [76]. Tyramine, putrescine, and other biogenic amines are found in fish and are produced by bacteria by the decarboxylation of homologous free amino acids [77]. It should be noted that the amount of tyramine and spermine increases when the fish is half-dried [78]. Thus, tyramine and other biogenic amines play an important role in SFP, especially when associated with semi-dried fish.
Other biogenic amines that can potentiate SFP are tryptamine and phenylethylamine. Tryptamine may inhibit DAO, and phenylethylamine inhibits both DAO and HNMT. Although only tyramine may reach concentrations high enough in fish to be toxicologically significant, the synergetic action of all these amines may cause a SFP reaction while eating fish [79].
Also, numerous studies have been conducted to identify the amines which are produced by the Lactobacillus buchneri colonizing cheese. Such amines as histamine, cadaverine, putrescine, tryptamine, and phenylethylamine are found in many sorts of cheese [21]. Experiments with E. faecalis EF37 have shown that increasing the temperature from 16 to 44 °C is correlated with a more rapid and intensive accumulation of tyramine [22]. In 2015, Bargossi et al. investigated the temperature most favorable for tyrosine decarboxylase derived from E. faecalis and found out that it functioned most intensively in the temperature range from 30 to 37 °C [80,81].
SFP의 병리학적인 메커니즘에 관한 연구에서 연구자들은
제품의 높은 히스타민 함량으로 인한
반응에 주목했습니다.
그러나
부패성(카데바린, 트립타민, 티라민, 세로토닌 등) 및/또는
폴리아민(푸트레신, 스페르민 등)으로 알려진
다른 생물성 아민도 인체에서 히스타민의 대사에 영향을 주어
유사한 반응을 유발할 수 있다는 점을 고려해야 합니다[71].
티라민은
최초로 발견된 모노아민 산화효소(MAO)의 기질로,
치즈에서 추출되었습니다.
티라민 산화효소는
장 점막에서 고농도로 발견됩니다.
아미노산의 박테리아 탈카르복실화의 결과로
바소프레서 아민이 형성됩니다 [72].
티라민은
히스타민에 대한 간접적 인 영향 외에도
MAO 억제제의 부작용에도 영향을 미칩니다.
이러한 반응에는
두통과 고혈압 위기가 포함됩니다 [73].
와인,
맥주,
일부 과일 및 채소(사우어크라우트, 바나나 껍질, 아보카도, 콩)[74,75],
생선[76] 섭취 후
티라민 및 기타 생물성 아민이 포함된 제품 섭취로 인한
고혈압 위기가 보고된 바도 있습니다.
티라민, 푸트레신 및 기타 생물성 아민은
생선에서 발견되며
박테리아가 상동 유리 아미노산을 탈카르복실화하여 생성합니다[77].
생선이 반 건조되면
티라민과 스페르민의 양이 증가한다는 점에 유의해야합니다 [78].
따라서
티라민 및 기타 생물성 아민은
특히 반건조 생선과 관련된 경우 SFP(히스타민 중독증)에서 중요한 역할을 합니다.
SFP를 강화할 수 있는 다른 생물성 아민으로는
트립타민과 페닐에틸아민이 있습니다.
트립타민은
DAO를 억제할 수 있고
페닐에틸아민은 DAO와 HNMT를 모두 억제합니다.
티라민만이
어류에서 독성학적으로 유의할 만큼 높은 농도에 도달할 수 있지만,
이러한 모든 아민의 상승 작용으로 인해
생선을 섭취하는 동안 SFP 반응을 일으킬 수 있습니다 [79].
또한 치즈에 서식하는
락토바실러스 부흐네리균이 생성하는 아민을 확인하기 위한
수많은 연구가 수행되었습니다.
히스타민,
카데베린,
푸트레신,
트립타민,
페닐에틸아민과 같은 아민은
여러 종류의 치즈에서 발견됩니다 [21].
대장균 EF37을 사용한 실험에 따르면
온도를 16°C에서 44°C로 올리면
티라민이 더 빠르고 집중적으로 축적되는 것으로 나타났습니다 [22].
2015년에 Bargossi 등은
대장균에서 추출한 티로신 탈카르복실효소에 가장 유리한 온도를 조사한 결과,
30~37°C의 온도 범위에서
가장 집중적으로 기능한다는 사실을 발견했습니다[80,81].
Tyramine acts by the mechanism as follows in Figure 4.
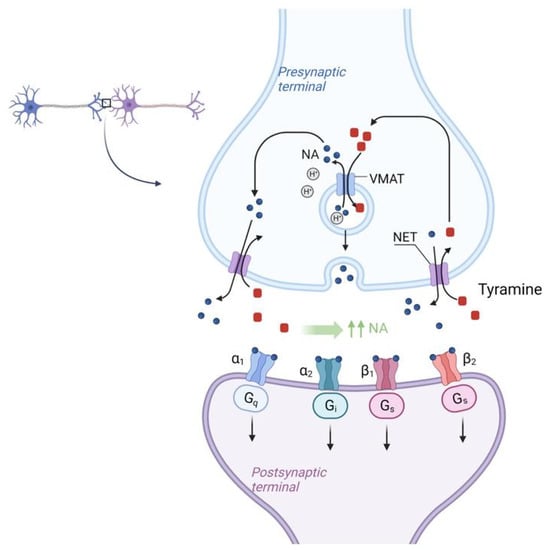
Figure 4. Molecular mechanism of NET (Norepinephrine transporter) activation by tyramine in SFP. Tyramine from the synaptic cleft is transported to the synaptic bulb by the NET (Norepinephrine transporter) in exchange for NE itself. Once inside the synaptic bulb, tyramine is transferred inside the vesicle by the vMAT (vesicular monoamine transporter), located on the membrane of these vesicles, in exchange for NE. This NE then enters the synaptic cleft again via the NET in exchange for tyramine and the cycle repeats. As a result, there is a sharp increase in the concentration of NE in the synaptic cleft. NET—norepinephrine transporter, vMAT—vesicular monoamine transporter.
There is the NET (Norepinephrine transporter) on the membrane of the synaptic bulb of adrenergic neurons. Tyramine from the synaptic cleft is transported to the synaptic bulb by the NET (Norepinephrine transporter) in exchange for NE itself. Once inside the synaptic bulb, tyramine is transferred inside the vesicle by the vMAT (vesicular monoamine transporter) located on the membrane of these vesicles, in exchange for NE. This NE then enters the synaptic cleft again via the NET in exchange for tyramine and the cycle repeats. As a result, there is a sharp increase in the concentration of NE in the synaptic cleft [82]. According to the mechanism of tyramine action, it should be assumed that tyramine, by increasing NE concentration and vasoconstriction, may mitigate the systemic effects of histamine, which is the main BAS responsible for the development of SFP.
아드레날린성 뉴런의 시냅스 전구 막에는
NET(노르에피네프린 수송체)가 있습니다.
시냅스 틈새에서 나온 티라민은
NET(노르에피네프린 수송체)에 의해
NE 자체와 교환하여 시냅스 구근으로 운반됩니다.
시냅스 전구 내부로 들어간 티라민은
소포 막에 있는 vMAT(소포성 모노아민 수송체)에 의해
소포 내부로 옮겨져 NE와 교환됩니다.
이 NE는 티라민과 교환하여
NET를 통해 다시 시냅스 틈새로 들어가고
이 사이클이 반복됩니다.
결과적으로
시냅스 틈새에서
NE의 농도가 급격히 증가합니다 [82].
티라민 작용 메커니즘에 따르면
티라민은 NE 농도와 혈관 수축을 증가시킴으로써
SFP의 발달을 담당하는
주요 BAS 인 히스타민의 전신 효과를 완화 할 수 있다고 가정해야합니다.
3.3. The Role of the Complement System
In many cases of SFP, there is an activation of the complement system, and they are extremely clinically significant because complement activation-related pseudo-allergy (CARPA) has an unpredictable course and may result in a lethal outcome [83].
The complement system participates in natural immune response, but it may also be involved in SFP pathogenesis and damage the cells with anaphylatoxins: C3a, C5, and the membrane attack complex (C5b-C9). There are nanoparticles that can activate the complement without IgE but with anaphylatoxins C3a and C5a that bind with the mast cells and induce the release of various vasoactive mediators that lead to the clinical presentation of the type 1 hypersensitivity reaction, but actually it is CARPA. These nanoparticles are found in some drugs and food products [84,85].
The complement cascade in CARPA may be realized in different pathways: the classic pathway (CP), the lectin pathway (LP), or the alternative pathway (AP) (Figure 5) [20].
SFP의 많은 경우 보체 시스템의 활성화가 있으며,
보체 활성화 관련 유사 알레르기(CARPA)는 예측할 수 없는 경과를 보이며
치명적인 결과를 초래할 수 있기 때문에 임상적으로 매우 중요합니다 [83].
보체 시스템은
자연 면역 반응에 참여하지만
SFP 발병에도 관여하여
아나필라톡신으로 세포를 손상시킬 수 있습니다:
C3a, C5 및 막 공격 복합체(C5b-C9). IgE 없이
보체를 활성화할 수 있지만
비만세포와 결합하여 다양한 혈관 활성 매개체의 방출을 유도하여
제1형 과민반응의 임상 증상을 유발하는
아나필라톡신 C3a 및 C5a를 가진 나노 입자가 있는데,
실제로는 CARPA입니다.
이러한 나노 입자는 일부 약물과 식품에서 발견됩니다 [84,85].
CARPA의 보체 캐스케이드는 고전적 경로(CP), 렉틴 경로(LP) 또는 대체 경로(AP)와 같은 다양한 경로에서 실현될 수 있습니다(그림 5) [20].
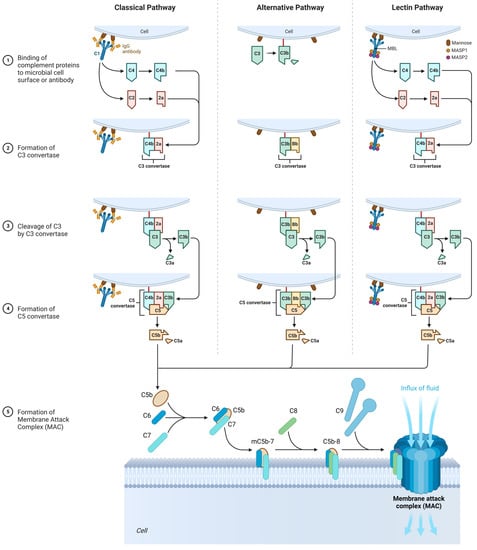
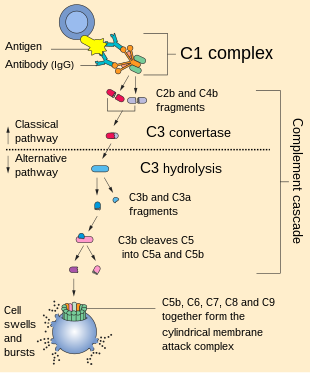
Figure 5. The role of the complement system in the SFP mechanism. Classical pathway: C1 component of complement cleaves C4 and C2 into two fragments. C3 convertase is formed. Under the action of C3 convertase, C3a will be formed. C3a, being an anaphylotoxin, will lead to the basophil degranulation. At the end, MAC is formed that leads to cellular damage. Alternative pathway: C3 spontaneously decomposes to C3a and C3b. After that, C3bBbP complex is formed (C3 convertase of the alternative pathway), and more C3 decomposes to C3a and C3b. The end step is MAC formation. Lectin pathway: All the characteristics are similar to those in classical pathway, except initiation factor. In this case, initiation factor is MBL.
3.3.1. Classic Pathway
Upon activation, C1 component of complement cleaves C4 into two fragments: C4a (small fragment) and C4b (large fragment). C1 then proceeds to cleave the C2 component of the complement into C2a (large fragment) and C2b (small fragment). There are glycoproteins on the cell membrane with which C4b binds first, and C4b then binds to C2a to form the C4bC2a complex, which is called C3 convertase. C3 convertase in turn cleaves C3 into C3a (small fragment) and C3b (large fragment). C3a, being an anaphylotoxin, binds to C3a receptors on the membrane of mast cells and promotes their degranulation.
C3b binds to the free-circulating C5 component of complement. This results in conformational changes in C5, so that it becomes susceptible to the C4bC2a complex, which splits it into C5a (small fragment) and C5b (large fragment). C5a is also an anaphylotoxin and binds to C5a receptors on the membrane of mast cells, contributing to their degranulation [86,87].
C5b binds to C6, forming the C5bC6 complex. Further, C7 and then C8 attach to this complex, forming the C5bC6C7C8 complex. Eventually, this complex is incorporated into the cell membrane and 10 to 18 C9 proteins attach to it, polymerizing with each other and forming a pore on the cell membrane. This whole structure is called MAC (Membrane Attack Complex). The formation of the pore on the cell membrane leads to cell death [86,87].
활성화되면 보체의 C1 성분은
C4를 두 개의 조각으로 나눕니다:
C4a(작은 조각)와 C4b(큰 조각).
그런 다음 C1은
보체의 C2 성분을 C2a(큰 조각)와 C2b(작은 조각)로 쪼개는 과정을 진행합니다.
세포막에는 C4b가 먼저 결합하는 당단백질이 있고, C4b는 C2a와 결합하여 C3 전환 효소라고 하는 C4bC2a 복합체를 형성합니다. C3 전환 효소는 차례로 C3를 C3a(작은 조각)와 C3b(큰 조각)로 절단합니다. 아나필로톡신인 C3a는 비만세포 막의 C3a 수용체에 결합하여 비만세포의 탈과립화를 촉진합니다.
C3b는 자유 순환하는 보체의 C5 성분에 결합합니다. 이로 인해 C5의 형태가 변화하여 C4bC2a 복합체에 취약해져 C5a(작은 조각)와 C5b(큰 조각)로 나뉘게 됩니다. C5a는 또한 아나필로톡신이며 비만세포 막의 C5a 수용체와 결합하여 비만세포의 탈과립화에 기여합니다[86,87].
C5b는 C6에 결합하여 C5bC6 복합체를 형성합니다. 또한 C7과 C8이 이 복합체에 부착하여 C5bC6C7C8 복합체를 형성합니다. 결국 이 복합체는 세포막에 통합되고 10~18개의 C9 단백질이 여기에 부착되어 서로 중합하여 세포막에 기공을 형성합니다. 이 전체 구조를 MAC(막 공격 복합체)라고 합니다. 세포막에 기공이 형성되면 세포 사멸로 이어집니다 [86,87].
3.3.2. Alternative Pathway
It is important to mention that the C3 component of complement is an unstable molecule and spontaneously decomposes to C3a and C3b in the resting state in the blood plasma. However, only a small amount of C3 undergoes this reaction [86,87].
C3b binds to glycoproteins on the cell membrane. After that, C3b is joined by B protein. Then, under the action of factor D, B protein is split into two fragments—Bb (a large fragment), which remains bound to C3b, and Ba (a small fragment), which is released into the extracellular space. As a result, factor P (properdin) is attached to the C3bBb complex, which stabilizes this complex. The C3bBbP complex is also called the C3 convertase of the alternative pathway, which in turn begins to break down other C3 molecules in the blood plasma. This produces a large amount of C3b, which is a “+” feedback mechanism. Next, another C3b molecule joins the C3bBbP complex to form the C3bBbPBb—C5 convertase of the alternative pathway. Further steps are similar to those of the classical pathway [86,87].
보체의 C3 성분은
불안정한 분자이며
혈장 내 휴식 상태에서 C3a 및 C3b로 자발적으로 분해된다는 점을 언급하는 것이 중요합니다.
그러나
소량의 C3만이 이 반응을 거칩니다[86,87].
C3b는 세포막의 당단백질에 결합합니다. 그 후 C3b는 B 단백질과 결합합니다. 그런 다음 인자 D의 작용으로 B 단백질은 C3b에 결합 된 상태로 유지되는 두 개의 단편 (큰 단편)과 세포 외 공간으로 방출되는 Ba (작은 단편)로 나뉩니다. 그 결과, 인자 P(프로퍼딘)가 C3bBb 복합체에 부착되어 이 복합체를 안정화시킵니다. C3bBbP 복합체는 대체 경로의 C3 전환 효소라고도 하며, 이는 혈장 내 다른 C3 분자를 분해하기 시작합니다. 이것은 "+" 피드백 메커니즘인 다량의 C3b를 생성합니다. 다음으로, 또 다른 C3b 분자가 C3bBbP 복합체에 결합하여 대체 경로의 C3bBbPBb-C5 전환효소를 형성합니다. 추가 단계는 고전적 경로와 유사합니다[86,87].
3.3.3. Lectin Pathway
In contrast to the classical complement pathway, lectin pathway is not initiated by antigen–antibody complex. Initiation of lectin pathway starts with binding of mannose-binding lectin (MBL) with terminal carbohydrate residues of D-mannose and other sugars with 3- and 4-OH groups placed in the equatorial plane, that can be found on the surfaces of certain pathogens. The function of MBL in lectin pathway is recognition of pathogen-associated carbohydrate patterns and activation of complement system [86,87].
Multimers of MBL form a complex with MBL-Associated Serine Proteases (MASP1 and MASP2), that are very similar to C1r and C1s molecules of the classical complement pathway. MASPs are protease zymogens. When the carbohydrate-recognizing heads of MBL bind to specifically arranged sugar residues on the surface of a pathogen, MASP1 and MASP2 start to cleave complement components C4 and C2. Cleavaged C4b binds to a bacterial cell, combines with C2a, and forms classical C3 convertase (C4bC2a) [86,87].
고전적인 보체 경로와
달리 렉틴 경로는
항원-항체 복합체에 의해 시작되지 않습니다.
렉틴 경로의 시작은
특정 병원체의 표면에서 발견되는
적도 평면에 3-OH 및 4-OH기가 있는 D-만노스 및 기타 당의
말단 탄수화물 잔기와 만노스 결합 렉틴(MBL)이 결합하는 것으로 시작됩니다.
렉틴 경로에서
MBL의 기능은 병원체와 관련된 탄수화물 패턴을 인식하고
보체 시스템을 활성화하는 것입니다[86,87].
MBL의 멀티머는
고전적인 보체 경로의 C1r 및 C1s 분자와 매우 유사한
MBL-관련 세린 프로테아제(MASP1 및 MASP2)와 복합체를 형성합니다.
MASP는 프로테아제 효소 유전자입니다. MBL의 탄수화물 인식 헤드가 병원체 표면에 특별히 배열된 당 잔류물에 결합하면 MASP1과 MASP2가 보체 성분 C4와 C2를 절단하기 시작합니다. 절단된 C4b는 박테리아 세포에 결합하고 C2a와 결합하여 고전적인 C3 전환효소(C4bC2a)를 형성합니다[86,87].
3.4. The Role of Histamine Liberators
Another important mechanism in the pathogenesis of SFP is the influence of histamine liberators on the mast cells. These substances induce the release of endogenous histamine from the secretory cells. In recent decades, many histamine-liberating amines, amides, guanines, guanidines, and other organic compounds have been discovered. Research regarding such substances has been conducted up to now, so the list of histamine liberators is constantly expanding [88].
It is noteworthy that mast cells may be activated directly by substances with low molecular mass—histamine liberators. Their activation is usually associated with systemic pseudoallergenic or peptide food supplements (the latter are usually given parenterally because digestive enzymes destroy them when given orally). Some molecules, for instance compound 48/80 (a polymeric amine, product of condensation between formaldehyde and p-methoxy-phenethyl-methylamine), may stimulate the activity of phospholipase D that activates synthesis of endogenous phospholipids which bind with the lysophosphatide acid receptor [89]. This process facilitates the activation of G-proteins, which may induce the release of mediators from the mast cells, synthesis of phosphatidylinositol-3-kinase, and the formation of arachidonate metabolites. Phosphatidylinositol-3-kinase stimulator activates phospholipase C gamma that stimulates phosphatidylinositol-4,5-bisphosphate hydrolysis that results in the formation of inositol-1,4,5-triphosphate and diacylglycerol [90]. These mediators induce the elevation of intracellular Ca2+ levels, which activates protein kinase C. This series of reactions leads to degranulation of the mast cells and the release of inflammatory mediators that are generated in the PTK-PLA2 pathway. These events lead to histamine leaving the cell and the development of the FPA [91].
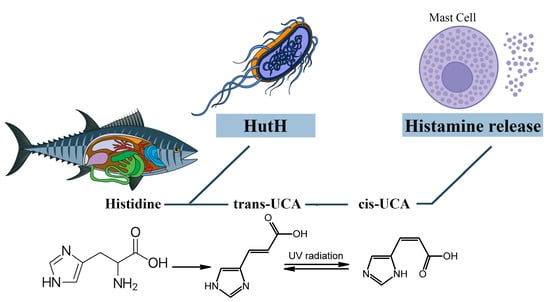
It is assumed that cis-urocanic acid is the histamine liberator in the mechanism of SFP [92,93,94,95]. Cis-urocanic acid is a degradation product of the protein amino acid L-histidine. Deaminated histidine is converted to the more soluble cis isomer by UV [96]. Based on the assumptions of Wille et al., urocanic acid is a mast cell degranulator or histamine liberator [92]. Norval et al.’s studies have shown that the administration of cis-urocanoic acid, regardless of the route of administration, induces histamine release [97,98]. The structure of cis-urocanoic acid has been found to be similar to that of serotonin, having the property of binding to the 5-HT2a receptor and activating immune suppression [99,100]. The ability of cis-urocanoic acid to induce mast cell degranulation has been demonstrated in human skin cultures, and depletion of the content of mast cell granules has been recorded [96].
Cis-urocanic acid in the pathogenesis of SFP is formed from the histidine of spoiled fish. Under the action of the putrefactive bacterial enzyme histidine ammonia-lyase (histidase), histidine is converted into trans-urocanic acid, which accumulates in fish [101,102]. Over time, the isomerization of trans-urocanic acid to cis-urocanic acid occurs. This process amplifies the ultraviolet radiation with its peak at 345 nm, e.g., storing fish in the sun [103,104,105]. It means that the concentration of histamine in SFP is elevated not only because of the exogenous histamine of the fish but also because of the endogenous histamine released from the mast cells [93] (Figure 6).
SFP의 발병 기전에서
또 다른 중요한 메커니즘은
비만 세포에 대한 히스타민 해방제의 영향입니다.
이러한 물질은
분비 세포에서 내인성 히스타민의 방출을 유도합니다.
최근 수십 년 동안
많은 히스타민 방출 아민, 아미드, 구아닌, 구아니딘 및 기타 유기 화합물이 발견되었습니다.
이러한 물질에 관한 연구는
지금까지 수행되어 왔으므로
히스타민 해방제 목록은 지속적으로 확장되고 있습니다 [88].
비만 세포는
저 분자량 히스타민 해방제를 가진 물질에 의해
직접 활성화 될 수 있다는 점은 주목할 만합니다.
이들의 활성화는
일반적으로 전신 유사 알레르기 또는
펩타이드 식품 보충제와 관련이 있습니다
(후자는 경구 투여 시 소화 효소에 의해 파괴되기 때문에 일반적으로 비경구 투여).
예를 들어
화합물 48/80(고분자 아민, 포름알데히드와 p-메톡시-페닐-메틸아민 사이의 축합 생성물)과 같은 일부 분자는
리소포스파티드산 수용체와 결합하는 내인성 인지질의 합성을 활성화하는
포스포리파제 D의 활성을 자극할 수 있습니다[89].
이 과정은
비만 세포에서 매개체의 방출,
포스파티딜이노시톨-3-키나아제 합성 및 아라키도네이트 대사 산물의 형성을 유도할 수 있는
G 단백질의 활성화를 촉진합니다.
포스파티딜이노시톨-3-키나아제 자극제는
포스파티딜이노시톨-4,5-비스포스페이트 가수분해를 자극하여
이노시톨-1,4,5-트리인산염과 디아실글리세롤을 형성하는
포스포리파제 C 감마를 활성화합니다[90].
이러한 매개체는
세포 내 Ca2+ 수준의 상승을 유도하여
단백질 키나아제 C를 활성화합니다.
이러한 일련의 반응은
비만 세포의 탈과립화와 PTK-PLA2 경로에서 생성되는
염증 매개체의 방출로 이어집니다.
이러한 사건은
히스타민이 세포를 떠나고
FPA의 발달로 이어집니다 [91].
시스-우르칸산은
SFP의 메커니즘에서
히스타민 해방제라고 가정합니다 [92,93,94,95].
시스-우르 칸산은
단백질 아미노산 L- 히스티딘의 분해 산물입니다.
탈아민화된 히스티딘은
자외선에 의해 더 잘 녹는 시스 이성질체로 전환됩니다 [96].
Wille 등의 가정에 따르면
우로칸산은
비만 세포 탈과립제 또는 히스타민 해방제입니다 [92].
Norval 등의 연구에 따르면
시스-우로카노산의 투여는 투여 경로에 관계없이
히스타민 방출을 유도하는 것으로 나타났습니다 [97,98].
시스-우르카노산의 구조는
세로토닌과 유사한 것으로 밝혀졌으며
5-HT2a 수용체에 결합하여
면역 억제를 활성화하는 성질을 가지고 있습니다 [99,100].
시스-우르카노산이 비만 세포 탈과립화를 유도하는 능력은 인간 피부 배양에서 입증되었으며, 비만 세포 과립의 함량이 고갈되는 것으로 기록되었습니다 [96].
SFP의 발병 기전에서
시스-우로칸산은 부패한 생선의 히스티딘에서 형성됩니다.
부패성 박테리아 효소 히스티딘 암모니아 분해 효소 (히스티다 제)의 작용으로
히스티딘은 트랜스 우르 칸산으로 전환되어 어류에 축적됩니다 [101,102].
시간이 지남에 따라
트랜스-우르칸산이 시스-우르칸산으로 이성질화됩니다.
이 과정은 햇볕에 생선을 보관하는 것과 같이 345nm에서 피크가 높은 자외선을 증폭시킵니다 [103,104,105]. 이는 물고기의 외인성 히스타민뿐만 아니라 비만 세포에서 방출되는 내인성 히스타민으로 인해 SFP의 히스타민 농도가 높아진다는 것을 의미합니다 [93] (그림 6).
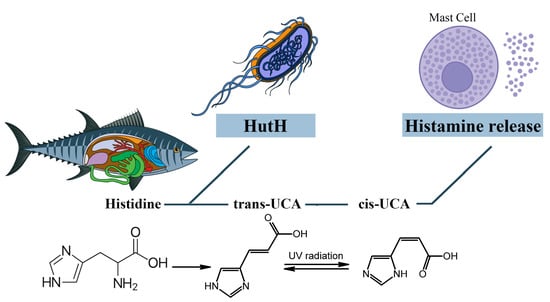
Figure 6. The role of cis-urocanic acid in the SFP mechanism. Histidine is converted into trans-urocanic acid, which accumulates in fish. Over time, the isomerization of trans-urocanic acid to cis-urocanic acid occurs, which plays the role of histamine liberator. HutH—histidine ammonia-lyase, UCA—urocanic acid.
However, Zara D. et al. found that, in the case of tuna fish, the key link in the pathogenesis is histamine and not cis-urocanoic acid. When storing tuna fish at a temperature of 3 °C for 15 days, the level of histamine increased, while the level of cis-urocanoic acid remained low [106]. It can be concluded that fish storage temperature and exposure to UV are important in the mechanism of cis-urocanoic acid formation. Due to the insufficient study of trans- and cis-urocanoic acids in the food industry and the consequences of consuming cis-urocanoic acid exclusively for the human body, the limits of permissible concentration for this compound have not yet been established.
그러나 Zara D. 등은
참치 생선의 경우 병인의 핵심 연결 고리는
시스 우르카노산이 아니라 히스타민이라는 것을 발견했습니다.
참치 생선을
3°C의 온도에서 15일 동안 보관했을 때
히스타민 수치는 증가한 반면
시스-우르카노산 수치는 낮게 유지되었습니다 [106].
생선 보관 온도와 자외선 노출이 시스-우르카노산 형성 메커니즘에서 중요하다는 결론을 내릴 수 있습니다.
식품 산업에서 트랜스 및 시스 우로카노산에 대한 연구가 불충분하고 시스 우로카노산을 인체에 독점적으로 섭취 한 결과로 인해이 화합물에 대한 허용 농도 한계는 아직 확립되지 않았습니다.
3.5. The Role of Associated Diseases and Disorders in the SFP Mechanism
Chronic gastrointestinal diseases, such as irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and gastroesophageal reflux disease (GERD), may disrupt the function of the intestinal mucosa and increase the absorption of histamine liberators into the mast cells of the mucosa. Because of that, these diseases play an important role in the development of FPA, such as SFP [56]. In that case, SFP is associated with individual sensitivity to products with a high content of cis-urocanic acid rather than a high amount of exogenous histamine and other biogenic amines in food products [93].
Another suggested pathological mechanism of SFP to a large amount of consumed exogenous histamine is the disruption of function of the catabolic enzyme DAO (DAO defect) in some people [71,107] (Figure 5). Petersen in 2002 and Schwelberger in 2004 discovered populations of patients genetically predisposed to gastrointestinal diseases with mutations in the SNP gene that codes for DAO [108,109]. It is possible that DAO synthesized in the intestines may have a crucial role in the development of histamine intolerance and the susceptibility of certain people to SFP [71]. This means that histamine intolerance may develop because of an enzyme deficiency or abnormal functioning. This is still an assumption because there are no reliable studies that show that decreased DAO activity might be a reason for the reaction to the ingested histamine. Many factors must be taken into consideration. Firstly, there is an alternative pathway of histamine metabolism in the human body, methylation by histamine-N-methyltransferase. More than that, histamine levels in food products vary depending on the conditions of storage, timing, and method of processing.
The leading role in the pathogenesis of SFP belongs to exogenous histamine. Histamine, in addition to its systemic effect, which leads to the development of the main symptoms of SPF (flushing, swelling, itchy rash, hypotension, acute pulmonary edema, bronchoconstriction) has an effect on the immune system. Histamine stimulates the secretion of large amounts of IL-4 and IL-5 by Th2-helper cells. IL-4 stimulates the proliferation of B cells, while IL-5 promotes the differentiation of B cells into plasma cells. It is important to know that the excessive release of IL-4, which occurs under the influence of histamine, leads to the selective synthesis of IgE by plasma cells. In turn, IgE will lead to prolonged mast cell activation, which may underlie the pathogenesis of patients developing mast cell activation syndrome and intestinal mastocytosis [110,111,112,113].
The molecular mechanism by which mast cell activation occurs under the action of exogenous histamine is shown in Figure 7. The mast cell membrane contains FcεRI receptors, which consist of one α, one β and two γ subunits. The cytoplasmic tails of the β and γ subunits contain immunoreceptor tyrosine-based activation motifs (ITAMs) that become phosphorylated upon FcεRI aggregation. This phosphorylation is mediated primarily by the Src family tyrosine kinase Lyn. Further, the Src homology (SH2) domain of Syk protein binds to the inorganic phosphate of γ subunits, and the Lyn molecule binds to the inorganic phosphate of β subunits with the same SH2 domain. Lyn phosphorylates Syk and thereby activates its kinase enzymatic function [114,115]. The linker for activation of T cells (LAT) protein is built into the membrane of mast cells and is a target for Syk and Lyn molecules, which phosphorylate LAT on multiple tyrosine residues. The inorganic phosphates on LAT protein serve as docking sites for SH2 domains of GRB2-related adaptor protein (GADS), growth-factor receptor-bound protein 2 (GRB2), and phospholipase Cγ1 (PLCγ1) [114,115].
과민성 대장 증후군(IBS),
염증성 장 질환(IBD),
위식도 역류 질환(GERD)과 같은 만성 위장 질환은
장 점막의 기능을 방해하고
점막의 비만 세포로
히스타민 해방제의 흡수를 증가시킬 수 있습니다.
따라서
이러한 질병은
SFP와 같은 FPA의 발병에 중요한 역할을 합니다 [56].
이 경우 SFP는
식품에 다량의 외인성 히스타민 및 기타 생체 아민보다는 시스-우로칸산 함량이 높은 제품에 대한 개별 민감도와 관련이 있습니다 [93].
다량의 외인성 히스타민 섭취에 대한 SFP의
또 다른 제안 된 병리학 적 메커니즘은
일부 사람들의 이화 효소 DAO (DAO 결함)의 기능 장애입니다 [71,107] (그림 5).
2002년 피터슨과 2004년 슈벨버거는
유전적으로 위장병에 걸리기 쉬운 환자 집단에서 DAO를 코딩하는
SNP 유전자의 돌연변이를 발견했습니다[108,109].
장에서 합성되는 DAO가
히스타민 과민증의 발병과 특정 사람들의
SFP 감수성에 중요한 역할을 할 수 있습니다 [71].
이는 효소 결핍이나 기능 이상으로 인해
히스타민 과민증이 발생할 수 있음을 의미합니다.
DAO 활성 감소가 섭취한 히스타민에 대한 반응의 원인이 될 수 있다는 신뢰할 수 있는 연구가 없기 때문에
이는 여전히 가정일 뿐입니다. 여러 가지 요인을 고려해야 합니다.
첫째,
인체에는 히스타민 대사의 대체 경로인
히스타민-N-메틸전달효소에 의한 메틸화라는 경로가 있습니다. 그
외에도 식품의 히스타민 수치는 보관 조건, 시기 및 가공 방법에 따라 달라집니다.
SFP의 발병 기전에서 주
도적인 역할을 하는 것은 외인성 히스타민입니다.
히스타민은
SPF의 주요 증상 (홍조, 부기, 가려운 발진, 저혈압, 급성 폐부종, 기관지 수축)의 발달로 이어지는
전신 효과 외에도 면역 체계에 영향을 미칩니다.
히스타민은
Th2 헬퍼 세포에 의해 다량의 IL-4 및 IL-5 분비를 자극합니다.
IL-4는 B 세포의 증식을 자극하고
IL-5는 B 세포가 형질 세포로 분화하는 것을 촉진합니다.
히스타민의 영향으로 발생하는
IL-4의 과도한 방출은 혈장 세포에 의한 IgE의 선택적 합성으로 이어진다는 것을 아는 것이 중요합니다.
결과적으로
IgE는 장기간의 비만 세포 활성화로 이어져
비만 세포 활성화 증후군 및 장 비만 세포증이 발생하는 환자의 병인의 기초가 될 수 있습니다 [110,111,112,113].
외인성 히스타민의 작용으로
비만 세포 활성화가 일어나는 분자 메커니즘은
그림 7에 나와 있습니다.
비만 세포막에는 α 1개, β 1개, γ 2개 서브유닛으로 구성된 FcεRI 수용체가 포함되어 있습니다. β 및 γ 서브유닛의 세포질 꼬리에는 면역 수용체 티로신 기반 활성화 모티프(ITAM)가 포함되어 있으며, 이는 FcεRI 응집 시 인산화됩니다. 이 인산화는 주로 Src 계열 티로신 키나아제 Lyn에 의해 매개됩니다. 또한, Syk 단백질의 Src 상동성(SH2) 도메인은 γ 서브유닛의 무기 인산염에 결합하고, Lyn 분자는 동일한 SH2 도메인을 가진 β 서브유닛의 무기 인산염에 결합합니다. Lyn은 Syk를 인산화하여 키나아제 효소 기능을 활성화합니다[114,115]. T세포 활성화용 링커(LAT) 단백질은 비만세포의 막에 내장되어 있으며, 여러 티로신 잔기에서 LAT를 인산화하는 Syk 및 Lyn 분자의 표적입니다. LAT 단백질의 무기 인산염은 GRB2 관련 어댑터 단백질(GADS), 성장 인자 수용체 결합 단백질 2(GRB2), 포스포리파아제 Cγ1(PLCγ1)의 SH2 도메인을 위한 도킹 부위 역할을 합니다[114,115].
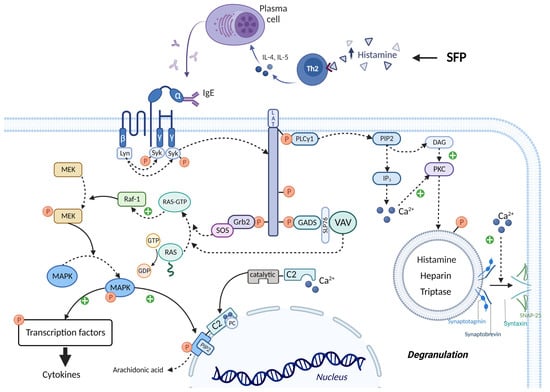
Figure 7. IgE-mediated activation of mast cells in SFP. IgE binds to the FcεRI receptors and leads to the activation of Lyn and Syn. Syn phosphorylates LAT, thereby creating a platform for recruitment of Grb2, GADS, and PLCγ1. Grb2 and GADS activate biochemical cascades which lead to MAPK activation and cytokines production. PLCγ1 activates IP3 system and leads to the mast cell degranulation and arachidonic acid production. ITAMs—immunoreceptor tyrosine-based activation motifs, SH2—Src homology domain, LAT—linker for activation of T cells, GADS—GRB2-related adaptor protein, GRB2—growth-factor receptor-bound protein 2, PLCγ1—phospholipase Cγ1, SOS—the Son of Sevenless, GEFs—guanine nucleotide exchange factors, RAF-1—proto-oncogene serine/threonine-protein kinase, MEK—MAPK/ERK kinase, MAPK—mitogen-activated protein kinase, SLP76—SH2-domain-containing leukocyte protein of 76 kDa, cPLA2α—cytoplasmic phospholipase A2α.
The SH3 domain of the GRB2 molecule is bound to the proline-rich region of the Son of Sevenless (SOS) protein, which belongs to guanine nucleotide exchange factors (GEFs). In the quiescent state in the cell cytoplasm, there is RAS protein bound to GDP—inactive state. SOS detaches the GDP molecule from RAS and attaches GTP in its place—active state. Then RAS-GTP goes to the RAF-1 protein (proto-oncogene serine/threonine-protein kinase) and activates it. In turn, RAF-1 phosphorylates and thereby activates the enzyme MEK (MAPK/ERK kinase). MEK phosphorylates the mitogen-activated protein kinase (MAPK) molecule by the OH group of serine/threonine (mitogen-activated protein kinase) and thereby activates it. Ultimately, MAPK phosphorylates and activates specific transcription factors that increase the expression of genes responsible for cytokine synthesis (such as TNF-α, IL-8, and MCP-1) [114,115]. The SH3 domain of the GADS protein binds to SH2- domain-containing leukocyte protein of 76 kDa (SLP76), which is an adaptor protein for the attachment and activation of VAV. In turn, VAV being GEF performs the same function as SOS—MAPK activation [114,115].
PLCγ1 activates the inositol trisphosphate system by increasing the concentration of calcium in the cytoplasm and activating PKC. Calcium promotes the fusion of the vesicle membrane containing biologically active substances (BAS) with the cell membrane. This occurs by the following mechanism: the Soluble NSF attachment receptor (vSNARE) proteins Synaptobrevin and Synaptotagmin are on the vesicle membrane, and the tSNARE proteins Syntaxin and SNAP-25 are on the mast cell membrane. Under the influence of calcium, the vSNARE proteins intertwine with the tSNARE proteins, bringing the vesicle membrane closer to the cell membrane and eventually promoting their fusion. That is, vesicle exocytosis occurs and BAS enters the extracellular space. In addition, PKC itself phosphorylates the vesicles and thus promotes their fusion with the mast cell membrane. One of the molecules released during mast cell degranulation is histamine. This leads to a vicious circle and exacerbates the allergic reaction [114,115,116].
It is important to know that calcium ions and MAPK activate cPLA2α, increasing the production by mast cells of prostaglandins, thromboxanes, leukotrienes, lipoxins, resolvins, and eoxins. This occurs by the following mechanism: the molecule Cytoplasmic phospholipase A2α (cPLA2α) has a C2 domain and a catalytic domain. Two calcium cations bind to the C2 domain of cPLA2α, stimulating the interaction of this domain with phosphatidylcholine (PC) located on the perinuclear membrane. Thus, cPLA2α gains access to the phospholipids of the perinuclear membrane, hydrolyzing them to form arachidonic acid. But calcium alone is not sufficient for prolonged activation of cPLA2α. MAPK phosphorylates cPLA2α by the OH group of serine/threonine, which changes the conformation of this molecule so that the catalytic domain of cPLA2α binds to Phosphatidylinositol-4,5-bisphosphate (PIP2), a constituent of the perinuclear membrane, which ensures long-term binding of cPLA2α to the membrane, even in the absence of calcium ions [114,115].
As we discussed previously, plasma cells are forced to synthesize IgE under the influence of a huge amount of exogenous histamine. IgE activates mast cells that secrete many biological active mediators, which include β-Hex, HIS, and pro-inflammatory cytokines (such as TNF-α, IL-8, and MCP-1). All these molecules, and especially TNF-α, lead to the development of the inflammation, mainly through the activation of NF-κB pathway [117]. There are two main NF-κB pathways: canonical and non-canonical (Figure 8).
GRB2 분자의 SH3 도메인은
구아닌 뉴클레오티드 교환 인자(GEF)에 속하는
아들 오브 세븐리스(SOS) 단백질의 프롤린이 풍부한 영역에 결합되어 있습니다.
세포질 내 휴지 상태에서는 RAS 단백질이 GDP 비활성 상태로 결합되어 있습니다. SOS는 RAS에서 GDP 분자를 분리하고 그 자리에 활성 상태의 GTP를 부착합니다. 그런 다음 RAS-GTP는 RAF-1 단백질(원시 종양 유전자 세린/트레오닌 단백질 키나아제)로 이동하여 활성화합니다. 차례로 RAF-1은 인산화되어 효소 MEK(MAPK/ERK 키나아제)를 활성화합니다. MEK는 세린/트레오닌(미토겐 활성화 단백질 키나아제)의 OH기에 의해 미토겐 활성화 단백질 키나아제(MAPK) 분자를 인산화하여 이를 활성화합니다. 궁극적으로 MAPK는 인산화되어 사이토카인 합성을 담당하는 유전자(예: TNF-α, IL-8, MCP-1)의 발현을 증가시키는 특정 전사인자를 활성화합니다[114,115]. GADS 단백질의 SH3 도메인은 VAV의 부착 및 활성화를 위한 어댑터 단백질인 76kDa의 SH2 도메인 함유 백혈구 단백질(SLP76)에 결합합니다. 결과적으로 GEF가 된 VAV는 SOS-MAPK 활성화와 동일한 기능을 수행합니다[114,115].
PLCγ1은 세포질 내 칼슘 농도를 증가시키고 PKC를 활성화하여 이노시톨 삼인산 시스템을 활성화합니다. 칼슘은 생물학적 활성 물질(BAS)을 함유한 소포막과 세포막의 융합을 촉진합니다. 이는 다음과 같은 메커니즘에 의해 발생합니다: 수용성 NSF 부착 수용체(vSNARE) 단백질인 시냅토브레빈과 시냅토택민은 소포막에, tSNARE 단백질인 신탁신과 SNAP-25는 비만세포막에 존재합니다. 칼슘의 영향을 받아 vSNARE 단백질은 tSNARE 단백질과 서로 얽혀서 소포막을 세포막에 더 가깝게 만들고 결국 융합을 촉진합니다. 즉, 소포 외세포화가 일어나고 BAS가 세포 외 공간으로 들어갑니다. 또한 PKC 자체는 소포체를 인산화하여 비만 세포막과의 융합을 촉진합니다. 비만 세포 탈과립 과정에서 방출되는 분자 중 하나는 히스타민입니다. 이것은 악순환으로 이어지고 알레르기 반응을 악화시킵니다 [114,115,116].
칼슘 이온과 MAPK가 cPLA2α를 활성화하여 프로스타글란딘, 트롬복산, 류코트리엔, 리폭신, 레졸빈 및 에옥신의 비만 세포 생성을 증가시킨다는 사실을 아는 것이 중요합니다. 이는 다음과 같은 메커니즘에 의해 발생합니다: 세포질 포스포리파제 A2α(cPLA2α) 분자는 C2 도메인과 촉매 도메인을 가지고 있습니다. 두 개의 칼슘 양이온이 cPLA2α의 C2 도메인에 결합하여 이 도메인과 핵 주위 막에 위치한 포스파티딜콜린(PC)의 상호 작용을 자극합니다. 따라서 cPLA2α는 핵 주위막의 인지질에 접근하여 이를 가수분해하여 아라키돈산을 형성합니다. 그러나 칼슘만으로는 cPLA2α의 장기적인 활성화에 충분하지 않습니다. MAPK는 세린/트레오닌의 OH기에 의해 cPLA2α를 인산화하여 이 분자의 형태를 변화시켜 cPLA2α의 촉매 도메인이 핵막 주위막의 구성 성분인 포스파티딜이노시톨-4,5-비스포스페이트(PIP2)에 결합하여 칼슘 이온이 없는 경우에도 cPLA2α가 막에 장기간 결합할 수 있도록 합니다[114,115].
앞서 설명했듯이 혈장 세포는 엄청난 양의 외인성 히스타민의 영향을 받아 IgE를 합성해야 합니다. IgE는 β-Hex, HIS 및 전 염증성 사이토카인(예: TNF-α, IL-8, MCP-1)을 포함한 많은 생물학적 활성 매개체를 분비하는 비만 세포를 활성화합니다. 이러한 모든 분자, 특히 TNF-α는 주로 NF-κB 경로의 활성화를 통해 염증을 일으킵니다 [117]. NF-κB 경로에는 표준 경로와 비표준 경로의 두 가지 주요 경로가 있습니다(그림 8).
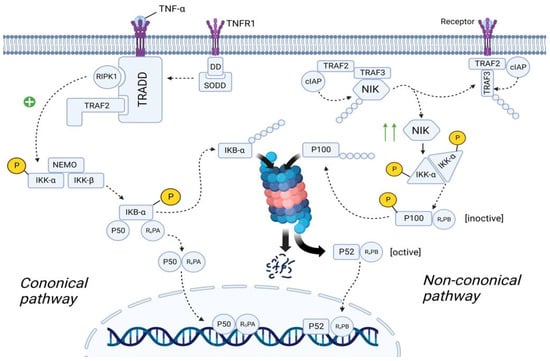
Figure 8. The role of the NF-κB in the SFP mechanism. Canonical pathway: TNF-α binds to TNFR1 and TNFR1 undergoes homotrimerization. Then, SODD dissociates from the TNFR1, which will lead to the recruitment of TRADD. In turn, TRADD recruits RIPK1 and IKK. RIPK1 phosphorylates and activates IKK. IKK starts phosphorylating the IkBα subunit of the NF-kβ and thereby promotes ubiquitination and further proteosomal degradation of IkBα. As a result, NF-kβ is transported to the nucleus. Non-canonical pathway: In the resting state, TRAF2/TRAF3/cIAP/NIK complex exists. Formation of this complex will lead to the proteosomal degradation of NIK. After ligand–receptor interaction, TRAF2/TRAF3/cIAP complex dissociates from NIK, preventing its proteosomal degradation. The number of NIK in the cell increases. NIK starts phosphorylating and activating the IKKα. IKKα leads to the formation of the p52/RelB complex and its translocation to the nucleus. TNFα—tumor necrosis factor α, TNFR1—tumor necrosis factor receptor1, DD—death domain, SODD—silence of death domain, TRADD—tumor necrosis factor receptor type 1-associated death domain protein, RIPK1—receptor-interacting serine/threonine-protein kinase 1, TRAF2—TNF receptor-associated factor 2, IKK—IκB kinase, NEMO—NF-kappa-B essential modulator, IkBα—inhibitor of nuclear factor (NF)-Κb α isoform, NLS—nuclear localization sequence, cIAP—cellular inhibitor of apoptosis protein 1, NIK—NF-kappa-B-inducing kinase.
3.5.1. Canonical NF-κB Pathway
In the quiescent state, TNFR1 (tumor necrosis factor receptor) is embedded in the cell membrane as monomers. The cytoplasmic part of TNFR1 contains DD (Death Domain), to which the molecule SODD (Silence Of Death Domain) is linked, blocking the activity of DD. When TNF-α binds to TNFR1, TNFR1 undergoes homotrimerization. In this state, TNFR1 changes its conformation, which leads to the detachment of SODD from the DD of TNFR1. Further, the adaptor protein TRADD (Tumor necrosis factor receptor type 1-associated DEATH domain protein) binds to DD. In turn, TRADD recruits the molecule RIPK1 (Receptor-interacting serine/threonine-protein kinase 1) and TRAF2 (TNF receptor-associated factor 2). TRAF2 then recruits the molecule IKK (IκB kinase), which consists of three subunits: IKK-α, IKK-β, and IKK-γ (NEMO—NF-kappa-B essential modulator). RIPK1 phosphorylates IKK-β by the OH-groups of the serine/threonine residues, leading to activation of the IKK molecule [118,119].
The cell cytosol contains the NF-κb molecule, which consists of three subunits: IkBα (Inhibitor of Nuclear Factor (NF)-Κb α isoform), p50, and RelA. In the resting state, IkBα masks the NLS (nuclear localization sequence) of p50 and RelA proteins, preventing transport of these subunits as a heterodimer from the cell cytosol into the nucleoplasm. However, upon activation, IKK starts phosphorylating the IkBα subunit by the OH-groups of the serine/threonine residues. Further, the enzyme E3 ubiquitin ligase binds to the phosphorylated serine/threonine residues, promoting ubiquitination and further proteosomal degradation of IkBα. As a result, NLS is unmasked and the p50/RelA heterodimer is transferred from the cell cytoplasm into the nucleoplasm [119,120].
휴면 상태에서는
TNFR1(종양괴사인자 수용체)이 단량체 형태로 세포막에 내장되어 있습니다.
TNFR1의 세포질 부분에는 DD(죽음 도메인)가 포함되어 있으며, 여기에 SODD(실런스 오브 데스 도메인)라는 분자가 연결되어 DD의 활성을 차단합니다. TNF-α가 TNFR1에 결합하면 TNFR1은 호모트리머화를 거칩니다. 이 상태에서 TNFR1은 그 형태가 변화하여 TNFR1의 DD에서 SODD가 분리됩니다. 또한 어댑터 단백질 TRADD(종양 괴사 인자 수용체 1형 관련 사멸 도메인 단백질)가 DD에 결합합니다. 차례로 TRADD는 RIPK1(수용체 상호 작용 세린/트레오닌 단백질 키나아제 1) 및 TRAF2(TNF 수용체 관련 인자 2) 분자를 모집합니다. 그런 다음 TRAF2는 세 개의 서브유닛으로 구성된 IKK(IκB 키나아제) 분자를 모집합니다: IKK-α, IKK-β, IKK-γ(NEMO-NF-kappa-B 필수 조절인자)로 구성됩니다. RIPK1은 세린/트레오닌 잔기의 OH 그룹에 의해 IKK-β를 인산화하여 IKK 분자의 활성화를 유도합니다[118,119].
세포질에는 세 개의 서브유닛으로 구성된 NF-κb 분자가 포함되어 있습니다: IkBα(핵 인자(NF)-Κb α 이소형 억제제), p50 및 RelA. 휴면 상태에서 IkBα는 p50 및 RelA 단백질의 NLS(핵 위치 서열)를 마스킹하여 이 서브유닛이 세포질에서 핵질로 헤테로다이머로 운반되는 것을 방지합니다. 그러나 활성화되면 IKK는 세린/트레오닌 잔기의 OH 그룹에 의해 IkBα 서브유닛을 인산화하기 시작합니다. 또한 효소 E3 유비퀴틴 리가제는 인산화된 세린/트레오닌 잔기에 결합하여 유비퀴틴화를 촉진하고 IkBα의 프로테오솜 분해를 촉진합니다. 그 결과, NLS가 마스크를 벗고 p50/RelA 헤테로다이머가 세포질에서 핵질로 옮겨집니다[119,120].
3.5.2. Non-Canonical NF-κB Pathway
In the quiescent state of the cell, cytosol contains TRAF2 molecule, to which cIAP (Cellular inhibitor of apoptosis protein 1) and TRAF3 molecules are bound. In turn, the NIK (NF-kappa-B-inducing kinase) molecule is linked to TRAF3. cIAP performs ubiquitination of the NIK, contributing to its proteosomal degradation. This suggests that in the resting state, the concentration of NIK in the cell cytosol is very low [118,120].
TRAF2 in complex with TRAF3 and cIAP are recruited to the receptor. NIK dissociates from this complex and is free in the cytoplasm of the cell. Since NIK is free, cIAP cannot ubiquitinate it, resulting in an increase in the concentration of NIK in the cell cytosol. Recruitment of the TRAF2/TRAF3/cIAP complex to the receptor causes cIAP to begin to ubiquitinate TRAF3 within this complex, contributing to its proteosomal degradation. This leads to a decrease in the concentration of TRAF3 in the cell cytoplasm, thus preventing the assembly of a new TRAF2/TRAF3/cIAP/NIK complex, resulting in the inability to ubiquitinate NIK and, consequently, an increase in NIK concentration [119,121].
The cell cytosol contains the NF-κB molecule, which consists of two subunits: p100 and RelB. However, this is the inactive state of NF-κB. NIK starts phosphorylating the IKKα/IKKα homodimer by the OH-groups of the serine/threonine residues, leading to its activation. It is important to note that this complex exists without NEMO protein. In turn, IKKα, on the one hand, phosphorylates NIK, which leads to its ubiquitination and further complete proteosomal degradation (negative feedback mechanism), and on the other hand, phosphorylates p100, which leads to its ubiquitination and partial proteosomal degradation. As a result, p100 is converted into p52 and the p52/RelB complex is formed, which is transported into the nucleoplasm [120,121].
Both p52/RelB and p50/RelA complexes are transcription factors that increase the expression of the genes responsible for the synthesis of cytokines (TNF-α, IFN-β, IL-β, IL-2, IL-3, IL-6, and etc.), chemokines (IL-8, RANTES, MCP-1), adhesion molecules (E-selectin, ICAM-1, and VCAM-1), acute response protein, complement, growth factor (GM-CSF, G-CSF, and M-CSF), etc. [119].
세포의 대기 상태에서 세포질에는 TRAF2 분자가 포함되어 있으며, 여기에는 cIAP(세포 사멸 단백질 1의 세포 억제제)와 TRAF3 분자가 결합되어 있습니다. 차례로 NIK(NF-kappa-B 유도 키나아제) 분자는 TRAF3와 연결되어 있습니다. cIAP는 NIK의 유비퀴틴화를 수행하여 프로테오솜 분해에 기여합니다. 이는 휴식 상태에서 세포질 내 NIK의 농도가 매우 낮다는 것을 시사합니다[118,120].
TRAF3 및 cIAP와 복합체를 이루는 TRAF2는 수용체에 모집됩니다. NIK는 이 복합체에서 해리되어 세포의 세포질에서 자유로워집니다. NIK가 자유롭기 때문에 cIAP는 이를 유비퀴틴화할 수 없으며, 그 결과 세포질에서 NIK의 농도가 증가합니다. TRAF2/TRAF3/cIAP 복합체가 수용체에 결합하면 cIAP가 이 복합체 내에서 TRAF3를 유비퀴틴화하기 시작하여 프로테오솜 분해에 기여하게 됩니다. 이로 인해 세포질에서 TRAF3의 농도가 감소하여 새로운 TRAF2/TRAF3/cIAP/NIK 복합체의 조립이 방지되어 NIK를 유비퀴틴화할 수 없게 되고 결과적으로 NIK 농도가 증가하게 됩니다[119,121].
세포질에는 p100과 RelB의 두 가지 서브유닛으로 구성된 NF-κB 분자가 포함되어 있습니다. 그러나 이것은 NF-κB의 비활성 상태입니다. NIK는 세린/트레오닌 잔기의 OH 그룹에 의해 IKKα/IKKα 동이합체를 인산화하기 시작하여 활성화로 이어집니다. 이 복합체는 NEMO 단백질 없이 존재한다는 점에 유의하는 것이 중요합니다. 한편으로 IKKα는 NIK를 인산화하여 유비퀴틴화와 추가적인 완전한 프로테오솜 분해(음성 피드백 메커니즘)를 유도하고, 다른 한편으로 p100을 인산화하여 유비퀴틴화와 부분적인 프로테오솜 분해를 유도합니다. 그 결과 p100이 p52로 전환되고 p52/RelB 복합체가 형성되어 핵질로 운반됩니다[120,121].
p52/RelB 및 p50/RelA 복합체는 모두 사이토카인(TNF-α, IFN-β, IL-β, IL-2, IL-3, IL-6 등), 케모카인(IL-8, RANTES, MCP-1), 접착 분자(E-selectin, ICAM-1, VCAM-1), 급성 반응 단백질, 보체, 성장 인자(GM-CSF, G-CSF 및 M-CSF) 등의 합성을 담당하는 유전자의 발현을 높이는 전사인자입니다[119]. [119].
4. Key Points in the Relief of the SFP
Despite the specificity of the molecular mechanisms of SFP, treatment approaches remain extremely similar, as in the management of IgE-associated food allergies. The following suggestions for managing patients with SFP should be taken into consideration. The patients may have both a slow development of symptoms and an acute current, such as an anaphylactoid reaction. This will determine the tactics to be used. In either case, the trigger—fish and fish products—must be eliminated.
In case of an anaphylactoid reaction (with bronchospasm, airway edema, or distributive shock), the patients should be treated for anaphylaxis with epinephrine (adrenaline) and methylprednisolone [4,32,61]. In case of hypotension, low doses of vasopressors can be added to therapy [61].
In most cases, treatment is supportive. In case of mild severity of symptoms, the effective approach to treatment is an immediate administration of antihistamines for 1 or 2 days. H1-Antihistamines are the mainstay of treatment [122]. For mild to moderate symptoms, oral H1 histamine antagonists such as diphenhydramine, cetirizine, and chlorpheniramine are effective [61]. H2 receptor antagonists (cimetidine, famotidine, or ranitidine) may also be added to therapy [61]. With this treatment, symptoms should resolve in 6–8 h [32]. However, there are no trials with quality control that can validate this recommendation or preference of one histamine antagonist or combination over others [61].
Intravenous administration of antihistamines, such as diphenhydramine, famotidine, and ranitidine, can be performed if patient does not tolerate oral antihistamines. Nausea and vomiting can be treated with intravenous promethazine. For patients experiencing dehydration, intravenous fluid should be administrated [61].
According to the symptoms, beta-2-adrenergic agonists, ipratropium bromide, and steroids may also be prescribed [4]. In addition, there is insufficient data to support the prophylactic use of mast cell stabilizers [123]. Another focus of attention for us should be the comorbidities that play a role in the SFP mechanism. The optimal treatment consists of a multidisciplinary and multifaceted approach, which encompasses long-term management strategies in order to minimize recurrences of reactions and improve quality of life [124].
SFP의 분자 메커니즘의 특이성에도 불구하고 치료 접근법은 IgE 관련 식품 알레르기의 관리와 마찬가지로 매우 유사합니다. SFP 환자 관리를 위한 다음 제안 사항을 고려해야 합니다. 환자는 증상이 느리게 진행되거나 아나필락시스 반응과 같은 급성으로 진행될 수 있습니다. 이에 따라 사용해야 할 전술이 결정됩니다. 두 경우 모두 유발 요인인 생선 및 생선 제품을 제거해야 합니다.
아나필락시스 반응(기관지 경련, 기도 부종 또는 분포성 쇼크)이 있는 경우 에피네프린(아드레날린)과 메틸프레드니솔론으로 아나필락시스 치료를 받아야 합니다[4,32,61]. 저혈압의 경우 저용량의 혈관압박제를 치료에 추가할 수 있습니다[61].
대부분의 경우 치료는 보조적입니다. 증상이 경미한 경우 효과적인 치료 방법은 항히스타민제를 하루 또는 이틀 동안 즉시 투여하는 것입니다. H1-항히스타민제는 치료의 주축입니다[122]. 경증에서 중등도 증상의 경우 디펜히드라민, 세티리진, 클로르페니라민과 같은 경구용 H1 히스타민 길항제가 효과적입니다[61]. H2 수용체 길항제(시메티딘, 파모티딘 또는 라니티딘)도 치료에 추가할 수 있습니다[61]. 이 치료법을 사용하면 6-8시간 내에 증상이 해결됩니다 [32]. 그러나 이러한 권장 사항이나 다른 히스타민 길항제 또는 조합에 대한 선호도를 검증 할 수있는 품질 관리를 통한 임상 시험은 없습니다 [61].
환자가 경구 항히스타민제에 내약성이 없는 경우 디펜히드라민, 파모티딘, 라니티딘과 같은 항히스타민제의 정맥 투여를 시행할 수 있습니다. 메스꺼움과 구토는 정맥 프로메타진으로 치료할 수 있습니다. 탈수를 경험하는 환자의 경우 정맥 수액을 투여해야합니다 [61].
증상에 따라 베타 -2- 아드레날린 작용제, 이프라트로피움 브로마이드 및 스테로이드도 처방 될 수 있습니다 [4]. 또한 비만 세포 안정제의 예방적 사용을 뒷받침하는 데이터는 충분하지 않습니다 [123]. 우리가 주목해야 할 또 다른 초점은 SFP 메커니즘에서 중요한 역할을 하는 동반 질환입니다. 최적의 치료는 반응의 재발을 최소화하고 삶의 질을 개선하기 위해 장기적인 관리 전략을 포함하는 다학제적이고 다각적인 접근 방식으로 구성됩니다 [124].
5. Conclusions
The molecular mechanisms of SFP include a complex system of interactions between the body and chemical triggers such as exogenous histamine, other biogenic amines, and cis-urocanic acid. The onset and development of the molecular mechanism of SFP are influenced by external contributing factors (fish species, saprophytic microorganism species that convert histidine to histamine, and environmental conditions) and internal individual factors (exogenous histamine consumed, inactivation speed, individual sensitivity, etc.). The main roles in the molecular mechanisms of SFP are not only dose-dependent exogenous histamine intoxication but also the complement system, NET activation, NF-κB, etc. Important in the development of SFP are associated diseases and disorders, such as IBS, IBD, GERD, mast cell activation syndrome, intestinal mastocytosis, and others. Therefore, SFP is a systemic process realized by various mechanisms that still offers many challenges for hygienic regulations because aspects of the identification of maximum allowable concentrations of some substances and their influence on the pathogenesis of this disease remain unclear.
SFP의 분자 메커니즘에는 외인성 히스타민, 기타 생체 아민 및 시스-우로칸산과 같은 화학 유발 물질과 신체 간의 복잡한 상호 작용 시스템이 포함됩니다. SFP의 분자 메커니즘의 시작과 발달은 외부 기여 요인(어종, 히스티딘을 히스타민으로 전환하는 부생 미생물 종, 환경 조건 등)과 내부 개별 요인(소비된 외인성 히스타민, 비활성화 속도, 개인 민감도 등)에 의해 영향을 받습니다. SFP의 분자 메커니즘에서 주요 역할은 용량 의존적 외인성 히스타민 중독뿐만 아니라 보체 시스템, NET 활성화, NF-κB 등입니다. SFP의 발병에 중요한 것은 IBS, IBD, GERD, 비만 세포 활성화 증후군, 장 비만 세포증 등과 같은 관련 질병 및 장애입니다. 따라서 SFP는 다양한 메커니즘에 의해 실현되는 체계적인 과정으로, 일부 물질의 최대 허용 농도를 파악하는 측면과 이 질병의 발병에 미치는 영향이 불분명하기 때문에 위생 규제에 여전히 많은 과제가 남아 있습니다.
Author Contributions
Conceptualization, Y.V.Z.; writing—original draft preparation, M.Y.S., O.K.Z., E.E.S. and E.V.B.; writing—review and editing, K.I.K., I.A.F., M.I.K. and V.A.S.; visualization, S.O.V. and D.V.S.; supervision, E.A.S., O.V.M. and Y.V.Z.; funding acquisition, Y.V.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation (grant no. 21-73-20202 for advice on SFP preventing, and grant no. 22-75-10140 for content analysis of SFP molecular mechanisms).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhernov, Y.V.; Vysochanskaya, S.O.; Sukhov, V.A.; Zaostrovtseva, O.K.; Gorshenin, D.S.; Sidorova, E.A.; Mitrokhin, O.V. Molecular Mechanisms of Eosinophilic Esophagitis. Int. J. Mol. Sci. 2021, 22, 13183. [Google Scholar] [CrossRef] [PubMed]
- Moneret-Vautrin, D.A. False food allergies: Non-specific reactions to foodstuffs. Clin. React. Food 1983, 135–153. [Google Scholar] [CrossRef]
- Velázquez-Sámano, G.; Collado-Chagoya, R.; Cruz-Pantoja, R.A.; Velasco-Medina, A.A.; Rosales-Guevara, J. Reacciones de hipersensibilidad a aditivosalimentarios [Hypersensitivity reactions to food additives]. Revista alergia México 2019, 66, 329–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traylor, J.; Mathew, D. Histamine toxicity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499871/ (accessed on 20 December 2022).
- Marcus, E.N. Scombroid (Histamine) Poisoning. Up to Date. [Literature Review Current through: April 2022.|This Topic Last Updated: 9 February 2022.]. Available online: http://www.uptodate.com/contents/scombroid-histamine-poisoning (accessed on 20 December 2022).
- Bulula, N.; Mugoyela, V.; Kaale, E. Investigation of Contributing Factors to Scombroid Fish Poisoning among Dar es Salaam City Residents in Tanzania. Open Access Libr. J. 2017, 4, 1–16. [Google Scholar] [CrossRef]
- Pinzer, T.C.; Tietz, E.; Waldmann, E.; Schink, M.; Neurath, M.F.; Zopf, Y. Circadian profiling reveals higher histamine plasma levels and lower diamine oxidase serum activities in 24% of patients with suspected histamine intolerance compared to food allergy and controls. Allergy 2018, 73, 949–957. [Google Scholar] [CrossRef] [Green Version]
- Gargano, D.; Appanna, R.; Santonicola, A.; De Bartolomeis, F.; Stellato, C.; Cianferoni, A.; Casolaro, V.; Iovino, P. Food Allergy and Intolerance: A Narrative Review on Nutritional Concerns. Nutrients 2021, 13, 1638. [Google Scholar] [CrossRef]
- Brock, I.; Eng, N.; Maitland, A. Adult-onset mast cell activation syndrome following scombroid poisoning: A case report and review of the literature. J. Med. Case Rep. 2021, 15, 620. [Google Scholar] [CrossRef]
- Lalmalani, R.M.; Gan Hs, J.; Stacey, S. Two Case Reports of Scombroid in Singapore: A Literature Review. Cureus 2022, 14, e22580. [Google Scholar] [CrossRef]
- Taylor, R.; Burg, J.; Opara, N. The Sea Was Angry That Day My Friends: An Inland Case of Acute Scombroid Poisoning with a Twist. Cureus 2021, 13, e18394. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, P.; Bian, L.; Hong, S. Rare Death via Histamine Poisoning Following Crab Consumption: A Case Report. J. Forensic Sci. 2018, 63, 980–982. [Google Scholar] [CrossRef]
- Hernandez Garcilazo, N.; Prasad, R.M.; Varghese, M.; Kemnic, T. Scombroid pancreatitis from mahi-mahi consumption. BMJ Case Rep. 2021, 14, e240261. [Google Scholar] [CrossRef] [PubMed]
- Van Hage, M.; Hamsten, C.; Valenta, R. ImmunoCAP assays: Pros and cons in allergology. J. Allergy Clin. Immunol. 2017, 140, 974–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupinek, C.; Wollmann, E.; Baar, A.; Banerjee, S.; Breiteneder, H.; Broecker, B.M.; Bublin, M.; Curin, M.; Flicker, S.; Garmatiuk, T.; et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: The MeDALL allergen-chip. Methods 2014, 66, 106–119. [Google Scholar] [CrossRef] [Green Version]
- Garib, V.; Rigler, E.; Gastager, F.; Campana, R.; Dorofeeva, Y.; Gattinger, P.; Zhernov, Y.; Khaitov, M.; Valenta, R. Determination of IgE and IgG reactivity to more than 170 allergen molecules in paper-dried blood spots. J. Allergy Clin. Immunol. 2019, 143, 437–440. [Google Scholar] [CrossRef] [Green Version]
- Karsonova, A.; Riabova, K.; Villazala-Merino, S.; Campana, R.; Niederberger, V.; Eckl-Dorna, J.; Fröschl, R.; Perkmann, T.; Zhernov, Y.V.; Elisyutina, O.G.; et al. Highly sensitive ELISA-based assay for quantification of allergen-specific IgE antibody levels. Allergy 2020, 75, 2668–2670. [Google Scholar] [CrossRef] [PubMed]
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: An updated practice parameter. Ann. Allergy Asthma Immunol. 2010, 105, 259–273. [Google Scholar] [CrossRef]
- Qiu, S.; Liu, Z.; Hou, L.; Li, Y.; Wang, J.; Wang, H.; Du, W.; Wang, W.; Qin, Y.; Liu, Z. Complement activation associated with polysorbate 80 in beagle dogs. Int. Immunopharmacol. 2013, 15, 144–149. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Q.; Shi, C.; Zhang, X. Drug-Induced Pseudoallergy: A Review of the Causes and Mechanisms. Pharmacology 2018, 101, 104–110. [Google Scholar] [CrossRef]
- Schirone, M.; Visciano, P.; Tofalo, R.; Suzzi, G. Histamine Food Poisoning. Handb. Exp. Pharmacol. 2017, 241, 217–235. [Google Scholar] [CrossRef]
- Maintz, L.; Novak, N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef]
- James, M. Hungerford, Histamine and Scombrotoxins. Toxicon 2021, 201, 115–126. [Google Scholar] [CrossRef]
- FAO/WHO [Food and Agriculture Organization of the United Nations/World Health Organization]. Public Health Risks of Histamine and other Biogenic Amines from Fish and Fishery Products. Meeting Report. 2013. Available online: https://www.who.int/publications/i/item/9789240691919 (accessed on 20 December 2022).
- Rodríguez-Caravaca, G.; Hijas-Gómez, A.I.; Tejedor-Alonso, M.Á.; Del-Moral-Luque, J.A.; Delgado-Iribarren, A.; Valverde-Cánovas, J.F.; Gil-de-Miguel, Á. Food poisoning caused by scombroids: A case-control study. J. Infect. Public Health 2019, 12, 591–593. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Joint FAO/WHO Literature Review: Histamine in Salmonids; Food & Agriculture Organization: Rome, Italy, 2018. [Google Scholar]
- Pereira, E.; Elliot, E.L.; Singleton, L.S.; Otto, M.; Tesfai, A.; Doyle, M.; Hawk, H.; Bloodgood, S.; Benner, R.A.; Ross, M.P.; et al. An Outbreak Investigation of Scombrotoxin Fish Poisoning Illnesses in the United States Linked to Yellowfin Tuna Imported from Vietnam-2019. J. Food Prot. 2021, 84, 962–972. [Google Scholar] [CrossRef]
- Zapata, R.; Acevedo, K.; Mella, M.; Mella, V.; Zapata, K. A clinical epidemiological onsite study of a massive outbreak of Scombroid fish poisoning after consumption of yellowtail kingfish in northern Chile. J. Food Safe Hyg. 2021, 6, 145–159. [Google Scholar] [CrossRef]
- Hwang, D.-F.; Chang, S.-H.; Shiau, C.-Y.; Cheng, C.-C. Biogenic Amines in the Flesh of Sailfish (Istiophorus plafypferus) Responsible for Scornbroid Poisoning. J. Food Sci. 1995, 60, 926–928. [Google Scholar] [CrossRef]
- James, M.J.; Martin, J.; Loessner, D.A. Golden Modern Food Microbiology, 7th ed.; Springer Science + Business Media, Inc.: New York, NY, USA, 2005; ISBN 0-387-23180-3. [Google Scholar]
- Dalgaard, P.; Madsen, H.L.; Samieian, N.; Emborg, J. Biogenic amine formation and microbial spoilage in chilled garfish (Belone belone belone)—Effect of modified atmosphere packaging and previous frozen storage. J. Appl. Microbiol. 2006, 101, 80–95. [Google Scholar] [CrossRef]
- Guergué-Díaz de Cerio, O.A.; Barrutia-Borque, J.; Gardeazabal-García, J. Scombroid Poisoning: A Practical Approach. Actas Dermo-Sifiliográficas (Engl. Ed.) 2016, 107, 567–571. [Google Scholar] [CrossRef]
- Chapter 3—Integrated Analytical Approaches for Food Safety and Environmental Sustainability. In Analytical Methods for Agricultural Contaminants; Maestroni, B.; Ochoa, V.; Cannavan, A. (Eds.) Academic Press: Cambridge, MA, USA, 2019; pp. 25–174. ISBN 9780128159408. [Google Scholar] [CrossRef]
- Bjornsdottir-Butler, K.; May, S.; Hayes, M.; Abraham, A.; Benner, R.A., Jr. Characterization of a novel enzyme from Photobacterium phosphoreum with histidine decarboxylase activity. Int. J. Food Microbiol. 2020, 334, 108815. [Google Scholar] [CrossRef] [PubMed]
- Kempkes, C.; Buddenkotte, J.; Cevikbas, F.; Buhl, T.; Steinhoff, M. 11 Role of PAR-2 in neuroimmune communication and itch. In Itch Mechanisms and Treatment; CRC Press: Boca Raton, FL, USA, 2014; Volume 193. [Google Scholar]
- Lin, C.-M.; Kung, H.-F.; Huang, Y.-L.; Huang, C.-Y.; Su, Y.-C.; Tsai, Y.-H. Histamine production by Raoultella ornithinolytica in canned tuna meat at various storage temperatures. Food Control 2012, 25, 723–727. [Google Scholar] [CrossRef]
- Kim, S.H.; Ben-Gigirey, B.; Barros-Velázquez, J.; Price, R.J.; An, H. Histamine and biogenic amine production by Morganella morganii isolated from temperature-abused albacore. J. Food Prot. 2000, 63, 244–251. [Google Scholar] [CrossRef]
- Kanki, M.; Yoda, T.; Tsukamoto, T.; Shibata, T. Klebsiella pneumoniae produces no histamine: Raoultella planticola and Raoultella ornithinolytica strains are histamine producers. Appl. Environ. Microbiol. 2002, 68, 3462–3466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, S.L.; Guthertz, L.S.; Leatherwood, M.; Lieber, E.R. Histamine production by Klebsiella pneumoniae and an incident of scombroid fish poisoning. Appl. Environ. Microbiol. 1979, 37, 274–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshinaga, D.H.; Frank, H.A. Histamine-producing bacteria in decomposing skipjack tuna (Katsuwonus pelamis). Appl. Environ. Microbiol. 1982, 44, 447–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjornsdottir-Butler, K.; McCarthy, S.; Burkhardt, W., 3rd; Benner, R.A., Jr. Importance of histamine-producing Clostridium perfringens in scombrotoxin-forming fish. J. Food Prot. 2013, 76, 1283–1287. [Google Scholar] [CrossRef]
- Gullian Klanian, M.; Delgadillo Díaz, M.; Sánchez Solís, M.J. Molecular Characterization of Histamine-Producing Psychrotrophic Bacteria Isolated from Red Octopus (Octopus maya) in Refrigerated Storage. High Throughput 2018, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Behling, A.R.; Taylor, S.L. Bacterial histamine production as a function of temperature and time of incubation. J. Food Sci. 1982, 47, 1311–1314. [Google Scholar] [CrossRef]
- Margareta, G.; Ratnawati, S.E.; Puspita, I.D. Growth Rate and Histamine Production of Citrobacter freundii CK01 in Various Incubation Temperatures. E3S Web Conf. 2020, 147, 03018. [Google Scholar] [CrossRef] [Green Version]
- Food and Drug Administration. Fish and Fishery Products Hazards and Controls Guidance, 4th ed.; FDA: Silver Spring, MD, USA, 2020. Available online: https://www.fda.gov/media/80637/download (accessed on 20 December 2022).
- EU. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Off. J. Eur. Union 2005, 338, 1–25. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32005R2073 (accessed on 20 December 2022).
- Durak-Dados, A.; Michalski, M.; Osek, J. Histamine and Other Biogenic Amines in Food. J. Vet. Res. 2020, 64, 281–288. [Google Scholar] [CrossRef]
- Barrett, K.A.; Nakao, J.H.; Tyalor, E.V.; Eggerts, C.; Gould, L.H. Fish-associated foodborne disease outbreaks: United States, 1998–2015. Foodborne Pathog. Dis. 2017, 14, 537–543. [Google Scholar] [CrossRef]
- González, M.C.; Díaz, A.C.; Moncayo, J.G.; Marín, J.A. Scombroid poisoning secondary to tuna ingestion: A case report. Biomedica 2020, 40, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Scombroid (histamine) poisoning. Prepared for the Ministry of Health by ESR Ltd. Issued May 2001. Available online: https://www.mpi.govt.nz/dmsdocument/26078-Scombroid-Histamine-poisoning (accessed on 20 December 2022).
- Chung, B.Y.; Park, S.Y.; Byun, Y.S.; Son, J.H.; Choi, Y.W.; Cho, Y.S.; Kim, H.O.; Park, C.W. Effect of Different Cooking Methods on Histamine Levels in Selected Foods. Ann. Dermatol. 2017, 29, 706–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broczek, M.; Rautenstrauch, D.; Windyga, B.; Ścieżyńska, H.; Jędra, M.; Badowski, P.; Karłowska, B. The content of histamine and tyramine depending on the microbiological quality of salted herrings, stored at different temperatures. Roczn. PZH 2003, 54, 87–95. [Google Scholar]
- Buyuktiryaki, B.; Masini, M.; Mori, F.; Barni, S.; Liccioli, G.; Sarti, L.; Lodi, L.; Giovannini, M.; du Toit, G.; Lopata, A.L.; et al. IgE-Mediated Fish Allergy in Children. Medicina 2021, 57, 76. [Google Scholar] [CrossRef]
- Torres Borrego, J.; Martínez Cuevas, J.F.; Tejero García, J. Reactividad cruzada entre pescados y mariscos [Cross reactivity between fish and shellfish]. Allergol. Immunopathol. 2003, 31, 146–151. (In Spanish) [Google Scholar] [CrossRef]
- Szebeni, J. Complement activation-related pseudoallergy. In The Complement System; Szebeni, J., Ed.; Springer: Boston, MA, USA, 2004. [Google Scholar] [CrossRef]
- Mahdavinia, M. Food Allergy in Adults. Med. Clin. N. Am. 2019, 104, 145–155. [Google Scholar] [CrossRef]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef] [Green Version]
- Manjur, J.E.; Ciuffo, M.M.; Burdowski, J.; George, S.; Kalogeropoulos, A.; Chen, O. Scombroid Fish Poisoning Leading to Vasospastic Angina: A Rare Etiology of Chest Pain. JACC Case Rep. 2019, 1, 322–326. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Fish and Fishery Products Hazards and Controls Guide; U.S. Food and Drug Administration: Washington, DC, USA; Center for Food Safety and Applied Nutrition: Washington, DC, USA; Office of Seafood: Washington, DC, USA, 1998; pp. 245–248.
- Feldman, K.A.; Werner, S.B.; Cronan, S.; Hernandez, M.; Horvath, A.R.; Lea, C.S.; Au, A.M.; Vugia, D.J. A large outbreak of scombroid fish poisoning associated with eating escolar fish (Lepidocybium flavobrunneum). Epidemiol. Infect. 2005, 133, 29–33. [Google Scholar] [CrossRef]
- Feng, C.; Teuber, S.; Gershwin, M.E. Histamine (Scombroid) Fish Poisoning: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 64–69. [Google Scholar] [CrossRef]
- Dessauer, C.W.; Ostrom, R.; Seifert, R.; Watts, V.J. Adenylyl cyclases (ACs) (version 2019.4) in the IUPHAR/BPS Guide to Pharmacology Database. IUPHAR/BPS Guide Pharmacol. CITE 2019, 4. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Santos, M.D.; Álvarez-González, M.; Estrada-Soto, S.; Bazán-Perkins, B. Regulation of Myosin Light-Chain Phosphatase Activity to Generate Airway Smooth Muscle Hypercontractility. Front. Physiol. 2020, 11, 701. [Google Scholar] [CrossRef] [PubMed]
- Raszewski, J.A.; Sharma, S. Physiology, Ryanodine Receptor. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538323/ (accessed on 20 December 2022).
- Frank, K.; Kranias, E.G. Phospholamban and cardiac contractility. Ann. Med. 2000, 32, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Primeau, J.O.; Armanious, G.P.; Fisher, M.E.; Young, H.S. The Sarco Endoplasmic Reticulum Calcium ATPase. Subcell Biochem. 2018, 87, 229–258. [Google Scholar] [CrossRef]
- Kania, E.; Roest, G.; Vervliet, T.; Parys, J.B.; Bultynck, G. IP3 Receptor-Mediated Calcium Signaling and Its Role in Autophagy in Cancer. Front. Oncol. 2017, 7, 140. [Google Scholar] [CrossRef] [Green Version]
- Fleming, I. Molecular mechanisms underlying the activation of eNOS. Pflug. Arch. 2010, 459, 793–806. [Google Scholar] [CrossRef]
- Kovalev, I.V.; Popov, A.G.; Panov, A.A.; Borodin, I.U.L.; Afinogenova, I.A.D.; Kapilevich, L.V.; Baskakov, M.B.; Medvedev, M.A. Issledovanie mekhanizmov NO-zavisimogo rasslableniia gladkikh myshts aorty krysy s pomoshch’iu nitrosoedineniĭ [Mechanisms of NO-dependent relaxation in smooth muscles of the rat aorta with nitro compounds]. Eksp. Klin. Farmakol. 2001, 64, 33–36. (In Russian) [Google Scholar]
- Schnittler, H. Contraction of endothelial cells: 40 years of research, but the debate still lives. Histochem. Cell Biol. 2016, 146, 651–656. [Google Scholar] [CrossRef]
- Hungerford, J.M. Scombroid poisoning: A review. Toxicon 2010, 56, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Blackwell, B.; Mabbitt, L.A. Tyramine in Cheese Related to Hypertensive Crises after Monoamine-Oxidase Inhibition. Lancet 1965, 1, 938–940. [Google Scholar] [CrossRef]
- Staruszkiewiez, W.F.; Bond, J.F. Gas chromatographid determination of cadaverine, putrescine, and histamine in foods. J. Assoc. Off. Anal. Chem. 1981, 64, 584–591. [Google Scholar]
- Moret, S.; Smela, D.; Populin, T.; Conte, L.S. A survey on free biogenic amine content of fresh and preserved vegetables. Food Chem. 2005, 89, 355–361. [Google Scholar] [CrossRef]
- Stratton, J.E.; Hutkins, R.W.; Taylor, S.L. Biogenic Amines in Cheese and other Fermented Foods: A Review. J. Food Prot. 1991, 54, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Visciano, P.; Schirone, M.; Paparella, A. An Overview of Histamine and Other Biogenic Amines in Fish and Fish Products. Foods 2020, 9, 1795. [Google Scholar] [CrossRef] [PubMed]
- Prester, L. Biogenic amines in fish, fish products and shellfish: A review. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2011, 28, 1547–1560. [Google Scholar] [CrossRef]
- Doeun, D.; Shin, H.S.; Chung, M.S. Effects of storage temperatures, vacuum packaging, and high hydrostatic pressure treatment on the formation of biogenic amines in Gwamegi. Appl. Biol. Chem. 2016, 59, 51–58. [Google Scholar] [CrossRef]
- Voigt, M.N.; Eitenmiller, R.R. Role of Histidine and Tyrosine Decarboxylases and Mono- and Diamine Oxidases in Amine Build-Up in Cheese. J. Food Prot. 1978, 41, 182–186. [Google Scholar] [CrossRef]
- Taylor, S.L. Histamine Poisoning Associated with Fish, Cheese and Other Foods; Report VPH/FOS/85.1; World Health Organization Press: Geneva, Switzerland, 1985; pp. 1–48.
- Bargossi, E.; Gardini, F.; Gatto, V.; Montanari, C.; Torriani, S.; Tabanelli, G. The Capability of Tyramine Production and Correlation between Phenotypic and Genetic Characteristics of Enterococcus faecium and Enterococcus faecalis Strains. Front. Microbiol. 2015, 6, 1371. [Google Scholar] [CrossRef] [Green Version]
- Burns, C.; Kidron, A. Biochemistry, Tyramine. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563197/ (accessed on 20 December 2022).
- Szebeni, J. Complement activation-related pseudoallergy: A stress reaction in blood triggered by nanomedicines and biological. Mol. Immunol. 2014, 61, 163–173. [Google Scholar] [CrossRef]
- Macdougall, I.C.; Vernon, K. Complement Activation-Related Pseudo-Allergy: A Fresh Look at Hypersensitivity Reactions to Intravenous Iron. Am. J. Nephrol. 2017, 45, 60–62. [Google Scholar] [CrossRef] [Green Version]
- Neun, B.W.; Ilinskaya, A.N.; Dobrovolskaia, M.A. Analysis of Complement Activation by Nanoparticles. Methods Mol. Biol. 2018, 1682, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Nesargikar, P.N.; Spiller, B.; Chavez, R. The complement system: History, pathways, cascade and inhibitors. Eur. J. Microbiol. Immunol. 2012, 2, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part I—Molecular Mechanisms of Activation and Regulation. Front. Immunol. 2015, 6, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uvnas, B. The mechanism of histamine liberation. J. Pharm. Pharmacol. 1958, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Brunet, C.; Bédard, P.M.; Hébert, J. Analysis of compound 48/80-induced skin histamine release and leukotriene production in chronic urticaria. J. Allergy Clin. Immunol. 1988, 82 Pt 1, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Quan, P.L.; Sabaté-Brescó, M.; Guo, Y.; Martín, M.; Gastaminza, G. The Multifaceted Mas-Related G Protein-Coupled Receptor Member X2 in Allergic Diseases and Beyond. Int. J. Mol. Sci. 2021, 22, 4421. [Google Scholar] [CrossRef]
- Kumar, M.; Duraisamy, K.; Chow, B.K. Unlocking the Non-IgE-Mediated Pseudo-Allergic Reaction Puzzle with Mas-Related G-Protein Coupled Receptor Member X2 (MRGPRX2). Cells 2021, 10, 1033. [Google Scholar] [CrossRef]
- Wille, J.J.; Kydonieus, A.F.; Murphy, G.F. Cis-urocanic acid induces mast cell degranulation and release of preformed TNF-alpha: A possible mechanism linking UVB and cis-urocanic acid to immunosuppression of contact hypersensitivity. Skin Pharmacol. Appl. Skin Physiol. 1999, 12, 18–27. [Google Scholar] [CrossRef]
- Lehane, L.; Olley, J. Histamine fish poisoning revisited. Int. J. Food Microbiol. 2000, 58, 1–37. [Google Scholar] [CrossRef]
- Khalil, Z.; Townley, S.L.; Grimbaldeston, M.A.; Finlay-Jones, J.J.; Hart, P.H. Cis-Urocanic acid stimulates neuropeptide release from peripheral sensory nerves. J. Inves.t Dermatol. 2001, 117, 886–891. [Google Scholar] [CrossRef]
- Zare, D.; Muhammad, K.; Bejo, M.H.; Ghazali, H.M. Development and validation of an ion-pair chromatographic method for simultaneous determination of trans- and cis-urocanic acid in fish samples. J. Chromatogr. A 2012, 1256, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Grimbaldeston, M.A.; Finlay-Jones, J.J. Mast cells in UV-B-induced immunosuppression. J. Photochem. Photobiol. B 2000, 55, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Norval, M.; El-Ghorr, A.A. Studies to determine the immunomodulating effects of cis-urocanic acid. Methods 2002, 28, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Norval, M.; Gibbs, N.K.; Gilmour, J. The role of urocanic acid in UV-induced immunosuppression: Recent advances (1992–1994). Photochem. Photobiol. 1995, 62, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.E. 5.11—Skin immunology and immunotoxicity. In Comprehensive Toxicology, 2nd ed.; McQueen, C.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 217–234. ISBN 9780080468846. [Google Scholar] [CrossRef]
- Walterscheid, J.P.; Nghiem, D.X.; Kazimi, N.; Nutt, L.K.; McConkey, D.J.; Norval, M.; Ullrich, S.E. Cis-urocanic acid, a sunlight-induced immunosuppressive factor, activates immune suppression via the 5-HT2A receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 17420–17425. [Google Scholar] [CrossRef] [Green Version]
- Zare, D.; Muhammad, K.; Ghazali, H.M. The manner of urocanic acid accumulation in fish by tracking histidine ammonia lyase activity during storage of vacuum-packed, eviscerated, and whole fish. J. Food Process Preserv. 2021, 45, e15288. [Google Scholar] [CrossRef]
- Wu, P.C.; Kroening, T.A.; White, P.J.; Kendrick, K.E. Histidine ammonia-lyase from Streptomyces griseus. Gene 1992, 115, 19–25. [Google Scholar] [CrossRef]
- Hug, D.H.; Dunkerson, D.D.; Hunter, J.K. The degradation of L-histidine and trans- and cis-urocanic acid by bacteria from skin and the role of bacterial cis-urocanic acid isomerase. J. Photochem. Photobiol. B 1999, 50, 66–73. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Elias, P.M.; Man, M.Q.; Hupe, M.; Selden, C.; Sundberg, J.P.; Tschachler, E.; Eckhart, L.; Mauro, T.M.; Feingold, K.R. Is the filaggrin-histidine-urocanic acid pathway essential for stratum corneum acidification? J. Invest. Dermatol. 2010, 130, 2141–2144. [Google Scholar] [CrossRef] [Green Version]
- Mackie, I.M.; Fernandez-Salguéro, J. Histidine metabolism in fish. Urocanic acid in mackerel (Scomber scombrus). J. Sci. Food Agric. 1977, 28, 935–940. [Google Scholar] [CrossRef]
- Zare, D.; Muhammad, K.; Bejo, M.H.; Ghazali, H.M. Determination of trans- and cis-urocanic acid in relation to histamine, putrescine, and cadaverine contents in tuna (Auxis Thazard) at different storage temperatures. J. Food Sci. 2015, 80, T479–T483. [Google Scholar] [CrossRef] [PubMed]
- Comas-Basté, O.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Lator-re-Moratalla, M.; Vidal-Carou, M.D.C. Histamine Intolerance: The Current State of the Art. Biomolecules 2020, 10, 1181. [Google Scholar] [CrossRef]
- Petersen, J.; Drasche, A.; Raithel, M.; Schwelberger, H.G. Analysis of genetic polymorphisms of enzymes involved in histamine metabolism. Inflamm. Res. 2003, 52 (Suppl. S1), S69–S70. [Google Scholar] [CrossRef] [PubMed]
- Schwelberger, H.G.; Falus, A. Diamine oxidase (DAO) enzyme and gene. In Histamine: Biology and Medical Aspects; Spring Med Publishing: Budapest, Hungary, 2004; pp. 43–52. [Google Scholar]
- Palma, A.M.; Hanes, M.R.; Marshall, J.S. Mast Cell Modulation of B Cell Responses: An Under-Appreciated Partnership in Host Defence. Front. Immunol. 2021, 12, 718499. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Mehta, A. Review Article—Role of Cytokines in Pathophysiology of Asthma. Iran. J. Pharmacol. Ther. 2006, 5, 1–4. [Google Scholar]
- Packard, K.A.; Khan, M.M. Effects of histamine on Th1/Th2 cytokine balance. Int. Immunopharmacol. 2003, 3, 909–920. [Google Scholar] [CrossRef]
- Hsieh, F.H. Gastrointestinal Involvement in Mast Cell Activation Disorders. Immunol. Allergy Clin. N. Am. 2018, 38, 429–441. [Google Scholar] [CrossRef]
- Gilfillan, A.M.; Tkaczyk, C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 2006, 6, 218–230. [Google Scholar] [CrossRef]
- Colgan, J.; Rothman, P. Manipulation of signaling to control allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 2007, 7, 51–56. [Google Scholar] [CrossRef]
- Rognlien, K.T.; Woodbury, D.J. Chapter 16—Reconstituting SNARE proteins into BLMs. In Membrane Science and Technology; Tien, H.T., Ottova-Leitmannova, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 7, pp. 479–488. ISBN 9780444509406. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Y.; Zhang, J.; Hong, T. Allantoin Inhibits Compound 48/80-Induced Pseudoallergic Reactions In Vitro and In Vivo. Molecules 2022, 27, 3473. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Begalli, F.; Bennett, J.; Capece, D.; Verzella, D.; D’Andrea, D.; Tornatore, L.; Franzoso, G. Unlocking the NF-κB Conundrum: Embracing Complexity to Achieve Specificity. Biomedicines 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trask, O.J., Jr. Nuclear Factor Kappa B (NF-κB) Translocation assay development and validation for high content screening. In Assay Guidance Manual [Internet]; Markossian, S., Grossman, A., Brimacombe, K., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. Available online: https://www.ncbi.nlm.nih.gov/books/NBK100914/figure/nfkb.F2/ (accessed on 26 December 2022).
- Sun, S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Harmelin, Y.; Hubiche, T.; Pharaon, M.; Del Giudice, P. Three cases of scombroid poisoning. Ann. Dermatol. Venereol. 2018, 145, 29–32. [Google Scholar] [CrossRef] [PubMed]
- De Silva, D.; Geromi, M.; Panesar, S.S.; Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Cardona, V.; Dubois, A.E.; Halken, S.; et al. Acute and long-term management of food allergy: Systematic review. Allergy 2014, 69, 159–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; duToit, G.; Eigenmann, P.; et al. EAACI food allergy and anaphylaxis guidelines: Diagnosis and management of food allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef] [PubMed]