
https://www.youtube.com/watch?v=45qMEOAkpug


Biomolecules. 2020 Aug; 10(8): 1181.
Published online 2020 Aug 14. doi: 10.3390/biom10081181
PMCID: PMC7463562
PMID: 32824107
Histamine Intolerance: The Current State of the Art
Oriol Comas-Basté,1,2,3 Sònia Sánchez-Pérez,1,2,3 Maria Teresa Veciana-Nogués,1,2,3 Mariluz Latorre-Moratalla,1,2,3 and María del Carmen Vidal-Carou1,2,3,*
Author information Article notes Copyright and License information PMC Disclaimer
Abstract
Histamine intolerance, also referred to as enteral histaminosis or sensitivity to dietary histamine, is a disorder associated with an impaired ability to metabolize ingested histamine that was described at the beginning of the 21st century. Although interest in histamine intolerance has considerably grown in recent years, more scientific evidence is still required to help define, diagnose and clinically manage this condition. This article will provide an updated review on histamine intolerance, mainly focusing on its etiology and the existing diagnostic and treatment strategies. In this work, a glance on histamine intoxication will also be provided, as well as the analysis of some uncertainties historically associated to histamine intoxication outbreaks that may be better explained by the existence of interindividual susceptibility to ingested histamine.
히스타민은
다양한 식품에 매우 다양한 농도로 존재하며,
이는 이 화합물의 주요 외인성 공급원입니다 [31].
식품에서
히스타민이 형성되는 주요 경로는
박테리아 유래 효소인 L-히스티딘 탈카르복실화 효소의 작용을 통한
히스티딘의 탈카르복실화입니다[32,33].
히스타민 외에도 식품에는
주로
티라민(4-하이드록시-페네틸아민),
푸트레신(1,4-디아미노부탄) 및
카데베린(1,5-디아미노펜탄)과 같은 다른 생체 아민도 포함될 수 있으며,
이는 각각
아미노산 티로신,
오르니틴(and/or 아그마틴) 및
라이신의 효소 탈아민화를 통해 형성됩니다 [31,34].



식품에 이러한 화합물이 축적되는 것은
미생물에 의한 아미노산 변형의 결과이며
전구체 아미노산의 가용성,
성장에 유리한 환경 조건 및/또는
박테리아 탈카르복실효소 활성과 같은 다양한 요인에 따라 달라집니다 [31,34,35].
Keywords: histamine, food intolerance, histamine intolerance, histaminosis, histamine intoxication, diamine oxidase (DAO), low-histamine diet, food supplement

1. Introduction
In 2011, the European Food Safety Authority (EFSA) issued a scientific report warning that the levels of biogenic amines found in foods marketed in European Union countries may still entail a consumer health risk [1]. Among them, histamine has the highest toxic potential, along with tyramine, and is therefore of great interest in terms of food safety. First described more than 60 years ago, the deleterious effects of excessive histamine ingestion were initially referred to as scombroid fish poisoning or scombrotoxicosis, as they were associated with the consumption of fish in this family, but the condition is now known as histamine intoxication or histamine poisoning. In recent years, another disorder associated with histamine intake, arising from an enzymatic deficiency, has been described. The inability of certain individuals to metabolize histamine in the intestine, resulting in sensitivity to normal or even low histamine levels in food, may help to explain some of the uncertainties historically associated with histamine intoxication.
During the last decade, histamine intolerance has gained social and scientific recognition, with a significant increase in the interest of researchers to investigate this disorder. This review aims to analyze the pathophysiological relevance of dietary histamine, giving special focus to the adverse effects derived from histamine intake and, in particular, to the state of the art concerning the etiology, diagnosis and treatment of histamine intolerance.
2011년 유럽식품안전청(EFSA)은
유럽 연합 국가에서 판매되는 식품에서 발견되는
바이오제닉 아민 수치가 여전히 소비자 건강에 위험을 초래할 수 있다고 경고하는
과학 보고서를 발표했습니다[1].

과학 문헌과 유럽연합 관련 설문조사, 보고서 및 소비 데이터의 데이터를 사용하여
발효 식품의 바이오제닉 아민(BA)에 대한
정성적 위험 평가를 실시했습니다.
히스타민과 티라민은
가장 독성이 강하고
식품 안전과 관련이 있는 것으로 간주되며,
발효 식품은
미생물 활동과 BA 형성 가능성이 높기 때문에
특히 BA가 우려되는 식품입니다.
식품의 평균 함량과 소비자 노출 데이터를 기반으로
히스타민과 티라민과 관련하여
발효 식품 카테고리의 순위가 매겨졌지만 현
재 이용 가능한 정보는 개별적으로 또는 조합하여
BA의 정량적 위험 평가를 수행하기에 불충분합니다.
BA 위험 완화 옵션과 관련하여 특히
원료에서 BA 생성 미생물의 발생을 최소화하기 위한
위생 조치, 추가 미생물 제어, BA 비생산 스타터 배양액 사용 등이 관련성이 높습니다.
공개된 제한적인 정보에 따르면, 식품에 함유된 다음 수준의 BA에 노출된 후 건강에 미치는 영향은 관찰되지 않았습니다:
a) 건강한 사람의 경우 히스타민 50mg, 히스타민 과민증이 있는 사람의 경우 검출 한계 이하,
b) 모노아미노 산화효소 억제제(MAOI) 약물을 복용하지 않는 건강한 사람의 경우 티라민 600mg, 3세대 MAOI 약물 복용자의 경우 50mg, 기존 MAOI 약물 복용자의 경우 6mg,
c) 퍼트레신과 카데빈에 대해서는 해당 정보가 불충분했습니다.
현재 고성능 액체 크로마토그래피(HPLC) 기반 방법만이 식품 내 모든 BA를 동시에 고감도로 정량화할 수 있으므로 모니터링 및 관리 목적에 가장 적합합니다. 생산 과정과 먹이사슬을 따라 발효 식품의 BA 농도를 모니터링하면 관리 및 추가 지식 확보에 도움이 될 것입니다. 특히 독성 및 관련 농도, 생산 공정 기반 제어 조치, 추가 공정 위생 및/또는 식품 안전 기준 개발, 분석 방법의 검증 등 발효 식품의 BA에 대한 추가 연구가 필요합니다.
그 중에서도
히스타민은
티라민과 함께 독성이 가장 높기 때문에
식품 안전 측면에서 큰 관심을 받고 있습니다.
60여 년 전에 처음 설명된 과도한 히스타민 섭취의 해로운 영향은
처음에는 이 과에 속하는 생선 섭취와 관련이 있어
스콤브로이드 생선 중독 또는 스콤브로독증이라고 불렸지만,
현재는 히스타민 중독 또는 히스타민 중독으로 알려져 있습니다.
최근에는
효소 결핍으로 인해 발생하는
히스타민 섭취와 관련된 또 다른 장애가 설명되었습니다.
특정 개인이
장에서 히스타민을 대사하지 못하여
음식의 정상 또는
낮은 히스타민 수치에 민감하게 반응하는 것은
역사적으로 히스타민 중독과 관련된 불확실성 중 일부를 설명하는 데 도움이 될 수 있습니다.
지난 10년 동안
히스타민 과민증은
사회적, 과학적으로 인정을 받으면서
이 질환을 조사하려는 연구자들의 관심이 크게 증가했습니다.
이 리뷰는
식이 히스타민의 병리 생리학적 관련성을 분석하고
히스타민 섭취로 인한 부작용,
특히 히스타민 과민증의 원인, 진단 및 치료에 관한 최신 지견에 초점을 맞추고자 합니다.
2. Histamine
Histamine (2-[4-imidazolyl]ethylamine) is a bioactive amine that is synthesized by decarboxylation of its precursor amino acid, histidine, in an enzymatic reaction first described by Windaus and Vogt in 1907 involving L-histidine decarboxylase (EC 4.1.1.22) (Figure 1) [2]. Due to its chemical structure and number of functional groups, histamine can be defined as a heterocyclic diamine with an imidazole ring and ethylamine (i.e., an organic compound that provides a functional group in the form of a primary amine) [1,3].
히스타민(2-[4- 이미다졸릴]에틸아민)은
1907년 Windaus와 Vogt가
L-히스티딘 탈카르복실효소(EC 4.1.1.22)와 관련된 효소 반응에서
전구체 아미노산인
히스티딘의 탈카르복실화에 의해 합성되는
생리 활성 아민 bioactive amine 입니다[그림 1](2) [그림 1].
히스타민은
화학 구조와 작용기의 수로 인해
이미다졸 고리와
에틸아민(즉, 일차 아민 형태의 작용기를 제공하는 유기 화합물)을 가진
헤테로사이클릭 디아민으로 정의할 수 있습니다[1,3].
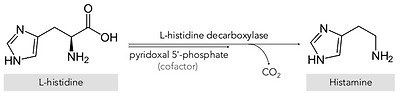
Synthesis of histamine by decarboxylation of its precursor amino acid.
The physiological and pathophysiological effects of histamine on the body were first described in 1910 by Dale and Laidlaw, two pioneering researchers who studied the functions of this organic compound at the Wellcome Physiological Research Laboratories [4,5,6]. Specifically, histamine is synthesized and stored in high concentrations in secretory granules, mainly in basophils and mast cells, and also in gastric enterochromaffin cells, lymph nodes and the thymus [1,7]. Functionally, this amine is involved in various immune and physiological mechanisms, stimulating gastric acid secretion, inflammation, smooth muscle cell contraction, vasodilation and cytokine production, among other processes [8,9,10,11]. In addition, histamine functions as a neurotransmitter, being synthesized by neurons located in the posterior region of the hypothalamus whose axons extend through the brain [12]. These wide-ranging physiological effects occur by interaction with four G-protein-coupled receptors with seven transmembrane domains (H1, H2, H3 and H4), which activate signal transduction pathways upon perceiving their ligand, histamine [7,12].
Two main histamine metabolic pathways are known in humans, involving the enzymes diamine oxidase (DAO) and histamine-N-methyltransferase (HNMT) (Figure 2) [10,11,13]. DAO (EC 1.4.3.22), also called histaminase or amiloride-binding protein, is a copper-dependent amino oxidase encoded by the AOC1 gene located on chromosome 7 (7q34-36) [14,15,16]. This functional enzyme, a homodimer with two isoforms, catalyzes the oxidative deamination of the primary amine group of histamine [14,16,17]. On the other hand, histamine can be metabolized to 1-methylhistamine by the enzyme HNMT (EC 2.1.1.8), a small monomeric protein encoded by a gene located on chromosome 2q22.1 [18]. HNMT catalyzes the methylation of the secondary amine group of the histamine imidazole aromatic heterocycle by a reaction requiring the S-adenosyl methionine cosubstrate as a methyl group donor [11,13,19].
히스타민이
신체에 미치는 생리적 및 병리 생리학적 영향은
1910년 웰컴 생리 연구소에서 이 유기 화합물의 기능을 연구한 선구적인 두 연구자
데일과 레이드로에 의해 처음 설명되었습니다[4,5,6].
특히 히스타민은
주로
호염기구와 비만 세포,
그리고 위장관 크롬마핀 세포,
림프절,
흉선 등에서
고농도로 합성되어 분비 과립에 저장됩니다[1,7].
basophils and mast cells, and also in gastric enterochromaffin cells, lymph nodes and the thymus [
기능적으로 이 아민은
다양한 면역 및 생리적 메커니즘에 관여하여
위산 분비,
염증,
평활근 세포 수축,
혈관 확장 및
사이토카인 생성을 자극합니다[8,9,10,11].
https://www.sciencedirect.com/science/article/pii/S1534580717302964

또한
히스타민은
뇌를 통해 축삭이 뻗어 있는
시상하부 후부에 위치한 뉴런에 의해 합성되는
신경 전달 물질로 기능합니다[12].



이러한
광범위한 생리학적 효과는
7개의 막 통과 도메인(H1, H2, H3, H4)을 가진
4개의 G단백질 결합 수용체와의 상호작용을 통해 발생하며,
이 수용체는 리간드인 히스타민을 감지하면
신호 전달 경로를 활성화합니다[7,12].
four G-protein-coupled receptors with seven transmembrane domains (H1, H2, H3 and H4),
which activate signal transduction pathways upon perceiving their ligand, histamine


인간에게는
두 가지 주요 히스타민 대사 경로가 알려져 있으며,
효소인 디아민 산화효소(DAO)와
히스타민-N-메틸전달효소(HNMT)가 관여합니다[그림 2][10,11,13].
히스타미나아제 또는
아밀로라이드 결합 단백질이라고도 하는
DAO(EC 1.4.3.22)는
7번 염색체(7q34-36)에 위치한 AOC1 유전자에 의해 암호화되는
구리 의존성 아미노 산화효소입니다[14,15,16].
참고) 구리 결핍 또는 과잉은 DAO 효소 기능을 망가뜨림.




이 기능성 효소는
두 개의 이성질체를 가진 동이합체로,
히스타민의 일차 아민기의 산화 탈아민화를 촉매합니다[14,16,17].
반면에
히스타민은
염색체 2q22.1에 위치한 유전자에 의해 코딩되는
작은 단량체 단백질인 효소 HNMT(EC 2.1.1.8)에 의해
1-메틸히스타민으로 대사될 수 있습니다[18].
HNMT는
메틸기 공여체로서 S-아데노실 메티오닌 코기질을 필요로 하는 반응에 의해
히스타민 이미다졸 방향족 헤테로사이클의
이차 아민기의 메틸화를 촉매합니다[11,13,19].
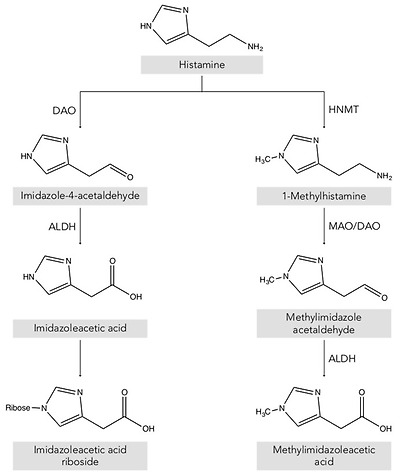
Histamine metabolism in humans. DAO: diamine oxidase; HNMT: histamine-N-methyltransferase; ALDH: aldehyde dehydrogenase; MAO: monoamine oxidase.
Thus, depending on its location, the histamine present in the body is deaminated or methylated by the action of the enzymes DAO and HNMT, respectively [1,10,20]. DAO is a secretory protein stored in vesicular structures of the plasma membrane and is responsible for the degradation of extracellular histamine [1,15]. In mammals, the expression of DAO is restricted to certain tissues, mainly the small intestine, ascending colon, placenta and kidneys [14,21]. In the intestine, DAO activity increases progressively from the duodenum to the ileum and is located mainly in the intestinal villi [22]. In contrast, the enzyme HNMT is expressed in a wide range of human tissues, above all in the kidneys and liver, and also the spleen, colon, prostate, ovaries, spinal cord cells and the trachea and respiratory tract [10,13]. HNMT is a cytosolic protein responsible for the inactivation of intracellular histamine and can be synthesized in the cell itself or incorporated from the extracellular space by binding to a receptor or by membrane transporters [7,18]. Regarding substrates, HNMT is highly selective for histamine, whereas DAO can also metabolize other biogenic amines such as putrescine and cadaverine, although it shows a preference for histamine [14,16,23]. The affinity of DAO and HNMT for histamine is very similar, although the latter shows a slightly lower Michaelis–Menten enzymatic constant (KM: 6–13 μmol/L) than DAO (KM: 20 μmol/L) [10].
The gateway for dietary histamine in the body is the intestinal epithelium. Therefore, although HNMT is also present in the gastrointestinal tract, the more highly expressed DAO plays the major role in protecting the body against exogenous histamine, whether originating from ingested food or generated by the intestinal microbiota [24,25,26]. The protective effect of DAO has been demonstrated in animal experimentation models that were administered aminoguanidine for irreversible and selective DAO inhibition, followed by a dose of histamine [24,27,28]. The development of anaphylaxis symptoms in DAO-inhibited pigs and sheep compared to control groups indicates that the enzyme exerts a significant barrier effect against the absorption of exogenous histamine into the systemic circulation [1,13,19,24,29]. The HNMT enzyme ranks second to DAO in protecting against the absorption of dietary histamine from the intestinal lumen, but appears to be more effective against intravenously or intradermally supplied histamine [13,30].
따라서
그 위치에 따라
체내에 존재하는 히스타민은
효소 DAO와 HNMT의 작용에 의해
각각 탈아민화되거나 메틸화됩니다 [1,10,20].
DAO는
혈장막의 소포 구조에 저장된 분비 단백질이며
세포 외 히스타민의 분해를 담당합니다 [1,15].
포유류에서
DAO의 발현은
특정 조직, 주로 소장, 상행 결장, 태반 및 신장으로 제한됩니다 [14,21].
장에서 DAO 활성은
십이지장에서 회장까지 점진적으로 증가하며
주로 장 융모에 위치합니다 [22].
이와 대조적으로
효소 HNMT는 광범위한 인체 조직,
특히 신장과 간, 비장, 대장, 전립선, 난소, 척수 세포,
기관 및 호흡기에서도 발현됩니다 [10,13].
HNMT는
세포 내 히스타민의 비활성화를 담당하는
세포질 단백질로 세포 자체에서 합성되거나 수용체 또는
막 수송체에 결합하여 세포 외 공간에서 통합될 수 있습니다 [7,18].
기질과 관련하여 HNMT는
히스타민에 대해 매우 선택적인 반면,
DAO는 히스타민을 선호하지만
푸트레신 및 카데베린과 같은 다른 생체 아민도 대사할 수 있습니다 [14,16,23].
히스타민에 대한 DAO와 HNMT의 친화도는
매우 유사하지만,
후자는 DAO(KM: 6-13 μmol/L)보다 약간 낮은 마이클리스-멘텐 효소 상수(KM: 20 μmol/L)를 보입니다[10].
체내 식이 히스타민의 관문은
장 상피입니다.
따라서
HNMT도 위장관에 존재하지만,
더 많이 발현되는 DAO는
섭취한 음식에서 유래하든 장내 미생물에 의해 생성되든
외인성 히스타민으로부터 신체를 보호하는 데 주요한 역할을 합니다 [24,25,26].
DAO의 보호 효과는
비가역적이고 선택적인 DAO 억제를 위해
아미노구아니딘을 투여한 후
히스타민을 투여한 동물 실험 모델에서 입증되었습니다 [24,27,28].
대조군에 비해
DAO가 억제된 돼지와 양에서
아나필락시스 증상이 나타난 것은
이 효소가 외인성 히스타민이 전신 순환계로 흡수되는 것에 대해
상당한 장벽 효과를 발휘한다는 것을 나타냅니다[1,13,19,24,29].
HNMT 효소는
장 내강에서 식이 히스타민의 흡수를 방지하는 데 있어
DAO에 이어 두 번째이지만 정맥 또는
피내 공급 히스타민에 대해서는
더 효과적인 것으로 보입니다 [13,30].
3. Histamine in Foods
Histamine is present in a wide range of foods in highly variable concentrations, which are the main exogenous source of this compound [31]. The main route for histamine formation in food is the decarboxylation of histidine through the action of L-histidine decarboxylase, an enzyme of bacterial origin [32,33]. Apart from histamine, food can also contain other biogenic amines, mainly tyramine (4-hydroxy-phenethylamine), putrescine (1,4-diaminobutane) and cadaverine (1,5-diaminopentane), which are formed through enzymatic deamination of the amino acids tyrosine, ornithine (and/or agmatine) and lysine, respectively [31,34]. The accumulation of these compounds in food is the result of the transformation of amino acids by microorganisms and depends on various factors, such as the availability of the precursor amino acids and environmental conditions favorable for growth and/or the bacterial decarboxylase activity [31,34,35].
These decarboxylation reactions have been described as a survival strategy for microorganisms in acidic environments, as well as an alternative source of metabolic energy in situations of suboptimal substrate availability [1,9]. This enzymatic activity in bacteria is a species- and strain-dependent property [32]. Several Gram-positive and Gram-negative bacteria responsible for microbial spoilage or fermentative processes in food are able to produce histamine [1,36]. Specifically, the Enterobacteriaceae species Hafnai aluei, Morganella morganii and Klebsiella pneumonia have been identified as some of the most prolific histamine-forming bacteria in fish [9,37]. On the other hand, in cheeses, fermented meat, vegetable derivatives and fermented beverages, various lactic acid bacteria have also been described as histamine-producing microorganisms (e.g., Lactobacillus hilgardii, Lactobacillus buchnerii, Lactobacillus curvatus and Oenococcus oeni) as well as certain strains of Enterobacteriaceae [1,38,39].
Foods that potentially contain high levels of histamine are: a) those microbiologically altered, such as fish and meat, or derived products that may have been preserved or processed in unsuitably hygienic conditions; and b) fermented products, in which the bacteria responsible for the fermentation process may also have aminogenic capacity [3,40]. Table 1 shows histamine content in the different food categories from the Spanish market [31].
히스타민은
다양한 식품에 매우 다양한 농도로 존재하며,
이는 이 화합물의 주요 외인성 공급원입니다 [31].
식품에서 히스타민이 형성되는 주요 경로는
박테리아 유래 효소인 L-히스티딘 탈카르복실화 효소의 작용을 통한
히스티딘의 탈카르복실화입니다[32,33].
히스타민 외에도 식품에는
주로
티라민(4-하이드록시-페네틸아민),
푸트레신(1,4-디아미노부탄) 및
카데베린(1,5-디아미노펜탄)과 같은 다른 생체 아민도 포함될 수 있으며,
이는 각각 아미노산 티로신, 오르니틴(and/or 아그마틴) 및
라이신의 효소 탈아민화를 통해 형성됩니다 [31,34].
식품에 이러한 화합물이 축적되는 것은
미생물에 의한 아미노산 변형의 결과이며
전구체 아미노산의 가용성,
성장에 유리한 환경 조건 및/또는
박테리아 탈카르복실효소 활성과 같은 다양한 요인에 따라 달라집니다 [31,34,35].
이러한
탈카르복실화 반응은
산성 환경에서 미생물의 생존 전략이자
최적의 기질 가용성이 낮은 상황에서
대사 에너지의 대체 공급원으로 설명되어 왔습니다[1,9].
박테리아의 이러한 효소 활성은
종과 균주에 따라 달라지는 특성입니다[32].
식품의 미생물 부패 또는 발효 과정을 담당하는
여러 그람 양성 및 그람 음성 박테리아는
히스타민을 생성할 수 있습니다 [1,36].
특히,
장내 세균과에 속하는
하프나이 알루에이,
모르가넬라 모가니,
클렙시엘라 뉴모니아는
어류에서 가장 많은 히스타민을 생성하는
박테리아로 확인되었습니다 [9,37].
반면에
치즈,
발효 육류(소금에 절인 고기)
식물성 유도체 및 발효 음료에서는
다양한 유산균(예: 락토바실러스 힐가디, 락토바실러스 부흐네리, 락토바실러스 커바투스 및 오에노코커스 오에니)과
특정 균주의 장내 세균과도
히스타민 생성 미생물로 설명되었습니다 [1,38,39].
잠재적으로 높은 수준의 히스타민을 함유할 수 있는 식품은
a) 생선 및 육류와 같이 미생물학적으로 변형된 식품 또는 부적절한 위생 조건에서 보존 또는 가공되었을 수 있는 파생 제품,
b) 발효 과정을 담당하는 박테리아가 아미노겐 생성 능력을 가질 수 있는 발효 제품입니다[3,40].
표 1은
스페인 시장의 다양한 식품 카테고리에서
히스타민 함량을 보여줍니다[31].
Table 1
Histamine content in different food categories. Adapted from [31].
Food Histamine Content (mg/kg)nMean (SD)MedianMinimumMaximum

| Fruits, vegetables and plant-based products | |||||
| Fruits | 136 | 0.07 (0.20) | ND | ND | 2.51 |
| Nuts | 41 | 0.45 (1.23) | ND | ND | 11.86 |
| Vegetables | 98 | 2.82 (7.43) | ND | ND | 69.72 |
| Legumes | 11 | ND | ND | ND | ND |
| Cereals | 28 | 0.12 (0.33) | ND | ND | 0.89 |
| Chocolate | 25 | 0.58 (0.44) | 0.17 | 0.16 | 0.56 |
| Spices | 12 | ND | ND | ND | ND |
| Alcoholic beverages | |||||
| Beer | 176 | 1.23 (2.47) | 0.70 | ND | 21.60 |
| White wine | 83 | 1.24 (1.69) | 0.45 | 0.10 | 13.00 |
| Red wine | 260 | 3.81 (3.51) | 1.90 | 0.09 | 55.00 |
| Fish and seafood products | |||||
| Fresh fish | 136 | 0.79 (0.71) | ND | ND | 36.55 |
| Canned fish | 96 | 14.42 (16.03) | 5.93 | ND | 657.05 |
| Semipreserved fish | 49 | 3.48 (3.37) | 2.18 | ND | 34.90 |
| Meat and meat products | |||||
| Fresh meat | 6 | ND | ND | ND | ND |
| Cooked meat | 48 | 0.30 (0.26) | ND | ND | 4.80 |
| Cured meat | 23 | 12.98 (37.64) | 0.80 | ND | 150.00 |
| Dry-fermented sausages | 209 | 32.15 (14.22) | 8.03 | ND | 357.70 |
| Dairy products | |||||
| Unripened cheese | 20 | ND | ND | ND | ND |
| Raw milk cheese | 20 | 59.37 (106.74) | 18.38 | ND | 389.86 |
| Pasteurized milk cheese 저온살균 우유 치즈 | 20 | 18.05 (38.23) | 4.59 | ND | 162.03 |
ND: not detected.

발효 소시지 또는 드라이 소시지는 다진 고기나 갈은 고기를 소금에 절여 수분을 제거하는 동시에 유익한 박테리아가 당분을 풍미 있는 분자로 분해하여 만드는 소시지의 일종입니다.
락토바실러스 종과 류코노스톡 종을 포함한 박테리아는
이러한 당분을 분해하여
소시지의 풍미에 영향을 주는
젖산을 생성할 뿐만 아니라
pH를 6.0에서 4.5~5.0으로 낮추어
소시지를 상하게 할 수 있는 박테리아의 성장을 방지합니다.
이러한 효과는
수분이 추출되면서
염분과 산도가 농축되기 때문에
건조 과정에서 더욱 극대화됩니다.
발효 소시지에는
고기, 지방, 박테리아 배양액, 소금, 향신료, 설탕,
아질산염 등의 성분이 들어갑니다.
아질산염은
일반적으로 발효 소시지에 첨가되어
고기의 숙성 속도를 높이고
매력적인 색상을 부여하는 동시에
보툴리누스 중독을 일으키는 클로스트리디움 보툴리눔 박테리아의 성장을 방지합니다.[1][2]
일부 전통 및 장인 생산자는 아질산염을 피합니다. 18시간에서 3일 동안의 발효 과정에서 박테리아의 젖산 생산을 돕기 위해 설탕을 첨가하는데, 발효 시간은 소시지를 보관하는 온도에 따라 달라지며 온도가 낮을수록 필요한 발효 기간이 길어집니다. 건조 과정에서 소시지 겉면에 하얀 곰팡이와 효모가 달라붙는 경우가 있습니다. 이 곰팡이는 소시지의 풍미를 더하고 해로운 박테리아가 소시지에 달라붙는 것을 방지하는 데 도움이 됩니다[3].
발효 소시지의 두 가지 주요 유형은 따뜻한 기후에서 발견되는 건조하고 소금에 절인 양념 소시지와 서늘하고 습한 기후에서 발견되는 발효 반건조 소시지입니다. 이탈리아, 스페인, 포르투갈과 같은 지중해 연안 국가의 건식 소시지는 25~35%의 수분과 4% 이상의 소금을 함유하고 있어 실온에서 보관할 수 있습니다. 북유럽의 소시지는 일반적으로 소금 함량(약 3%)이 적고 수분 함량이 40~50%이므로 독일과 같은 습한 기후에서는 잘 건조되지 않습니다.
미생물학
발효 소시지에는 다양한 젖산 생성 박테리아와 스타일에 따라 간혹 곰팡이가 서식하기도 합니다. 발효는 젖산 생성으로 pH를 낮출 뿐만 아니라 지방 분해를 통해 다양한 향미 화합물을 생성합니다. 단백질 분해와 수분 함량 감소로 식감이 달라집니다.[4]
다른 발효 식품과 마찬가지로 원하는 유형의 미생물을 도입하기 위해 잘 정의된 스타터 배양이 상업적으로 사용됩니다.[4] 라티락토바실러스 사케이,[5] 스타필로코커스 카르노서스,[6] 다양한 기타 스타필로코커스 종, 페니실륨 아우란티오그리세움 곰팡이가 스타터 배양의 성분으로 사용되었습니다[4].
4. Uncertainties Associated with Histamine Poisoning: A Paradigm Shift Towards Histamine Intolerance
Although histamine has important physiological functions in the body, it can pose a health risk when ingested in high levels [41]. The proper functioning of histamine degradation systems is key in preventing its accumulation. Histamine intoxication, a kind of food poisoning, may occur after the consumption of foods with an unusually high histamine content that overpowers the degradation mechanisms (generally higher than 500 mg/kg) [1,3,42].
Historically, histamine intoxication has also been termed scombroid fish poisoning or the mahi-mahi flush because of its repeated association with the consumption of fish in the Scombridae and Scomberesocidae families (e.g., tuna, herring and mackerel) [43]. Histamine was first identified in 1946 as the causative agent of the toxic effects of consuming poorly transported tuna, and for a long time histamine poisoning was associated almost exclusively with the consumption of spoiled fish [44,45]. Over the years, the World Health Organization (WHO) has recommended the use of the term histamine intoxication to better designate this pathology, as it can be caused by marine species from other families (e.g., Clupeidae, Engraulidae, Coriphaenidae and Pomatomidae) and even other foods, such as cheese [43]. A meta-analysis carried out in 2018 of the different scientific reports of histamine intoxication between 1959 and 2013 established that the causative food in 98% of cases was fish, the remaining percentage being attributed to cheese [46]. Currently, international health administrations consider histamine intoxication to be one of the main problems of global food security, both for its effects on human health and its impact on trade [47,48].
Histamine intoxication is characterized by occurring in outbreaks and having a short incubation period (i.e., 20–30 min post-ingestion), with symptoms that are generally of low/moderate severity and remit in a few hours [3]. The symptoms are closely linked to the various physiological functions of histamine in the body, affecting the skin (e.g., redness, rash, urticaria, pruritus, edema and local inflammation), the gastrointestinal tract (e.g., nausea, vomiting and diarrhea) and the hemodynamic (hypotension) and neurological (e.g., headache, palpitations and tingling) bodily functions [1,41]. The symptomatic similarity of histamine intoxication with allergy means it is likely to be underdiagnosed [43,48,49]. The diagnosis of histamine intoxication is based primarily on the determination of elevated plasma histamine levels and/or the identification of an ingested food with an unusually high histamine content [13]. In general, an outbreak of histamine poisoning tends to involve more than one individual, lasts a short period of time and a particular causative food is identified [38].
히스타민은
신체에서 중요한 생리적 기능을 수행하지만,
과다 섭취하면 건강에 위험을 초래할 수 있습니다[41].
히스타민 분해 시스템의 적절한 기능은
히스타민의 축적을 방지하는 데 핵심적인 역할을 합니다.
식중독의 일종인 히스타민 중독은
히스타민 함량이 비정상적으로 높아
분해 메커니즘을 압도하는 식품(일반적으로 500 mg/kg 이상)을 섭취한 후에
발생할 수 있습니다[1,3,42].
역사적으로 히스타민 중독은
참치, 청어, 고등어와 같은
스콤브리아과 및 스콤베레소키과 어류의 섭취와 반복적으로 연관되어
스콤브리아 어독 또는 마히마히 플러시라고도 불렸습니다[43].
히스타민은
1946년 제대로 운송되지 않은 참치 섭취로 인한
독성 영향의 원인 물질로 처음 확인되었으며,
오랫동안 히스타민 중독은
거의 독점적으로 부패한 생선 섭취와 관련이 있었습니다[44,45].
수년에 걸쳐 세계보건기구(WHO)는
히스타민 중독이라는 용어를 사용하여
이 병리를 더 잘 지정할 것을 권장했는데,
이는 다른 과(예: Clupeidae, Engraulidae, Coriphaenidae 및 Pomatomidae)의 해양 종이나
치즈와 같은 다른 식품에 의해서도 발생할 수 있기 때문입니다 [43].
1959년부터 2013년까지
히스타민 중독에 대한 다양한 과학적 보고에 대해
2018년에 수행된 메타 분석에 따르면
98%의 사례에서 원인 식품이 생선이고
나머지 비율은 치즈에 기인하는 것으로 밝혀졌습니다 [46].
현재 국제 보건 당국은
히스타민 중독이 인체 건강에 미치는 영향과
무역에 미치는 영향 모두에서
세계 식량 안보의 주요 문제 중 하나로 간주하고 있습니다 [47,48].
히스타민 중독은
집단적으로 발생하고 잠복기가 짧으며(즉, 섭취 후 20~30분),
일반적으로 증상이 중등도 이하이고 몇
시간 내에 완화되는 특징이 있습니다[3].
이러한 증상은
피부(예: 발적, 발진, 두드러기, 가려움증, 부종 및 국소 염증),
위장관(예: 오심, 구토 및 설사),
혈역학(저혈압) 및
신경학적(예: 두통, 심계항진 및 저림) 신체 기능에 영향을 미치는
신체 내 히스타민의 다양한 생리 기능과 밀접하게 연관되어 있습니다[1,41].
히스타민 중독과
알레르기의 증상적 유사성은
과소 진단될 가능성이 높다는 것을 의미합니다[43,48,49].
히스타민 중독의 진단은
주로 혈장 히스타민 수치 상승 및/또는
히스타민 함량이 비정상적으로 높은 섭취한 음식의 확인을
기반으로 합니다[13].
일반적으로
히스타민 중독은
한 명 이상의 개인에게 발생하는 경향이 있으며,
단기간 동안 지속되며 특정 원인 식품이 확인됩니다[38].
In terms of incidence, the data available for the European Union shows an increase in histamine intoxication outbreaks in the last ten years, unlike other types of food poisoning, and with an almost hegemonic predominance of fish as the causative agent (over 90% of cases) [42,50]. The most recent data from the EFSA and European Center for Disease Prevention and Control (ECDC) show that in 2017, there was a 22% increase in outbreaks compared to the previous year [50]. Specifically, in 2017, there were a total of 117 outbreaks of histamine intoxication involving 572 people, 9% of whom required hospitalization. Fortunately, no deaths have been attributed to histamine poisoning over the past decade [42]. The same trend is observed in the information provided by the European Union Food and Feed Warning System (RASFF), with a progressive rise in the number of cases of histamine poisoning linked to tuna consumption in 2014–2017 and a particularly high increase in 2017 [3].
Although histamine intoxication has been extensively studied in recent decades, unresolved questions remain, concerning, for example, the variable histamine concentrations in the foods triggering outbreaks, or the heterogeneity in the degree and type of adverse effects [46]. Furthermore, the fact that oral administration of histamine in doses equivalent to those normally found in foods causing illness does not produce the same range and/or severity of symptoms is a paradox that has led to multiple hypotheses [30].
Several authors have proposed that alcohol and certain food components, such as other biogenic amines, may have a potentiating effect on histamine toxicity [13,48]. Amines such as putrescine and cadaverine, which are usually found in foods along with histamine, can also act as DAO substrates. It has therefore been suggested that these amines could weaken the protective barrier against dietary histamine by competitively interacting with degradation enzymes in the intestine [3,49]. Other possible potentiators are alcohol and its metabolite acetaldehyde, as they compete with histamine for the enzyme aldehyde dehydrogenase (ALDH), which is simultaneously involved in histamine and alcohol metabolism [1,32]. The potentiation effect of these components could help explain the differences in absorption of the same dose of histamine when ingested in isolation or in a food matrix [48,49]. The FAO and WHO have acknowledged that the involvement of potentiators can alter the threshold dose for toxicity, and they recommend that future studies focus on clarifying the ambiguities in the pathogenesis of histamine intoxication [30].
Finally, several authors have reported considerable interindividual variability in histamine tolerance, which has been demonstrated in intervention studies [1,3,10,13]. After the oral administration of the same histamine dosage, not all participants showed symptoms, and those who did varied in symptom type and severity and even had different blood histamine levels [48,51,52]. These results indicate the existence of population subgroups with greater sensitivity and clinical responses to histamine, likely linked to a diminished histamine degradation capacity, which could explain some of the historical uncertainties associated with histamine intoxication outbreaks [1]. Without disputing the clinical entity of histamine intoxication, the paradigm shift lies precisely in moving the focus from food to the human body, maintaining histamine as the causative agent, but focusing on how each person is able to respond to the intake of variable levels of histamine from food. Thus, histamine intolerance is the clinical condition that describes the inability of certain individuals to degrade histamine and results in the onset of symptoms caused by its accumulation in the blood (Figure 3).
발생률 측면에서 유럽 연합의 데이터는
다른 유형의 식중독과 달리
지난 10년간 히스타민 중독 발생이 증가했으며,
원인 물질로 어류가 거의 헤게모니적으로 우세한 것으로 나타났습니다(90% 이상)[42,50].
유럽식품안전청(EFSA)과
유럽질병예방통제센터(ECDC)의 가장 최근 데이터에 따르면
2017년에는 전년 대비 22% 증가한 것으로 나타났습니다[50].
특히 2017년에는
총 117건의 히스타민 중독이 발생하여
572명이 입원 치료를 받았으며,
이 중 9%는 입원이 필요했습니다.
다행히도 지난 10년 동안
히스타민 중독으로 인한
사망자는 발생하지 않았습니다[42].
유럽 연합 식품 및 사료 경고 시스템(RASFF)에서 제공하는 정보에서도 같은 추세가 관찰되는데,
2014~2017년 참치 섭취와 관련된
히스타민 중독 사례가 점진적으로 증가했으며
특히 2017년에 높은 증가율을 보였습니다[3].
히스타민 중독은
최근 수십 년 동안 광범위하게 연구되어 왔지만,
발병을 유발하는 식품의 다양한 히스타민 농도 또는
부작용의 정도와 유형의 이질성과 관련하여 해결되지 않은 의문점이 남아 있습니다 [46].
또한,
질병을 유발하는 식품에서
일반적으로 발견되는 것과 동일한 용량의 히스타민을 경구 투여해도
증상의 범위 및/또는 심각도가 동일하게 나타나지 않는다는 사실은
여러 가설을 낳은 역설입니다 [30].
몇몇 저자는
알코올과
다른 생체 아민과 같은 특정 식품 성분이
히스타민 독성을 강화하는 효과가 있을 수 있다고 제안했습니다[13,48].
일반적으로
히스타민과 함께 식품에서 발견되는
푸트레신 및 카데베린과 같은 아민도
DAO 기질로 작용할 수 있습니다.
따라서
이러한 아민은
장내의 분해 효소와 경쟁적으로 상호 작용하여
식이 히스타민에 대한 보호 장벽을 약화시킬 수 있다고 제안되었습니다 [3,49].
알코올과
그 대사산물인 아세트알데히드는
히스타민과 경쟁하여
히스타민과 알코올 대사에 동시에 관여하는
알데히드 탈수소효소(ALDH) 효소와 경쟁하기 때문에
다른 가능한 강화제입니다[1,32].
이러한 성분의 강화 효과는
단독으로 섭취하거나
식품 매트릭스와 함께 섭취할 때
동일한 용량의 히스타민 흡수 차이를 설명하는 데 도움이 될 수 있습니다 [48,49].
FAO와 WHO는
강화제의 개입이 독성 역치를 변화시킬 수 있음을 인정했으며,
향후 연구에서는 히스타민 중독의 발병 기전의 모호성을 명확히 하는 데 초점을 맞출 것을 권장합니다[30].
마지막으로, 여러 저자들은
히스타민 내성에
상당한 개인 간 변동성이 있다고 보고했으며,
이는 중재 연구에서 입증되었습니다 [1,3,10,13].
동일한 히스타민 용량을 경구 투여한 후
모든 참가자에게 증상이 나타난 것은 아니었으며,
증상이 나타난 참가자들은
증상 유형과 심각도가 다양했고
심지어 혈중 히스타민 수치도 달랐습니다[48,51,52].
이러한 결과는
히스타민에 대한 민감도와
임상 반응이 더 큰 인구 하위 그룹의 존재를 나타내며,
이는 히스타민 분해 능력 감소와 관련이 있을 수 있으며,
히스타민 중독 발생과 관련된
역사적 불확실성 중 일부를 설명할 수 있습니다[1].
히스타민 중독의 임상적 실체에 대한 논쟁 없이,
패러다임의 전환은
초점을 음식에서 인체로 옮겨
히스타민을 원인 물질로 유지하되
각 개인이 음식에서 다양한 수준의 히스타민 섭취에 어떻게 반응할 수 있는지에
초점을 맞추는 데 있습니다.
따라서
히스타민 과민증은
특정 개인이 히스타민을 분해하지 못하고
혈액에 축적되어 증상이 시작되는 임상 상태를 설명하는 용어입니다(그림 3).
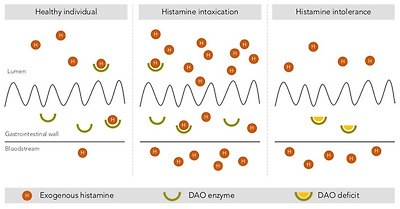
Intestinal degradation of histamine by the DAO enzyme in three different situations: in a healthy individual, with histamine intoxication and with histamine intolerance. Adapted from [13].
5. Histamine Intolerance
According to the 2003 review of allergy nomenclature by the World Allergy Organization, adverse reactions to food without an immunological basis should be referred to as nonallergic food hypersensitivity, in order to clearly differentiate them from food allergies initiated by a specific immune mechanism [53]. Nonallergic food hypersensitivity is commonly known as food intolerance, a response triggered by a food or any of its components at a dose normally tolerated by the healthy population [54]. While the prevalence of food allergies is estimated at 1–2% in adults, currently almost 20% of the Westernized world’s population suffers from some type of food intolerance, with lactose intolerance being the most common [54].
Histamine intolerance, also referred to as enteral histaminosis or sensitivity to dietary histamine, can be defined as a disorder arising from reduced histamine degradation capacity in the intestine due to impaired DAO activity, leading to its accumulation in plasma and the appearance of adverse effects [11,41,55].
The DAO enzyme was first identified back in 1929 by Charles H. Best in autolyzing lung tissue, which he called histaminase because of its ability to degrade histamine [56]. Years later, given its ability to also degrade other diamines, as described above, the more accurate designation of DAO was proposed [57,58]. Beyond its role in the intestinal degradation of histamine in humans, DAO is also present in microorganisms, plants and animals, where it also catalyzes the oxidative deamination of the primary amino group of histamine into its corresponding aldehyde, concomitantly producing stoichiometric amounts of ammonia and hydrogen peroxide (Figure 4) [14,59,60].
2003년
세계 알레르기 기구의 알레르기 명명법 검토에 따르면,
면역학적 근거가 없는 식품에 대한 부작용은
특정 면역 메커니즘에 의해 유발되는
식품 알레르기와 명확히 구분하기 위해
비알레르기성 식품 과민증으로 지칭해야 합니다 [53].
비알레르기성 식품 과민증은
일반적으로 식품 과민증으로 알려져 있으며,
건강한 사람이 일반적으로 견딜 수 있는 용량으로
식품 또는 그 성분에 의해 유발되는 반응입니다 [54].
식품 알레르기의 유병률은
성인의 경우 1~2%로 추정되지만,
현재 서구화된 세계 인구의 거의 20%가
어떤 종류의 식품 과민증을 앓고 있으며
유당 과민증이 가장 흔합니다[54].
장내 히스타민증 또는
식이 히스타민에 대한 민감성이라고도 하는
히스타민 과민증은
DAO 활동 장애로 인해
장내 히스타민 분해 능력이 감소하여
혈장에 축적되고 부작용이 나타나는 장애로 정의할 수 있습니다[11,41,55].
DAO 효소는
1929년 Charles H. Best에 의해
폐 조직 자가 분해에서 처음 확인되었는데,
그는 히스타민을 분해하는 능력 때문에 히
스타민 분해 효소라고 불렀습니다[56].
몇 년 후, 위에서 설명한 대로
다른 디아민도 분해할 수 있다는 점을 고려하여
DAO라는 보다 정확한 명칭이 제안되었습니다[57,58].
사람의 장에서 히스타민을 분해하는 역할 외에도
DAO는
미생물, 식물 및 동물에도 존재하며,
히스타민의 주요 아미노기가 해당 알데히드로 산화 탈아민화되는 것을 촉매하여
화학량론적 양의 암모니아와 과산화수소를 동시에 생성합니다(그림 4)[14,59,60].
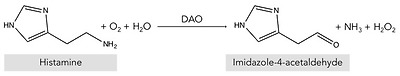
Oxidative deamination of histamine by the DAO enzyme.
Although the first scientific references to histamine intolerance date from more than 20 years ago, it is significant that almost 80% are from the last decade, reflecting the growing interest of researchers in this disorder (Figure 5). In 2011, EFSA already considered histamine intolerance as one of the risks associated with histamine intake, clinically differentiating it from histamine intoxication [1]. In a subsequent joint report, the WHO and FAO emphasized that the no observed adverse effect level (NOAEL) established for histamine was only valid for healthy people, and not for members of susceptible populations, such as those with histamine intolerance [30]. EFSA concluded that only foods with histamine levels below the detection limits are safe for individuals with histamine intolerance [1].
히스타민 과민증에 대한
최초의 과학적 언급은 20여 년 전의 일이지만,
거의 80%가 지난 10년간의 일이라는 것은
이 질환에 대한 연구자들의 관심이 증가하고 있음을 반영하는 중요한 사실입니다(그림 5).
2011년에
EFSA는
이미 히스타민 과민증을 히스타민 섭취와 관련된 위험 중 하나로 간주하여
임상적으로 히스타민 중독과 구분했습니다[1].
이후 공동 보고서에서 WHO와 FAO는
히스타민에 대해 설정된 관찰된 부작용 수준(NOAEL)은
건강한 사람에게만 유효하며
히스타민 과민증과 같은 취약한 집단에는 적용되지 않는다고 강조했습니다[30].
EFSA는
히스타민 수치가 검출 한도 이하인 식품만
히스타민 과민증이 있는 사람에게 안전하다고 결론지었습니다[1].
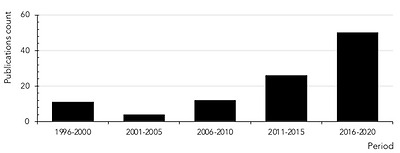
Count of scientific publications containing the keywords histamine intolerance or histaminosis, according to a search performed through the PubMed search engine at the MEDLINE bibliographic database (search performed in July 2020).
Clinical manifestations of histamine intolerance consist of a wide range of nonspecific gastrointestinal and extraintestinal symptoms, due to the ubiquitous distribution of the four histamine receptors in different organs and tissues of the body (Figure 6) [10,13,54,61]. In a very recently published study, a team of Austrian researchers comprehensively analyzed the symptoms experienced by 133 patients diagnosed with histamine intolerance [62]. The most frequent and severe manifestations were gastrointestinal, with abdominal distension observed in 92% of patients and postprandial fullness, diarrhea, abdominal pain and constipation in 55–73%. Impairments of the nervous and cardiovascular systems, such as dizziness, headaches and palpitations, were recorded in second place, followed by respiratory and dermatological symptoms. Highlighting the complexity of the clinical picture of histamine intolerance, combinations of three or more symptoms involving different organs were recorded in 97% of cases, with an average of 11 symptoms per patient. The low specificity and complex variability of symptoms undoubtedly contribute to the current difficulty in achieving consensus on the diagnostic criteria for histamine intolerance, as will be discussed in detail below [13]. A lack of data also makes it difficult to determine the current incidence of this condition, although some authors have estimated that it affects 1–3% of the population, a percentage that will possibly increase as more knowledge and diagnostic tools for histamine intolerance become available [10,13,63].
히스타민 과민증의 임상 증상은
신체의 여러 기관과 조직에
네 가지 히스타민 수용체가 어디에나 분포하기 때문에
광범위한 비특이적 위장관 및 장외 증상으로 구성됩니다(그림 6) [10,13,54,61].
최근에 발표된 연구에서
오스트리아 연구팀은
히스타민 과민증으로 진단받은
133명의 환자가 경험한 증상을 종합적으로 분석했습니다[62].
가장 빈번하고 심각한 증상은
위장관으로,
환자의 92%에서 복부 팽창이 관찰되었고
55-73%에서 식후 포만감, 설사, 복통 및 변비가 나타났습니다.
어지럼증, 두통, 심계항진 등
신경계 및 심혈관계 장애가 두 번째로 많았고
호흡기 및
피부과 증상이 그 뒤를 이었습니다.
히스타민 과민증의
임상 양상이 복잡하다는 점을 강조하기 위해
서로 다른 장기와 관련된
3가지 이상의 증상이 복합적으로 나타나는 경우가 97%에 달했으며,
환자당 평균 11가지의 증상이 나타났습니다.
아래에서 자세히 설명하는 것처럼
증상의 낮은 특이성과
복잡한 가변성은
의심할 여지없이 히스타민 과민증 진단 기준에 대한
합의를 달성하는 데 현재 어려움을 겪고 있는 원인입니다 [13].
일부 저자는
히스타민 과민증이
인구의 1~3%에 영향을 미치는 것으로 추정하고 있지만,
데이터 부족으로 인해 이 질환의 현재 발병률을 파악하기 어렵습니다[10,13,63].
히스타민 과민증에 대한
더 많은 지식과 진단 도구가 제공됨에 따라
그 비율은 증가할 가능성이 높습니다[10,13,63].
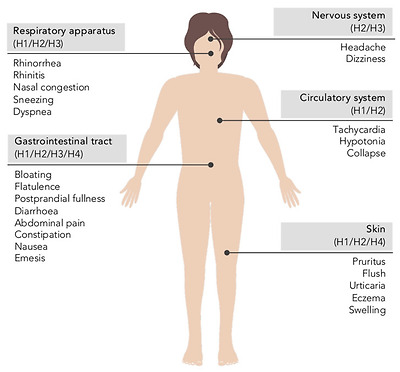
Main symptoms of histamine intolerance and possibly corresponding histamine receptors [10,64].
5.1. The Etiology of Histamine Intolerance
As mentioned in previous sections, the main barrier against exogenous histamine in the intestines is the DAO enzyme, which prevents its passage into the systemic circulation [10,13,65]. Numerous clinical studies have provided data on the prevalence of low plasma DAO levels in individuals showing symptoms of histamine intolerance, mainly headaches and gastrointestinal or dermatological disorders [66]. Although certain studies have limitations, either in the design or number of participants, the majority point to an association between symptoms and DAO deficiency, establishing a general trend that supports the key role of DAO in the etiology of these disorders. A DAO deficiency that predisposes a population subgroup to histamine intolerance may have a genetic, pathological or pharmacological origin [1,41].
Regarding the genetic background of histamine intolerance, several studies have analyzed in depth the polymorphisms in genes encoding the enzymes L-histidine decarboxylase, DAO and HNMT, as well as the different histamine receptors. More than 50 nonsynonymous single-nucleotide polymorphisms (SNPs) in the DAO-encoding gene have been identified, some of which can produce a protein with altered activity and lead to symptoms of histamine intolerance [67,68,69,70,71,72]. Specifically, the most relevant SNPs affecting DAO enzyme functionality in Caucasian individuals are rs10156191, rs1049742, rs2268999 and especially rs1049793 [69,71]. On the other hand, an SNP in the promoter region of the gene has also been identified that causes a lower transcriptional activity of the DAO-encoding gene (rs2052129), as well as several genetic variations responsible for enzyme deficiency in people of Asian or African origin (rs45558339 and rs35070995, respectively) [67,72]. In most cases, the effect of these genetic variations on DAO functionality is through changes in enzyme kinetics, the resulting increase in KM causing a reduction in the rate of histamine degradation [69]. In parallel, three SNPs have been identified as being responsible for enhanced DAO enzyme activity (rs2071514, rs1049748 and rs2071517) [72]. There is also evidence of DAO mutations in patients with certain cardiovascular, gastrointestinal and nervous system pathologies, although with contradictory results regarding positive/negative effects [68].
DAO deficiency can also be an acquired condition, caused by certain pathologies or interaction with drugs. Several inflammatory bowel pathologies affecting mucosal integrity are known to result in impaired DAO activity, the degree of which can be correlated with the severity of mucosal damage [73,74,75]. Thus, DAO activity has been proposed as a marker of integrity of the intestinal mucosa. Miyoshi et al. demonstrated that DAO activity can be a useful predictor of intestinal mucosal damage in patients receiving chemotherapy [76]. Additionally, DAO deficiency has also been linked to certain functional gastrointestinal disorders, such as carbohydrate malabsorption and nonceliac gluten sensitivity (NCGS) [63,73,77,78,79]. Enko et al. found that a concomitant reduction in DAO and lactase enzyme activities could be a consequence of mucosal damage in the small intestine due to gastrointestinal disorders (e.g., gastroenteritis, irritable bowel syndrome, short bowel syndrome and gastrointestinal surgery) [73]. Moreover, patients with lactose intolerance and plasma DAO deficit showed higher end-expiratory H2 levels and the appearance of more symptoms during the H2 breath test in comparison with lactose-intolerant individuals with normal DAO activity [79]. More recently, two works have suggested a potential relationship between a reduced DAO activity and the presence of NCGS. Schnedl et al. based this relationship on the broad parallelism between the symptomatology of NCGS and histamine intolerance, while the pilot study conducted by Griauzdaite et al. reported a strong association between reduced DAO activity and the presence of NCGS, although with a reduced number of patients [77,78]. In fact, Griauzdaite et al. found out that nine of 10 patients with NCGS had decreased serum DAO activity levels [78]. This recently indicated relationship between both disorders, NCGS and histamine intolerance, should be further explored as it may be of interest for the correct clinical management of affected patients.
Finally, DAO deficiency can be a temporary and reversible condition, caused by the inhibitory effect of substances such as biogenic amines and alcohol, as discussed above, as well as several widely used drugs (Table 2) [1,10]. It has been estimated that approximately 20% of the European population regularly take DAO-inhibiting drugs, which significantly increases the number of people susceptible to the adverse effects of dietary histamine [28]. In vitro experimental results show a potent inhibitory effect (greater than 90%) of chloroquine, a historical antimalarial active ingredient, and clavulanic acid, a β-lactam antibiotic widely used in combination with amoxicillin [80]. A significant inhibition of the enzymatic activity has also been observed with the antihypertensive drug verapamil and the histamine H2 receptor antagonist cimetidine, although the clinical use of the latter is currently anecdotal [23,80]. Other substances have also shown an inhibitory effect, albeit to a lesser extent (Table 2) [23,80,81]. In most cases, the structural similarity of the cited drugs with histamine could explain their potential to bind to the active site of DAO and reduce its enzymatic activity [23]. Along the same lines, substances with an inhibitory effect on other enzymes involved in any of the metabolic pathways of histamine in the body (i.e., HNMT, ALDH and MAO) may act as a trigger of histamine hypersensitivity [82].
이전 섹션에서 언급했듯이
장에서 외인성 히스타민에 대한 주요 장벽은
DAO 효소이며,
이는 전신 순환으로의 통과를 방지합니다 [10,13,65].
수많은 임상 연구에서
히스타민 과민증,
주로 두통과 위장 또는
피부 질환의 증상을 보이는
개인의 낮은 혈장 DAO 수치 유병률에 대한 데이터를 제공했습니다 [66].
일부 연구는
연구 설계나 참여자 수에 한계가 있지만,
대다수의 연구는 증상과
DAO 결핍 사이의 연관성을 지적하며
이러한 장애의 원인에서 DAO의 핵심 역할을 뒷받침하는 일반적인 추세를 확립하고 있습니다.
인구 하위 그룹이
히스타민 과민증에 걸리기 쉬운 DAO 결핍은
유전적, 병리적 또는 약리학적인 기원을 가질 수 있습니다[1,41].
히스타민 과민증의 유전적 배경과 관련하여
여러 연구에서
L- 히스티딘 탈카르복실 효소,
DAO 및 HNMT와 다양한 히스타민 수용체를 코딩하는
유전자의 다형성을 심층적으로 분석했습니다.
DAO 코딩 유전자에서
50개 이상의 비동일 단일염기다형성(SNP)이 확인되었으며,
이 중 일부는 활성이 변경된 단백질을 생성하고
히스타민 과민증 증상을 유발할 수 있습니다[67,68,69,70,71,72].
특히,
백인 개인의 DAO 효소 기능에 영향을 미치는 가장 관련성이 높은 SNP는
rs10156191, rs1049742, rs2268999, 특히 rs1049793입니다 [69,71].
한편, 유전자 프로모터 영역의 SNP는
DAO 암호화 유전자(rs2052129)의 전사 활성을 낮추고
아시아 또는 아프리카계 사람들의 효소 결핍을 유발하는
여러 유전적 변이(각각 rs45558339 및 rs35070995)도 확인되었습니다[67,72].
대부분의 경우,
이러한 유전적 변이가
DAO 기능에 미치는 영향은 효소 동역학의 변화를 통해 이루어지며,
그 결과 KM이 증가하여 히스타민 분해 속도가 감소합니다 [69].
이와 동시에 세 가지 SNP(rs2071514, rs1049748, rs2071517)가 DAO 효소 활성 강화에 관여하는 것으로 확인되었습니다[72]. 또한 특정 심혈관, 위장 및 신경계 병리를 가진 환자에서 DAO 돌연변이의 증거가 있지만, 긍정/부정적 영향에 관한 상반된 결과가 있습니다 [68].
DAO 결핍은
특정 병리 또는 약물과의 상호작용으로 인해 발생하는
후천적 질환일 수도 있습니다.
점막 완전성에 영향을 미치는
여러 염증성 장 병리는
DAO 활동 장애를 초래하는 것으로 알려져 있으며,
그 정도는 점막 손상의 심각성과 상관관계가 있을 수 있습니다 [73,74,75].
따라서
DAO 활성은
장 점막의 완전성을 나타내는 마커로 제안되었습니다.
미요시 등은
화학 요법을 받는 환자의 장 점막 손상에 대한
유용한 예측 인자가 될 수 있음을 입증했습니다 [76].
또한 DAO 결핍은
탄수화물 흡수 장애 및 비체강 글루텐 민감성(NCGS)과 같은
특정 기능성 위장 장애와도 관련이 있습니다[63,73,77,78,79].
Enko 등은
위장 장애(예: 위장염, 과민성 대장 증후군, 단장 증후군 및 위장 수술)로 인한
소장의 점막 손상으로 인해
DAO 및 락타아제 효소 활성의 수반되는 감소가 발생할 수 있음을 발견했습니다[73].
또한,
유당 불내증과
혈장 DAO 결핍이 있는 환자는
DAO 활동이 정상인 유당 불내증 환자에 비해
호기말 H2 수치가 더 높고
H2 호흡 테스트 중에 더 많은 증상이 나타나는 것으로 나타났습니다 [79].
최근에는 두 가지 연구에서
DAO 활동 감소와 NCGS의 존재 사이의
잠재적 관계를 제안했습니다.
Schnedl 등은
NCGS의 증상과
히스타민 과민증 사이의 광범위한 유사성을 근거로
이 관계를 설명했으며, Griauzdaite 등이 실시한 파일럿 연구에서는
비록 환자 수는 줄었지만
DAO 활성 감소와
NCGS의 존재 사이에 강력한 연관성이 있다고 보고했습니다 [77,78].
실제로 그리우즈다이트 등은
NCGS 환자 10명 중 9명이
혈청 DAO 활성도가 감소했다는 사실을 발견했습니다[78].
최근에 밝혀진 이 두 질환,
즉 NCGS와 히스타민 과민증 사이의 관계는
영향을 받는 환자의 올바른 임상 관리에 관심이 있을 수 있으므로 더 자세히 살펴봐야 합니다.
마지막으로,
DAO 결핍은
위에서 설명한 바와 같이
생체 아민 및 알코올과 같은 물질과 널리 사용되는
여러 약물(표 2)[1,10]의 억제 효과로 인해
일시적이고 가역적인 상태가 될 수 있습니다.
유럽 인구의 약 20%가 정기적으로
DAO 억제 약물을 복용하는 것으로 추정되며,
이는 식이 히스타민의 부작용에 취약한 사람들의 수를 크게 증가시킵니다 [28].
시험관 내 실험 결과에 따르면
과거 항말라리아 활성 성분인
클로로퀸과 아목시실린과 함께 널리 사용되는
β-락탐 항생제인 클라불란산은
강력한 억제 효과(90% 이상)를 보였습니다 [80].
항고혈압제 베라파밀과 히스타민 H2 수용체 길항제 시메티딘에서도
효소 활성의 현저한 억제가 관찰되었지만,
후자의 임상적 사용은 현재 일화적인 사례에 불과합니다 [23,80].
다른 물질도 덜하지만 억제 효과를 보였습니다(표 2) [23,80,81].
대부분의 경우,
인용된 약물과 히스타민의 구조적 유사성은
DAO의 활성 부위에 결합하여
효소 활성을 감소시킬 수 있는 잠재력을 설명할 수 있습니다 [23].
같은 맥락에서
체내 히스타민의 대사 경로에 관여하는
다른 효소(예: HNMT, ALDH 및 MAO)에 억제 효과가 있는 물질은
히스타민 과민증을 유발할 수 있습니다[82].
dge 및 히스타민 불내성에 대한 진단 도구를 사용할 수 있게 됩니다[10,13,63].
Table 2
Active ingredients with an experimentally demonstrated inhibitory effect on the DAO enzyme [23,28,80,81].
Active IngredientIndication
| Chloroquine | Antimalarial |
| Clavulanic acid | Antibiotic |
| Colistimethate | Antibiotic |
| Cefuroxime | Antibiotic |
| Verapamil | Antihypertensive |
| Clonidine | Antihypertensive |
| Dihydralazine | Antihypertensive |
| Pentamidine | Antiprotozoal |
| Isoniazid | Antituberculous |
| Metamizole | Analgesic |
| Diclofenac | Analgesic and anti-inflammatory |
| Acetylcysteine | Mucoactive |
| Amitriptyline | Antidepressant |
| Metoclopramide | Antiemetic |
| Suxamethonium | Muscle relaxant |
| Cimetidine | Antihistamine (H2 antagonist) |
| Prometazina | Antihistamine (H1 antagonist) |
| Ascorbic acid | Vitamin C |
| Thiamine | Vitamin B1 |
5.2. Prevalence of DAO Deficit in Persons with Symptoms Related to Histamine Intolerance
Several studies have evaluated the prevalence of DAO deficit in plasma of individuals with symptoms of histamine intolerance and/or diagnosis with certain chronic disorders.
Mušič et al. found DAO deficiency in 80% of 316 adult patients showing various symptoms associated with histamine intolerance (e.g., urticaria, pruritus, diarrhea, abdominal pain, vomiting, constipation, cough, rhinitis and headache), as well as significantly lower plasma DAO activity compared to the control group [83]. Similarly, in a retrospective study, Manzotti et al. evaluated DAO activity in 14 patients with a confirmed diagnosis of histamine intolerance who showed mainly gastrointestinal and dermatological symptoms, but also headaches [84]. In this case, patients showed a high prevalence of DAO deficit (71%) and a significantly lower mean DAO activity compared to healthy volunteers. A lower percentage of DAO deficiency in histamine-intolerant patients (24%) was reported by Pinzer et al. [63]. Those patients featured elevated histamine levels and constantly reduced DAO activities throughout the day.
In a study focused only on headache symptoms, Steinbrecher and Jarisch reported DAO deficiency in 23 of 27 patients (85%) [85]. In parallel, the authors described a significant increase in DAO activity after patients followed a low-histamine diet for four weeks, along with a remission or reduction in frequency of headaches in almost 90% of individuals. More recently, Izquierdo et al. studied the prevalence of DAO deficit in 137 patients diagnosed with a confirmed migraine diagnosis and in a control group of 61 nonmigraine individuals [66]. In this study, a high prevalence of DAO deficiency was observed in the migraine group (87%) and with a mean DAO activity significantly lower in comparison with that obtained from control volunteers. However, the prevalence of DAO deficiency in the control population amounted up to 44%, which was attributed to the fact that certain individuals could present other symptoms associated with histamine intolerance or DAO deficiency other than migraines. Another study with 44 migraine patients reported a 60% prevalence of DAO deficiency and a significant copresence of certain gastrointestinal disorders, such as celiac disease and NCGS [78].
In the field of dermatological symptomatology, several studies have monitored plasma DAO activity in patients with eczema, chronic idiopathic urticaria and atopic dermatitis. Overall, the reported prevalence of DAO deficiency ranges from 19 to 57%, with the exception of the study by Worm et al., who did not detect statistically significant differences in plasma DAO activity between control patients and those with atopic dermatitis [86,87,88,89].
Finally, regarding gastrointestinal symptoms, Honzawa et al. assessed the clinical significance of plasma DAO activity levels in 98 patients suffering inflammatory bowel disease [90]. This study showed that DAO activity in blood was significantly lower in patients with Crohn’s disease and ulcerative colitis compared to the control population, suggesting its potential importance as a marker of intestinal permeability. In a pediatric population under 15 years of age, Rosell-Camps et al. determined DAO deficiency in 88% of patients with abdominal pain, diarrhea and vomiting [91]. In contrast, in a more recent study by a group of Austrian researchers, DAO deficiency was found in only 8% of 394 children with chronic abdominal pain [92].
To date, little data is available on the prevalence of this enzymatic deficiency related to gender, and it is inconclusive. Klockler et al. found no differences in plasma DAO activity between men and women, although the number of individuals considered was scarce (n = 28) [93]. Likewise, the study performed by Izquierdo et al. reported similar percentages of DAO deficiency in migraine-suffering women (83%) and men (90%) [66]. On the contrary, García-Martín et al. did describe differences in plasma DAO activity by gender, with the prevalence of this enzyme deficiency being higher in women [94]. Significant fluctuations in DAO activity values have also been reported in women associated with different stages of the menstrual cycle [94,95].
One factor that could explain the discordance among the prevalence data of DAO deficit in patients with disorders associated with histamine intolerance is that the parameter considered in all of them was serum DAO activity, which, a priori, would not reflect an enzymatic deficiency derived from certain intestinal pathologies. Overall, in spite of the varying percentages in DAO deficiency, the currently available studies seem to indicate an etiological relationship between DAO deficiency and certain symptoms or disorders related to histamine intolerance. Nevertheless, more studies are needed to assess the clinical significance of the determination of plasma DAO activity, as well as to develop new diagnostic methods aimed at identifying individuals with histamine intolerance due to DAO deficiency.
여러 연구에서
히스타민 과민증 증상 및/또는
특정 만성 질환 진단을 받은 사람의 혈장에서
DAO 결핍의 유병률을 평가했습니다.
Mušič 등은
히스타민 과민증과 관련된 다양한 증상
(예: 두드러기, 가려움증, 설사, 복통, 구토, 변비, 기침, 비염 및 두통)을 보이는
성인 환자 316명 중 80%에서
DAO 결핍이 발견되었으며
대조군에 비해 혈장 DAO 활성도가 현저히 낮은 것으로 나타났습니다[83].
마찬가지로
후향적 연구에서 Manzotti 등은
주로 위장 및 피부과 증상뿐만 아니라
두통도 보이는 히스타민 과민증 진단을 받은
14명의 환자에서 DAO 활성을 평가했습니다[84].
이 경우 환자들은
건강한 지원자들에 비해
DAO 결핍 유병률(71%)이 높고
평균 DAO 활성도가 현저히 낮은 것으로 나타났습니다.
Pinzer 등[63]은
히스타민 불내성 환자에서
DAO 결핍의 비율이 더 낮다고 보고했습니다(24%).
이러한 환자들은
히스타민 수치가 높고
하루 종일 지속적으로 DAO 활동이 감소하는 특징을 보였습니다.
두통 증상에만 초점을 맞춘 연구에서
Steinbrecher와 Jarisch는
27명의 환자 중 23명(85%)에서 DAO 결핍을 보고했습니다[85].
이와 동시에 저자들은
환자가 4주 동안 저히스타민 식단을 따른 후
DAO 활동이 크게 증가했으며,
거의 90%의 두통이 완화되거나 빈도가 감소했다고 설명했습니다.
최근에는
편두통 진단을 받은 137명의 환자와
편두통이 없는 61명의 대조군[66]에서
DAO 결핍 유병률을 연구한 Izquierdo 등이 있습니다.
이 연구에서
편두통 그룹(87%)에서 DAO 결핍의 높은 유병률이 관찰되었으며,
대조군 지원자에서 얻은 결과와 비교하여
평균 DAO 활동이 현저히 낮았습니다.
그러나
대조군에서 DAO 결핍 유병률은 최대 44%에 달했는데,
이는 특정 개인이 편두통 외에
히스타민 과민증이나 DAO 결핍과 관련된
다른 증상을 보일 수 있기 때문인 것으로 추정됩니다.
44명의 편두통 환자를 대상으로 한 또 다른 연구에서는
DAO 결핍의 유병률이 60%에 달하고
셀리악병 및 NCGS와 같은 특정 위장 장애가
유의미하게 공존한다고 보고했습니다 [78].
피부과 증상 분야에서는
습진, 만성 특발성 두드러기,
아토피 피부염 환자의
혈장 DAO 활성을 모니터링한 여러 연구가 진행되었습니다.
대조군과
아토피 피부염 환자 간의
혈장 DAO 활성에 통계적으로 유의미한 차이를 발견하지 못한 Worm 등의 연구를 제외하고
전반적으로 보고된 DAO 결핍의 유병률은
19~57%입니다[86,87,88,89].
마지막으로,
위장 증상과 관련하여 Honzawa 등은
염증성 장 질환을 앓고 있는 98명의 환자에서
혈장 DAO 활성도의 임상적 중요성을 평가했습니다[90].
이 연구에 따르면
크론병과 궤양성 대장염 환자에서
혈중 DAO 활성도가 대조군에 비해 유의하게 낮았으며,
이는 장 투과성의 마커로서 잠재적인 중요성을 시사합니다.
15세 미만의 소아 환자군을 대상으로 한 연구에서는
복통, 설사, 구토를 동반한 환자의 88%에서
DAO 결핍이 확인되었습니다[91].
반면,
오스트리아 연구진의 최근 연구에서는
만성 복통을 앓는 어린이 394명 중
8%에서만 DAO 결핍이 발견되었습니다[92].
현재까지
성별과 관련된 이 효소 결핍의 유병률에 대한 데이터는 거의 없으며
결정적이지 않습니다.
Klockler 등은
고려된 개인의 수가 적었지만(n = 28),
남성과 여성 간의 혈장 DAO 활성에 차이가 없음을 발견했습니다[93].
마찬가지로 Izquierdo 등이 수행한 연구에서도
편두통을 앓는 여성(83%)과 남성(90%)에서
비슷한 비율의 DAO 결핍이 보고되었습니다 [66].
반대로
García-Martín 등은
이 효소 결핍의 유병률이 여성에서 더 높다는
성별에 따른 혈장 DAO 활성의 차이를 설명했습니다 [94].
월경 주기의 여러 단계와 관련된 여성에서도
DAO 활성도 값의 상당한 변동이 보고되었습니다 [94,95].
히스타민 과민증과 관련된 장애를 가진 환자에서
DAO 결핍의 유병률 데이터 간의 불일치를 설명할 수 있는 한 가지 요인은
이들 모두에서 고려된 파라미터가
특정 장 병리에서 파생된 효소 결핍을 반영하지 않는
혈청 DAO 활성도라는 점입니다.
전반적으로,
DAO 결핍의 비율은 다양하지만
현재 이용 가능한 연구에 따르면 DAO 결핍과
히스타민 과민증과 관련된 특정 증상 또는
장애 사이에 병인학적 관계가 있는 것으로 보입니다.
그럼에도 불구하고
혈장 DAO 활성도 측정의 임상적 중요성을 평가하고
DAO 결핍으로 인한 히스타민 과민증 환자를 식별하기 위한
새로운 진단 방법을 개발하기 위해서는 더 많은 연구가 필요합니다.
5.3. Diagnosis of Histamine Intolerance
Despite significant advances in the understanding of histamine intolerance, reaching a consensus on a diagnostic algorithm remains a pending challenge. The nonspecificity of symptoms and lack of validated diagnostic tools prompts many affected individuals to go “doctor shopping”; that is, to consult several medical specialists in search of an explanation and solution for their varied symptomatology [13,63]. In the absence of a consensual and clinically validated diagnosis, Figure 7 shows a schematic summary of the diagnostic algorithm for histamine intolerance based on the available scientific evidence reviewed below.
히스타민 과민증에 대한 이해가 크게 발전했음에도 불구하고
진단 알고리즘에 대한 합의에 도달하는 것은
여전히 미해결 과제로 남아 있습니다.
증상의 비특이성과 검증된 진단 도구의 부재로 인해
많은 피해자들이 '닥터 쇼핑',
즉 다양한 증상에 대한 설명과 해결책을 찾기 위해
여러 의료 전문가와 상담하게 됩니다[13,63].
합의되고 임
상적으로 검증된 진단이 없는 경우,
그림 7은 아래에 검토된 과학적 증거를 바탕으로 한 히스타민 과민증 진단 알고리즘의 개략적인 요약을 보여줍니다.
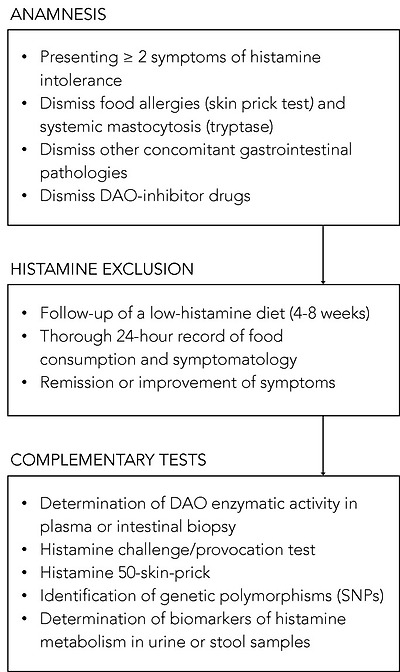
Summary of the described approaches to the diagnosis of histamine intolerance. SNPs: single-nucleotide polymorphisms.
The combination of diagnostic criteria currently in use includes the appearance of typical clinical manifestations and the exclusion of other related disorders [10,13,54]. All the authors who have proposed a diagnostic algorithm for histamine intolerance emphasize the need to initially rule out other potential causes of symptoms associated with an increase in plasma histamine [10,13,54]. For this purpose, it is advisable to carry out an intradermal skin allergy test (i.e., skin prick test) to discard IgE sensitization caused by food allergy, and to measure plasma tryptase to exclude an underlying systemic mastocytosis [10]. It is also important to know whether the patient is taking any medication with a possible inhibitory effect on DAO activity [55]. If these conditions are negative, the appearance of two or more typical symptoms of histamine intolerance and their improvement or remission after the following of a low-histamine diet (i.e., a diet excluding foods that, a priori, contain high histamine levels) will confirm the diagnosis of histamine intolerance [10,54,96,97]. In the diet follow-up, a thorough 24-h record of all the foods consumed and symptoms experienced is recommended in order to establish a relationship, if any, between a food and the onset of symptoms [10,13]. The duration of the low-histamine diet to confirm the diagnosis is not clearly stipulated, although some studies suggest a period of 4 to 8 weeks [54,97]. In addition to the diet, testing the effect of antihistamine treatment on symptoms has also been proposed, although its usefulness once dietary histamine is removed is unclear [10,54].
Once it has been established that dietary histamine is responsible for the intolerance-associated symptoms, the diagnosis of this disorder is virtually confirmed. A range of nonvalidated complementary tests have also been proposed by several authors with the aim of obtaining a marker to confirm the diagnosis [97]. However, it has to be taken into account that not all of the tests consider the different origins of DAO deficiency (i.e., genetic, pathological or pharmacological). Thus, a genetic origin would lead to a reduction of the DAO enzymatic activity in the whole organism. Likewise, the pharmacological blockade of DAO would take place in all tissues where the drug is distributed after entering the systemic circulation, although in a punctual manner upon the substance’s introduction. Lastly, the scope of a DAO deficit due to intestinal pathologies would be limited to the local intestinal environment.
Due to the genetic background of DAO deficiency, one of the strategies for the diagnosis could be the determination of genetic polymorphisms (SNPs) that characterize the population as genetically susceptible to histamine [54]. Currently, there is already the possibility of performing a noninvasive genetic analysis capable of identifying three of the SNPs associated with reduced DAO activity (i.e., rs10156191, rs1049742 and rs1049793) from a sample of the oral mucosa, although evidence-based studies on the diagnosis potential of this test are still needed. It is important to note that this test will only reflect the existence of a genetic DAO deficiency.
The most studied, and possibly also the most controversial, is the determination of plasma DAO activity. This analytical test consists of measuring the amount of histamine degraded in a blood sample in a given time interval. Two types of commercial testing kits are currently available on the market, one consisting of an ELISA-type immunoassay, and the other a radioimmunoassay using radioactively labeled putrescine [83,84]. The evidence for the validity of blood DAO activity measurements for the diagnosis of histamine intolerance is neither abundant nor conclusive. Some studies have proposed that determining blood DAO activity may be helpful in identifying subjects with symptoms associated with histamine intolerance [63,83,84]. In contrast, three studies did not find a significant relationship between the clinical history of patients with typical symptoms of histamine intolerance and blood DAO activity values, concluding that this technique cannot be recommended as a diagnostic tool in routine clinical practice until studies have validated its effectiveness [98,99,100]. Moreover, the work performed by Schnoor et al. also reported a high interassay variation in DAO activity values that made the proper classification of histamine-intolerant subjects impossible [100]. This controversy is described in a joint article published in 2017 by the German and Swiss allergology societies, which emphasizes the need for more research before giving plasma DAO activity a definitive diagnostic value for histamine intolerance [97].
A variant of the intradermal skin allergy test called the histamine 50-skin-prick test was also proposed by Kofler et al. to diagnose histamine intolerance [101]. In this technique, the results were read after 50 min (as opposed to the usual 20 min) and showed that, although the size of the wheal did not differ between the histamine intolerant and control groups, the time course was significantly different. Patients with symptoms of intolerance showed a delayed remission of the wheal induced by cutaneous administration of histamine, signaling a reduced degradation ability. The same results were obtained in a study recently published by Wagner et al., who re-evaluated this skin test as a diagnostic tool of histamine intolerance, also observing a correlation between the delay in wheal disappearance and a lower plasma DAO activity [102].
Both the determination of plasma DAO activity and the histamine 50-skin-prick test could be suitable tests to identify a DAO deficiency from genetic or pharmacological origin, but they would not be useful to determine a deficit secondary to certain intestinal diseases.
On the contrary, there are certain alternatives, such as the intestinal biopsy, the histamine provocation test or the histamine metabolomics in urine, that could make it possible to diagnose histamine intolerance due to DAO deficiency without excluding any of the possible etiological causes.
The measurement of intestinal DAO activity by a colon biopsy during endoscopic procedures has been studied as a possible diagnostic marker. The few available studies have shown a reduced intestinal DAO catabolic activity in patients with recurrent urticaria, food allergy and colon adenoma, accompanied by an increase in histamine levels [103,104,105,106]. Although this test has interesting diagnostic potential, more studies are needed to validate its clinical significance and its relationship with the symptoms of histamine intolerance [97]. If proven, this diagnostic test would be very adequate since this disorder originates from a reduced ability of the intestinal DAO enzyme to cope with dietary histamine.
Histamine challenge/provocation test has also been proposed by some authors as a diagnostic tool for intolerance, which would, at the same time, establish the individual tolerance threshold. This double-blind, placebo-controlled test involves oral administration of histamine and requires patient medical supervision and hospitalization. In the study by Wöhrl et al., half of the healthy volunteers developed symptoms after the administration of a solution containing 75 mg of histamine [107]. In contrast, the results of a multicenter study by Komericki et al. using the same oral dose of histamine indicated the challenge test was unreliable for diagnosing histamine intolerance due to a lack of intraindividual reproducibility of symptoms after two different provocation tests [108]. The application of this procedure is still limited because of the risk of serious adverse side effects and the absence of a standardized dose of histamine and properly established protocol [97].
Finally, in recent years, efforts have been made to identify a noninvasive marker to establish a solid and clinically irrefutable diagnostic criterion for histamine intolerance due to DAO deficiency. Currently, the application of metabolomics as a tool for the identification of biomarkers of histamine metabolism in urine is also being challenged as a possible new diagnostic strategy [11]. The hypothesis is that individuals with histamine intolerance could have a different excretion profile of histamine and its metabolites in urine than normal individuals. For this purpose, Comas-Basté et al. have recently proposed a chromatographic approach that allows for determining in a fast and unequivocal manner the urinary levels of histamine and its methylated metabolite, methylhistamine [11]. It is still necessary to validate the potential diagnostic utility of this approach in patients with histamine intolerance, as well as complementing the excretion profile with other histamine metabolites to obtain a more accurate image of the possible alterations produced in this intolerance.
히스타민 과민증 진단에 대한 설명 된 접근 방식 요약. SNP: 단일 뉴클레오티드 다형성.
현재 사용중인 진단 기준의 조합에는
전형적인 임상 증상의 출현과
다른 관련 장애의 배제가 포함됩니다 [10,13,54].
히스타민 과민증에 대한 진단 알고리즘을 제안한 모든 저자는
혈장 히스타민 증가와 관련된 증상의
다른 잠재적 원인을 처음에 배제해야 할 필요성을 강조합니다 [10,13,54].
이를 위해
피내 피부 알레르기 검사(즉, 피부 단자 검사)를 실시하여
음식 알레르기로 인한 IgE 감작을 배제하고
혈장 트립타아제를 측정하여
기저 전신 비만 세포증을 배제하는 것이 좋습니다[10].
환자가
DAO 활동을 억제할 수 있는 약물을 복용하고 있는지
여부를 아는 것도 중요합니다[55].
이러한 조건이 음성인 경우,
히스타민 과민증의 전형적인 증상이
두 가지 이상 나타나고
저히스타민 식단(즉, 선험적으로 히스타민 수치가 높은 음식을 제외한 식단)을 따른 후 개선 또는 완화되면
히스타민 과민증 진단을 확정할 수 있습니다[10,54,96,97].
식이 추적 조사에서는
음식과 증상 발병 사이의 관계를 파악하기 위해
섭취한 모든 음식과 경험한 증상을
24시간 동안 철저히 기록하는 것이 좋습니다[10,13].
진단을 확인하기 위한
저히스타민 식단의 기간은 명확하게 규정되어 있지 않지만
일부 연구에서는 4~8주 [54,97]의 기간을 제안합니다.
식이 요법 외에도
항히스타민 치료가 증상에 미치는 영향을 테스트하는 방법도 제안되었지만,
식이 히스타민이 제거되면
그 유용성은 불분명합니다 [10,54].
식이 히스타민이
과민성 관련 증상의 원인이라는 것이 확인되면
이 질환의 진단은 사실상 확정됩니다.
진단을 확인하기위한 마커를 얻기 위해
여러 저자에 의해 검증되지 않은
다양한 보완 테스트도 제안되었습니다 [97].
그러나
모든 검사가
DAO 결핍의 다양한 기원(예: 유전적, 병리적 또는 약리학적)을
고려하는 것은 아니라는 점을 고려해야 합니다.
따라서
유전적 기원은
전체 유기체에서
DAO 효소 활성의 감소로 이어질 수 있습니다.
마찬가지로
DAO의 약리학적 차단은
물질이 도입되는 즉시
약물이 전신 순환에 들어간 후 약
물이 분포하는 모든 조직에서 일어날 수 있습니다.
마지막으로,
장 병리로 인한 DAO 결핍의 범위는
국소 장 환경으로 제한됩니다.
DAO 결핍의 유전적 배경으로 인해
진단 전략 중 하나는
히스타민에 유 전적으로 취약한 집단을 특징 짓는
유전적 다형성 (SNP)을 결정하는 것일 수 있습니다 [54].
현재 구강 점막 샘플에서
DAO 활성 감소와 관련된
세 가지 SNP(즉, rs10156191, rs1049742 및 rs1049793)를 식별할 수 있는
비침습적 유전자 분석이 이미 가능하지만
이 검사의 진단 가능성에 대한 증거 기반 연구가 여전히 필요합니다.
이 검사는
유전적 DAO 결핍의 존재 여부만 반영한다는 점에 유의하세요.
가장 많이 연구되었지만
가장 논란의 여지가 있는 것은
혈장 DAO 활성도 측정입니다.
이 분석 검사는
주어진 시간 간격으로 혈액 샘플에서 분해된 히스타민의 양을 측정하는 것으로 구성됩니다.
현재 두 가지 유형의 상용 검사 키트가 시판되고 있는데, 하나는 ELISA 방식의 면역 분석법이고 다른 하나는 방사성 표지된 푸트레신을 사용하는 방사성 면역 분석법입니다[83,84]. 히스타민 과민증 진단을 위한 혈중 DAO 활성도 측정의 유효성에 대한 증거는 풍부하지도 결정적이지도 않습니다. 일부 연구에서는 혈중 DAO 활성도를 측정하는 것이 히스타민 과민증과 관련된 증상이 있는 피험자를 식별하는 데 도움이 될 수 있다고 제안했습니다[63,83,84]. 반면, 세 연구에서는 히스타민 과민증의 전형적인 증상이 있는 환자의 임상 병력과 혈중 DAO 활성도 값 사이에 유의미한 관계가 없다는 결론을 내렸으며, 이 기술은 그 효과가 검증될 때까지 일상적인 임상 진료에서 진단 도구로 권장할 수 없다는 결론을 내렸습니다[98,99,100]. 또한 슈누어 등이 수행한 연구에서도 DAO 활성도 값의 분석 간 편차가 커서 히스타민 과민성 피험자의 적절한 분류가 불가능하다고 보고했습니다[100]. 이러한 논란은 2017년 독일 및 스위스 알레르기 학회에서 발표한 공동 논문에 기술되어 있으며, 이 논문에서는 혈장 DAO 활성도를 히스타민 과민증의 확실한 진단 값으로 사용하기 전에 더 많은 연구가 필요하다고 강조하고 있습니다[97].
히스타민 과민증을 진단하기 위해
히스타민 50-스킨-픽 검사라는
피내 피부 알레르기 검사의 변형도 Kofler 등에 의해 제안되었습니다 [101].
이 기술에서는 일반적인 20 분과 달리 50 분 후에 결과를 읽었으며, 히스타민 과민증 그룹과 대조군 사이에 수포의 크기가 다르지 않았지만 시간 경과가 크게 다르다는 것을 보여주었습니다. 과민증 증상이 있는 환자는 히스타민의 피부 투여로 유발된 수포의 완화가 지연되어 분해 능력이 저하된 것으로 나타났습니다. 히스타민 과민증의 진단 도구로 이 피부 검사를 재평가한 Wagner 등이 최근 발표한 연구에서도 동일한 결과를 얻었으며, 수포 소실 지연과 혈장 DAO 활성도 저하 사이의 상관관계를 관찰했습니다[102].
혈장 DAO 활성도 측정과 히스타민 50-스킨픽 테스트는 모두 유전적 또는 약리학적인 원인으로 인한 DAO 결핍을 확인하는 데 적합한 검사가 될 수 있지만 특정 장 질환으로 인한 이차적인 결핍을 확인하는 데는 유용하지 않습니다.
반대로 장 생검, 히스타민 유발 검사 또는 소변 내 히스타민 대사체 검사와 같은 특정 대안이 있어 가능한 병인 원인을 배제하지 않고도 DAO 결핍으로 인한 히스타민 불내성을 진단할 수 있습니다.
내시경 시술 중 대장 생검을 통한 장내 DAO 활성도 측정이 가능한 진단 마커로 연구되고 있습니다. 이용 가능한 몇 가지 연구에 따르면 재발성 두드러기, 음식 알레르기 및 결장 선종 환자에서 히스타민 수치의 증가와 함께 장내 DAO 이화 작용이 감소하는 것으로 나타났습니다 [103,104,105,106]. 이 검사는 흥미로운 진단 잠재력을 가지고 있지만 임상적 중요성과 히스타민 과민증 증상과의 관계를 검증하기 위해서는 더 많은 연구가 필요합니다 [97]. 이 장애는 식이 히스타민에 대처하는 장내 DAO 효소의 능력 저하에서 비롯되므로 이 진단 검사가 입증된다면 매우 적절할 것입니다.
히스타민 도전/도발 테스트는 일부 저자에 의해 과민성 진단 도구로 제안되기도 했는데, 이는 동시에 개인의 내성 임계치를 설정할 수 있습니다. 이 이중맹검, 위약 대조 테스트는 히스타민을 경구 투여하는 방식으로 진행되며 환자의 의료 감독과 입원이 필요합니다. Wöhrl 등의 연구에서 건강한 지원자 중 절반이 히스타민 75mg을 함유한 용액을 투여한 후 증상이 발생했습니다[107]. 반면, 동일한 용량의 히스타민을 경구 투여한 Komericki 등의 다기관 연구 결과에 따르면 두 가지 다른 자극 테스트 후 증상의 개인 내 재현성이 부족하여 챌린지 테스트가 히스타민 과민증 진단에 신뢰할 수 없는 것으로 나타났습니다 [108]. 이 절차의 적용은 심각한 부작용의 위험과 표준화된 히스타민 용량 및 적절하게 확립된 프로토콜의 부재로 인해 여전히 제한적입니다 [97].
마지막으로,
최근 몇 년 동안 DAO 결핍으로 인한
히스타민 불내성에 대한
견고하고 임상적으로 반박 할 수없는 진단 기준을 확립하기 위해
비 침습적 마커를 식별하려는 노력이 이루어졌습니다.
현재 소변에서
히스타민 대사의 바이오마커를 식별하는 도구로
대사체학을 적용하는 것도 가능한 새로운 진단 전략으로 도전받고 있습니다[11].
히스타민 과민증이 있는 사람은
소변에서 히스타민과 그 대사 산물의 배설 프로필이
정상인과 다를 수 있다는 가설이 있습니다.
이를 위해 최근 Comas-Basté 등은
히스타민과 그 메틸화된 대사산물인 메틸히스타민의 소변 수치를
빠르고 명확하게 측정할 수 있는 크로마토그래피 접근법을 제안했습니다[11].
히스타민 과민증 환자에서
이 접근법의 잠재적 진단 유용성을 검증하고,
다른 히스타민 대사산물로 배설 프로필을 보완하여
과민증에서 발생할 수 있는 변화에 대한
보다 정확한 이미지를 확보하는 것이 여전히 필요합니다.
5.4. Treatment Approaches to Histamine Intolerance
Currently, the main strategy to avoid the symptoms of histamine intolerance is to follow a low-histamine diet. Supplementation with exogenous DAO has recently been postulated as a complementary treatment to enhance dietary histamine degradation in intolerant individuals who have a deficiency of this enzyme in the intestine [109,110].
현재
히스타민 과민증 증상을 피하기 위한 주요 전략은
저히스타민 식단을 따르는 것입니다.
최근 장에 이 효소가 결핍된 과민증 환자의 식이 히스타민 분해를 개선하기 위해
외인성 DAO를 보충하는 것이
보완적인 치료법으로 제시되고 있습니다[109,110].
5.4.1. Low-Histamine Diet
A low-histamine or histamine-free diet has been proposed as the main strategy for the preventive treatment of histamine intolerance [10,54,82,111]. Conceptually, these diets exclude a number of foods that patients associate with the onset of symptoms, primarily those that may contain high levels of histamine [82]. However, there is no a single dietary recommendation of a low-histamine diet. As it may be seen in Table 3, there is no coincidence in all the foods excluded in the different low-histamine diets found in the literature [10,87,91,112,113,114,115,116,117,118].
히스타민 과민증의
예방 치료를 위한 주요 전략으로
저히스타민 또는 히스타민 무함유 식단이 제안되었습니다 [10,54,82,111].
개념적으로 이러한 식단은
환자가 증상의 시작과 연관시키는 여러 가지 음식,
주로 높은 수준의 히스타민을 함유할 수 있는 음식을 배제합니다 [82].
그러나
저히스타민 식단에 대한
단일 식단 권장 사항은 없습니다.
표 3에서 볼 수 있듯이,
문헌에서 발견된 다양한 저히스타민 식단에서 제외된 모든 식품이 일치하지 않습니다[10,87,91,112,113,114,115,116,117,118].
Table 3
Foods excluded in the different low-histamine diets found in the literature [10,87,91,112,113,114,115,116,117,118].
Foods Excluded by Low-Histamine Diets
| <20% * | 20–60% * | >60% * |
| Milk | Shellfish | Cured and semicured cheese |
| Lentils | Eggs | Grated cheese |
| Chickpeas | Fermented soy derivatives | Oily fish |
| Soybeans | Eggplant | Canned and semipreserved oily fish derivatives |
| Mushrooms | Avocado | Dry-fermented meat products |
| Banana | Spinach | |
| Kiwi | Tomatoes | |
| Pineapple | Fermented cabbage | |
| Plum | Citrus | |
| Nuts | Strawberries | |
| Chocolate | Wine | |
| Beer |
* Percentage of low-histamine diets from the literature that exclude each foodstuff.
Histamine is widely distributed in different food categories and in highly variable concentrations, as its accumulation is influenced by multiple factors [3,119]. In fresh foods such as fish and meat, and in some derived products, the presence of histamine is due to a lack of freshness or an inadequately hygienic quality of raw materials and/or production processes [31]. For this reason, meat and fish can be consumed in the framework of a low-histamine diet, as long as their freshness is ensured. In contrast, fermented products are systematically excluded, due to a high probability of containing histamine [31]. Other foods such as spinach, eggplant and tomatoes should also be avoided for the same reason. In general, all these abovementioned foods are unanimously eliminated in most published low-histamine diets (Table 3).
On the other hand, there are certain foods that a priori do not contain histamine, but that patients associate with the appearance of symptoms. For these foods, there is much more variability when it comes to their exclusion from low-histamine diets (Table 3). The exclusion of foods could be based on their content of other biogenic amines, such as putrescine and cadaverine, which act as competitive substrates for DAO and may therefore inhibit intestinal degradation of histamine if present in significant quantities [1,82]. Thus, the onset of symptoms after the consumption of citrus fruits, mushrooms, soybeans, bananas and nuts may be due to high levels of other amines, specially putrescine [82]. These diets may also exclude certain foods free of histamine and with low enough concentrations of other amines to justify their exclusion. This is the case, for example, for papayas, kiwis, strawberries, pineapples and plums, which have been reported to trigger the release of endogenous histamine, although the mechanism responsible has not yet been elucidated [8,13].
The effectiveness of a low-histamine diet has been demonstrated in clinical studies, which report favorable results in terms of improvement or total remission of symptoms frequently associated with histamine intolerance and DAO deficiency (Table 4). As shown in Table 4, over the past three decades, various clinical studies have assessed the effect of a low-histamine diet on the evolution of various symptoms, mainly dermatological, gastrointestinal and neurological, including cases with more than one type. Although most studies have involved only a small group of patients (a mean of 38 per study, with a minimum of 10 and maximum of 157), they report an efficacy rate for the diet ranging from 33% to 100%. Specifically, 10 of the 13 studies reviewed found an improvement in symptoms in more than 50% of patients who followed the diet; two studies show success rates of less than 50% (33% and 46%), and only one did not observe any beneficial effects (Table 4). Most of the studies involved patients with dermatological symptoms, primarily chronic idiopathic urticaria, atopic dermatitis and eczema. In this field, a recent systematic literature review included a total of 1668 patients with chronic urticaria undergoing different exclusion diets, including low-histamine, pseudoallergen-free (i.e., without preservatives and artificial colors present in processed foods or aromatic compounds from certain natural products) and fish exclusion diets [120]. Overall, following any of the exclusion diets resulted in the total or partial remission of symptoms in 4.9% and 37.5% of patients, respectively. A low-histamine diet for an average of 3 weeks resulted in one of the highest remission rates. Despite the promising results of a low-histamine diet for the treatment of dermatological conditions, scientific societies of dermatology still consider this exclusion diet of unproven utility pending randomized, double-blind, placebo-controlled clinical trials to confirm its effectiveness [121].
히스타민은
여러 요인에 의해 축적되기 때문에
다양한 식품 범주와 매우 다양한 농도로 널리 분포되어 있습니다[3,119].
생선 및 육류와 같은
신선 식품과 일부 파생 제품에서 히스타민의 존재는
신선도가 부족하거나 원료 및/또는
생산 공정의 위생적 품질이 부적절하기 때문입니다 [31].
이러한 이유로
육류와 생선은
신선도가 보장되는 한 저히스타민 식단의 틀 안에서 섭취할 수 있습니다.
반대로
발효 제품은
히스타민을 함유 할 가능성이 높기 때문에
체계적으로 제외됩니다 [31].
시금치, 가지, 토마토와 같은
다른 음식도 같은 이유로 피해야 합니다.
일반적으로 위에서 언급한 모든 식품은
대부분의 저히스타민 식단에서 만장일치로 제외됩니다(표 3).
반면에
선험적으로 히스타민을 포함하지 않지만
환자가 증상 발현과 연관시키는 특정 음식이 있습니다.
이러한 식품의 경우
저히스타민 식단에서 제외할 때
훨씬 더 많은 변동성이 있습니다(표 3).
식품 제외는
DAO의 경쟁 기질로 작용하여
상당한 양이 존재할 경우 히스타민의 장내 분해를 억제할 수 있는
푸트레신 및 카데베린과 같은
다른 생체 아민의 함량에 따라 결정될 수 있습니다[1,82].
따라서
감귤류, 버섯, 대두, 바나나 및 견과류 섭취 후
증상이 시작되는 것은
높은 수준의 다른 아민, 특히 푸트레신 때문일 수 있습니다 [82].
이러한 식단에서는
히스타민이 없고
다른 아민 농도가 충분히 낮아 제외할 수 있는 특정 식품도 제외할 수 있습니다.
예를 들어
파파야, 키위, 딸기, 파인애플, 자두는
내인성 히스타민 방출을 유발하는 것으로 보고되었지만
그 메커니즘은 아직 밝혀지지 않았습니다 [8,13].
저히스타민 식단의 효과는
임상 연구에서 입증되었는데,
히스타민 과민증 및 DAO 결핍과 관련된 증상의 개선 또는
완전한 완화에 유리한 결과를 보고했습니다(표 4).
표 4에서 볼 수 있듯이
지난 30년 동안 다양한 임상 연구에서
저히스타민 식단이 주로
피부과, 위장, 신경과 등 다양한 증상의 발현에 미치는 영향을 평가했으며,
한 가지 이상의 유형을 가진 경우도 포함되었습니다.
대부분의 연구는
소수의 환자 그룹(연구당 평균 38명, 최소 10명에서 최대 157명)만을 대상으로 했지만,
33%에서 100%에 이르는 식단의 효능률을 보고하고 있습니다.
구체적으로 검토된 13개 연구 중
10개 연구에서 식이요법을 따른
환자의 50% 이상에서 증상이 개선된 것으로 나타났으며,
2개 연구에서는 성
공률이 50% 미만(33%, 46%)으로 나타났고 1
개 연구에서만 유익한 효과가 관찰되지 않았습니다(표 4).
대부분의 연구는
주로
만성 특발성 두드러기,
아토피 피부염,
습진 등 피부과적 증상이 있는 환자를 대상으로 진행되었습니다.
이 분야의 최근 체계적 문헌 고찰에서는
총 1668명의 만성 두드러기 환자를 대상으로
저히스타민, 유사 알레르겐 무함유
(즉, 가공식품에 존재하는 방부제와 인공 색소 또는 특정 천연물의 방향 화합물이 없는) 및
생선 배제 식단 등
다양한 배제 식단을 시행한 결과[120]를 포함했습니다.
전반적으로
이러한 배제 식단을 따랐을 때
각각 4.9%와 37.5%의 환자에서 증상이 완전히 또는
부분적으로 완화되는 결과를 얻었습니다.
평균 3주 동안의 저히스타민 식단은
가장 높은 관해율을 보였습니다.
피부과 질환 치료를 위한
저히스타민 식단의 유망한 결과에도 불구하고,
피부과학 학회에서는 여전히 이 배제 식단의 효과를 확인하기 위해
무작위, 이중맹검, 위약 대조 임상시험이 진행 중이므로
효용성이 입증되지 않은 것으로 간주하고 있습니다[121].
Table 4
Clinical studies on the efficacy of a low-histamine diet for the treatment of symptoms of histamine intolerance.
Design and Outcomes of the StudyNumber of Patients and SymptomsDurationPercentage of Patients with Improvement in the Study OutcomesReference
| Prospective study with evaluation of the evolution of the symptomatology | 28 patients with chronic headache and 17 with other dermatological and respiratory symptoms | 4 weeks | 68% reduction in chronic headache and 82% reduction in other symptoms | [124] |
| Prospective study with evaluation of the evolution of symptoms, plasma histamine levels and DAO activity | 10 patients with chronic idiopathic urticaria and 19 control individuals | 3 weeks | 100% reduction in symptoms, 100% reduction in plasma histamine and no changes in DAO activity | [122] |
| Prospective study with evaluation of the evolution of symptoms, plasma histamine levels and DAO activity | 35 patients with headache and other symptoms (urticaria, arrhythmia, diarrhea and asthma) | 4 weeks | 77% reduction in symptoms, 73% increase in DAO activity and no changes in plasma histamine levels | [85] |
| Prospective study with evaluation of the evolution of symptoms and DAO activity (in five of the patients) | 17 patients with DAO deficiency, atopic eczema and other symptoms (headache, flushing and gastrointestinal symptoms) | 2 weeks | 100% reduction in symptoms and 60% (three out of five) increase in DAO activity | [86] |
| Prospective study with evaluation of the evolution of symptoms and the use of antihistamine drugs | 13 patients with chronic idiopathic urticaria and 35 control patients (without diet) | 4 weeks | Lack of improvement in symptoms and no changes in the use of antihistamines | [125] |
| Prospective study with evaluation of the evolution of the symptomatology | 36 patients with atopic dermatitis and 19 control individuals | 2 weeks | 33% reduction in symptoms | [88] |
| Prospective study with evaluation of the evolution of the symptomatology and DAO activity | 20 patients with DAO deficiency and dermatological, gastrointestinal and respiratory symptoms | 6–12 months | 100% reduction in symptoms and 100% increase in DAO activity | [83] |
| Retrospective study with evaluation of the evolution of the symptomatology | 16 pediatric patients with diffuse abdominal pain, diarrhea, headache, vomiting and rash | 4 weeks | 100% reduction of symptoms | [91] |
| Prospective study with evaluation of the evolution of the symptomatology | 16 pediatric patients with chronic abdominal pain and DAO deficiency | 4 weeks | 88% reduction of symptoms | [92] |
| Retrospective study with evaluation of the evolution of the symptomatology | 157 patients with chronic idiopathic urticaria | 4 weeks | 46% reduction of symptoms | [126] |
| Prospective study with evaluation of the evolution of the symptomatology and DAO activity | 56 patients with chronic idiopathic urticaria and gastrointestinal symptoms | 3 weeks | 75% reduction in symptoms and no changes in DAO activity | [87] |
| Prospective study with evaluation of the evolution of symptoms, plasma histamine levels and DAO activity | 22 patients with chronic idiopathic urticaria | 4 weeks | 100% reduction in symptoms, 100% reduction in plasma histamine levels and no changes in DAO activity | [112] |
| Retrospective study with evaluation of the evolution of the symptomatology and DAO activity | 63 patients with gastrointestinal symptoms | 7–18 months | 79% reduction in symptoms and 52% increase in DAO activity | [123] |
In general, the duration of the dietary treatment considered in the different clinical studies ranges from 3 to 4 weeks, and no positive relationship could be established between a longer duration and the success rate in symptom remission (Table 4). As may also be seen in this table, some studies have also assessed the effect of diet on other variables, such as plasma histamine levels or plasma DAO activity [83,85,86,87,112,122,123]. Regarding DAO activity, the studies published by Steinbrecher et al., Maintz et al., Mušič et al. and Lackner et al. all point out an increase in plasma enzymatic activity in more than 50% of patients after the dietary intervention, although no explanatory hypothesis has been yet suggested [83,85,86,123]. In contrast, Guida et al., Wagner et al. and Son et al. reported no changes in serum DAO activity [87,112,122]. The inconsistency of these data highlights the need to develop more research in this specific field before conclusions can be drawn.
일반적으로
여러 임상 연구에서 고려된 식이 치료 기간은
3주에서 4주 사이이며,
더 긴 기간과 증상 완화 성공률 사이에는 긍
정적인 관계가 확립되지 않았습니다(표 4).
이 표에서 볼 수 있듯이
일부 연구에서는 혈장 히스타민 수치 또는
혈장 DAO 활성도와 같은 다른 변수에 대한 식단의 영향도 평가했습니다[83,85,86,87,112,122,123].
DAO 활성과 관련하여
Steinbrecher 등, Maintz 등, Mušič 등, Lackner 등이 발표한 연구는
모두 식이 중재 후 환자의 50% 이상에서
혈장 효소 활성의 증가를 지적하지만
아직 설명 가능한 가설은 제시되지 않았습니다 [83,85,86,123].
이와는 대조적으로
Guida 등, Wagner 등, Son 등은
혈청 DAO 활성에 변화가 없다고 보고했습니다[87,112,122].
이러한 데이터의 불일치는 결론을 도출하기 전에 이 특정 분야에 대한 더 많은 연구가 필요하다는 것을 강조합니다[21].
5.4.2. Exogenous DAO Supplementation
Similar to the current treatment for lactose intolerance, the possibility of oral supplementation with exogenous DAO has been proposed by several authors to facilitate dietary histamine degradation [13,127]. Improving intestinal DAO activity would allow a less restrictive diet, which could include foods with a tolerable dose of histamine [10,61]. In this context, in the update of the official list of novel foods in 2017, the European Commission gave the green light to the marketing of a DAO supplement as food supplement or as food for special medical purposes [128]. Specifically, European regulations authorize the formulation of porcine kidney protein extract with an enteric coating to ensure its integrity during its passage through the gastric environment [128]. In this specific regulation, the minimum DAO enzymatic capacity required for the supplement is determined through a radio extraction assay (REA). This technique, based on the radioactive labeling of putrescine and the scintillation counting of its consumption, is advantageous in terms of rapidity and sensitivity, but it was mainly conceived to be applicable to serum samples. Comas-Basté et al. have recently developed a rapid and reliable methodology through ultra-high performance liquid chromatography and fluorimetric detection (UHPLC-FL) for the in vitro determination of DAO activity specifically for the analysis of unpurified complex matrixes, such as porcine kidney extract and DAO supplements [129]. This methodological approach is based in the direct determination of histamine degradation and overcomes certain drawbacks in terms of matrix interferences and handling of radioactive materials.
Porcine kidneys are the main source of DAO enzyme, according to the literature. Several studies have demonstrated the capacity of this product to degrade histamine and other biogenic amines in vitro [57,129,130,131,132,133]. A wide variability of the DAO capacity of porcine kidney extracts has been reported (with values ranging from 0.1 to more than 100 mU/mg), depending on the purification grade applied to the matrix and/or the amine compound used as the reaction substrate [57,129,130,131,132,133]. In fact, many of these works sought the selective purification of the DAO enzyme to design biosensors for the biorecognition of biogenic amines as indicators of freshness in foods [134]. More recently, two studies have been published specifically focused in investigating the in vitro DAO activity of porcine kidney protein extract expressly used as active ingredient to formulate food supplements for the preventive treatment of histamine intolerance [129,130]. Comas-Basté et al. studied the in vitro enzymatic activity of 13 different production batches of porcine kidney protein extract used in the elaboration of food supplements, reporting a low influence of the raw material (porcine kidney) on the DAO activity of the extract, with a mean value of 0.23 ± 0.01 mU/mg [129]. Later, Kettner et al. obtained a porcine kidney crude extract with an in vitro DAO activity of 0.5 ± 0.06 mU/mg, and described a 10-fold increase of this enzymatic activity through the application of several consecutive purification steps [130].
Regarding the food supplement, divergent results have also been reported, since while certain authors discard its enzymatic capacity, DAO activity values ranging from 0.04 to 0.20 mU/mg have also been described for different commercial products available in the market [129,130]. Overall, these works coincide in the need to identify alternative sources to porcine kidney DAO for exogenous supplementation in histamine-intolerant individuals.
A higher catalytic capacity of DAO enzymes of plant origin in degrading certain amino substrates has been described by some authors in comparison with those of animal origin [129,135,136,137]. Specifically, the germinated sprouts of certain edible legumes have been pointed out as interesting sources of DAO enzyme. Germination is a physiological process that has been described as capable of increasing the DAO enzymatic capacity of sprouts by up to 250 times compared to ungerminated seeds [61,138]. The increased presence of DAO enzyme in legume sprouts could be associated with the importance of hydrogen peroxide, a byproduct of the deamination reaction, in the cell wall structuring, lignification and mobilization of seed reserves during germination [139,140,141,142]. In fact, it has been demonstrated that the germination of legume seeds for a period of 6–8 days in darkness provides the optimal environment to maximize the DAO activity of this plant-origin matrix [61,138,143]. From a commercial point of view, having a plant source of this enzyme would expand the target of this novel food for the vegetarian/vegan population, as well as those with religious restrictions on the consumption of pork products. In addition, obtaining DAO enzyme from legumes would be a practice in accordance with the current call for action of the Sustainable Development Goals.
Nowadays, only five published intervention studies (four from the last five years) have tested the clinical efficacy of exogenous DAO supplementation in patients with symptoms of histamine intolerance (Table 5). Although there is some variability, the available research points to the effectiveness of DAO supplements in reducing the appearance and intensity of symptoms. However, it is difficult to compare the different studies, since they differ in the design, the enzyme dosage, the intervention time and the measurement of efficacy outcomes. Komericki et al., Manzotti et al. and Schnedl et al. assayed the efficacy of DAO supplementation in patients with diverse symptoms associated with histamine intolerance (gastrointestinal, cardiovascular, respiratory and dermatological and/or neurological complaints) [84,108,109]. All three studies reported an important improvement in the intensity or frequency of symptoms, although they involved a small study population (14, 28 and 39 patients) and/or a reduced intervention time (from two to four weeks). Moreover, Schnedl et al. also evaluated the changes in plasmatic DAO activity, reporting a slight increase in 61% of patients during the intervention, which the authors linked to a possible improvement in the integrity of the intestinal mucosa due to the supplementation [109].
현재 유당 불내증 치료법과 유사하게,
식이 히스타민 분해를 촉진하기 위해
외인성 DAO를 경구 보충할 수 있는 가능성이
여러 저자에 의해 제안되었습니다 [13,127].
장내 DAO 활동을 개선하면
허용 가능한 용량의 히스타민을 함유한 식품을 포함하여
덜 제한적인 식단을 구성할 수 있습니다[10,61].
이러한 맥락에서 유럽위원회는
2017년에 새로운 식품의 공식 목록을 업데이트하면서 식
품 보조제 또는 특수 의료 목적의 식품으로서
DAO 보충제의 마케팅에 청신호를 보냈습니다[128].
특히, 유럽 규정은
돼지 신장 단백질 추출물이
위 환경을 통과하는 동안
무결성을 보장하기 위해 장용 코팅이 된 제형을 승인합니다 [128].
이 특정 규정에서 보충제에 필요한
최소 DAO 효소 용량은
방사성 추출 분석법(REA)을 통해 결정됩니다.
푸트레신의 방사성 표지 및 소비량의 섬광 계수를 기반으로 하는 이 기술은
신속성과 감도 측면에서 유리하지만
주로 혈청 샘플에 적용할 수 있는 것으로 생각되었습니다.
Comas-Basté 등은
최근 돼지 신장 추출물 및
DAO 보충제와 같은 정제되지 않은
복합 매트릭스의 분석을 위해 특히
DAO 활성의 시험관 내 측정을 위한 초고성능 액체 크로마토그래피 및 형광 검출(UHPLC-FL)을 통해
신속하고 신뢰할 수 있는 방법론을 개발했습니다[129].
이 방법론적 접근 방식은
히스타민 분해를 직접 측정하는 것을 기반으로 하며
매트릭스 간섭 및 방사성 물질 처리 측면에서 특정 단점을 극복합니다.
문헌에 따르면
돼지 신장은
DAO 효소의 주요 공급원입니다.
여러 연구에서 이 제품이
히스타민 및 기타 생체 아민을 시험관 내에서
분해하는 능력이 입증되었습니다[57,129,130,131,132,133].
매트릭스에 적용된 정제 등급 및/또는 반응 기질로 사용된
아민 화합물에 따라
돼지 신장 추출물의 DAO 용량의 광범위한 변동성이 보고되었습니다(0.1 ~ 100mU/mg 범위의 값) [57,129,130,131,132,133].
실제로 이러한 연구 중 다수는
식품의 신선도 지표로서 생체 아민의 생체 인식을 위한
바이오센서를 설계하기 위해
DAO 효소의 선택적 정제를 모색했습니다 [134].
최근에는 히스타민 과민증 예방 치료를 위한 식품 보충제를 제조하기 위해 활성 성분으로 명시적으로 사용되는 돼지 신장 단백질 추출물의 시험관 내 DAO 활성을 조사하는 데 초점을 맞춘 두 가지 연구가 발표되었습니다 [129,130]. Comas-Basté 등은 식품 보충제의 정교화에 사용되는 돼지 신장 단백질 추출물의 13가지 생산 배치의 시험관 내 효소 활성을 연구하여 추출물의 DAO 활성에 대한 원료(돼지 신장)의 낮은 영향을 평균값 0.23 ± 0.01 mU/mg으로 보고했습니다[129]. 나중에 Kettner 등은 0.5 ± 0.06 mU / mg의 시험관 내 DAO 활성을 가진 돼지 신장 조 추출물을 얻었으며 몇 가지 연속적인 정제 단계를 적용하여이 효소 활성이 10 배 증가했다고 설명했습니다 [130].
식품 보충제와 관련하여 일부 저자는 효소 용량을 무시하는 반면, 시중에서 판매되는 다른 상용 제품에 대해 0.04 ~ 0.20 mU/mg 범위의 DAO 활성 값이 설명되었기 때문에 다양한 결과도 보고되었습니다 [129,130]. 전반적으로 이러한 연구는 히스타민 불내성 환자의 외인성 보충을 위해 돼지 신장 DAO의 대체 공급원을 찾아야 한다는 점에서 일치합니다.
일부 저자들은 특정 아미노 기질을 분해하는 데 있어 식물 유래 DAO 효소의 촉매 능력이 동물 유래 효소에 비해 더 높다고 설명했습니다[129,135,136,137]. 특히, 특정 식용 콩과 식물의 발아된 새싹은 DAO 효소의 흥미로운 공급원으로 지적되어 왔습니다. 발아는 발아하지 않은 종자에 비해 새싹의 DAO 효소 용량을 최대 250배까지 증가시킬 수 있는 것으로 설명된 생리적 과정입니다[61,138]. 콩과 식물 새싹에서 DAO 효소의 증가는 탈아민화 반응의 부산물인 과산화수소가 발아 중 세포벽 구조화, 리그화 및 종자 비축량 동원에 중요하기 때문일 수 있습니다[139,140,141,142]. 실제로, 어둠 속에서 6-8일 동안 콩과 식물 종자를 발아시키면 이 식물 기원 매트릭스의 DAO 활성을 극대화하는 최적의 환경을 제공한다는 것이 입증되었습니다 [61,138,143]. 상업적인 관점에서 볼 때, 이 효소의 식물 공급원이 있다면 채식주의자/비건 인구와 돼지고기 제품 소비에 종교적 제한이 있는 사람들을 위한 이 새로운 식품의 타겟이 확대될 것입니다. 또한 콩과 식물에서 DAO 효소를 얻는 것은 현재 지속 가능한 개발 목표의 행동 요청에 따른 실천이 될 것입니다.
현재 발표된 5건의 중재 연구(지난 5년간 4건)만이 히스타민 과민증 환자를 대상으로 외인성 DAO 보충제의 임상적 효능을 테스트했습니다(표 5). 약간의 차이는 있지만, 현재 이용 가능한 연구에 따르면 DAO 보충제가 증상의 출현과 강도를 줄이는 데 효과적이라고 합니다. 그러나 연구 설계, 효소 복용량, 개입 시간, 효능 결과 측정 방식이 다르기 때문에 서로 다른 연구를 비교하기는 어렵습니다. Komericki 등, Manzotti 등, Schnedl 등은 히스타민 과민증과 관련된 다양한 증상(위장관, 심혈관, 호흡기 및/또는 피부과적, 신경학적 증상)을 가진 환자에서 DAO 보충제의 효능을 분석했습니다[84,108,109]. 세 연구 모두 소규모 연구 집단(14명, 28명, 39명) 및/또는 짧은 개입 시간(2주에서 4주)을 포함했지만 증상의 강도 또는 빈도가 유의미하게 개선되었다고 보고했습니다. 또한 Schnedl 등은 혈장 DAO 활성의 변화를 평가하여 개입 중 환자의 61%에서 약간의 증가를 보고했으며, 저자는 보충제로 인한 장 점막의 완전성 개선 가능성과 연관시켰습니다 [109].
Table 5
Studies on the efficacy of DAO enzyme supplementation for the treatment of symptoms of histamine intolerance.
DesignNumber of Patients and SymptomsDuration of DAO SupplementationEfficacy OutcomesReference
| Randomized, double-blind, placebo-controlled, crossover provocation study using histamine-containing and histamine-free tea in combination with DAO capsules or placebo | 39 patients with histamine intolerance (headache and gastrointestinal and skin complaints) | - | Statistically significant reduction of histamine-associated symptoms compared to placebo | [108] |
| Retrospective study with evaluation of the clinical response to DAO supplementation | 14 patients with diagnosis of histamine intolerance (headache and gastrointestinal, cardiovascular, respiratory and skin complaints) | 2 weeks | Reduction of at least one of the reported symptoms in 93% of patients | [84] |
| Double-blind, placebo-controlled, crossover study | 20 patients with chronic spontaneous urticaria | 1 month | Significant reduction of 7-Day Urticaria Activity Score (UAS-7) and slight significant reduction of daily antihistamine dose | [144] |
| Randomized, double-blind, placebo-controlled clinical trial | 100 patients with episodic migraine and serum DAO deficit | 1 month | Significant decrease in the duration of migraine attacks and decrease in triptans intake | [110] |
| Open-label interventional pilot study | 28 patients with histamine intolerance (gastrointestinal, cardiovascular, respiratory and skin complaints) and reduced serum DAO values | 1 month of intervention and 1 month of follow-up | Significant improvement in frequency and intensity of all symptoms. 61% of patients showed slightly increase in serum DAO values. During the follow-up period (without DAO supplementation), the symptoms sum scores increased and DAO levels decreased. | [109] |
The clinical trials developed by Yacoub et al. and Izquierdo-Casas et al. were focused on a single disorder related to histamine intolerance (chronic spontaneous urticaria and migraine, respectively) [110,144]. Yacoub et al. considered 20 patients with chronic spontaneous urticaria who showed a significant reduction in the severity of the complaint according to the Urticaria Activity Score (UAS-7) [144]. Regarding migraines, the randomized double-blind clinical trial conducted by Izquierdo et al. considered a larger number of patients (100 patients) and obtained a statistically significant decrease in the duration of pain attacks with no recorded adverse side effects [110]. However, the authors did not find statistical differences when considering other research outputs, such as the frequency and intensity of pain.
Overall, despite the promising results, more ambitious clinical studies with a rigorous experimental design, longer treatment periods and properly sized samples are essential to establish the clinical efficacy of this treatment.
6. Conclusions and Perspectives
Histamine intolerance is currently a clinical entity of increasing interest, which can appear due to the intake of histamine from foods, mainly caused by a deficiency of the DAO enzyme at the intestinal level. Novel knowledge and studies of histamine intolerance have helped clarify many of the uncertainties that were classically associated with histamine intoxication. Etiologically, various SNPs have been identified in the gene encoding the DAO enzyme related to lower enzyme activity. Moreover, certain inflammatory bowel diseases that limit enzyme secretion or some DAO-inhibiting drugs have also been identified as possible causes of DAO deficiency. This intolerance manifests through a plethora of nonspecific gastrointestinal and extraintestinal symptoms.
The diagnosis of histamine intolerance is usually performed after ruling out allergic symptoms and by the presence of at least two clinical manifestations and their improvement or remission after following a low-histamine diet. Various complementary tests are currently being proposed to improve the diagnosis of this intolerance based, among others, on determining the DAO activity in blood or intestinal biopsy samples or on identifying genetic or metabolic urinary markers by noninvasive techniques.
The clinical management is carried out mainly through the follow-up of a low-histamine diet, although there is no consensus on the list of foods to be excluded. Even so, there are different clinical studies that show the efficacy of this dietary intervention in improving the quality of life of patients with symptoms of histamine intolerance. Oral supplementation with exogenous DAO enzyme from porcine kidney is also being used to enhance the intestinal capacity to degrade dietary histamine. Although few works have assayed the clinical efficacy of this preventive treatment, promising results have been obtained so far. Research is currently also being made to identify new sources of DAO enzyme, especially of plant origin, due to its higher catalytic capacity and other potential productive and commercial advantages.
In this context, it is necessary to keep promoting the multidisciplinary study of this disorder, both from basic (i.e., analytical chemistry, food science, physiology and biochemistry) and clinically applied research, meant to increase the scientific base and the currently available diagnostic and treatment strategies for histamine intolerance.
히스타민 과민증은
현재 관심이 높아지고 있는 임상적 질환으로,
주로 장내 DAO 효소의 결핍으로 인해
음식에서 히스타민을 섭취할 때 나타날 수 있습니다.
히스타민 과민증에 대한 새로운 지식과 연구는
고전적으로 히스타민 중독과 관련된
많은 불확실성을 밝히는 데 도움이 되었습니다.
병인학적으로,
효소 활성 저하와 관련된 DAO 효소를 코딩하는 유전자에서
다양한 SNP가 확인되었습니다.
또한, 효소 분비를 제한하는 특정 염증성 장 질환이나 일부 DAO 억제 약물도 DAO 결핍의 가능한 원인으로 확인되었습니다. 이러한 과민증은 다양한 비특이적 위장 및 장외 증상을 통해 나타납니다.
히스타민 과민증 진단은 일반적으로 알레르기 증상을 배제한 후 최소 두 가지 이상의 임상 증상과 저히스타민 식단을 따른 후 개선 또는 완화를 통해 이루어집니다. 현재 혈액 또는 장 생검 샘플에서 DAO 활성을 측정하거나 비침습적 기술을 통해 유전적 또는 대사성 비뇨기 마커를 식별하는 등 다양한 보완 검사가 이 과민증의 진단을 개선하기 위해 제안되고 있습니다.
임상 관리는 주로 저히스타민 식단의 추적 관찰을 통해 이루어지지만, 제외해야 할 음식 목록에 대한 합의는 아직 없습니다. 그럼에도 불구하고 히스타민 과민증 증상이있는 환자의 삶의 질을 개선하는 데 이러한식이 개입의 효능을 보여주는 다양한 임상 연구가 있습니다. 돼지 신장에서 추출한 외인성 DAO 효소를 경구 보충하는 것도 식이 히스타민을 분해하는 장내 능력을 향상시키는 데 사용되고 있습니다. 이 예방 치료의 임상적 효능을 분석한 연구는 거의 없지만 지금까지 유망한 결과를 얻었습니다. 현재 더 높은 촉매 능력과 기타 잠재적 생산성 및 상업적 이점을 가진 식물 유래 DAO 효소의 새로운 공급원을 확인하기 위한 연구도 진행 중입니다.
이러한 맥락에서 히스타민 과민증에 대한 과학적 기반과 현재 이용 가능한 진단 및 치료 전략을 높이기 위해 기초(분석 화학, 식품 과학, 생리학 및 생화학) 및 임상 응용 연구 모두에서 이 질환에 대한 다학제적 연구를 계속 촉진할 필요가 있습니다.
Acknowledgments
Sònia Sánchez-Pérez is a recipient of a doctoral fellowship from the University of Barcelona (APIF2018).
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
1. EFSA Panel on Biological Hazards (BIOHAZ) Scientific Opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011;9:1–93. doi: 10.2903/j.efsa.2011.2393. [CrossRef] [Google Scholar]
2. Windaus A., Vogt W. Synthese des Imidazolyl-äthylamins. Berichte der Dtsch. Chem. Gesellschaft. 1907;40:3691–3695. doi: 10.1002/cber.190704003164. [CrossRef] [Google Scholar]
3. Comas-Basté O., Luz Latorre-Moratalla M., Sánchez-Pérez S., Teresa Veciana-Nogués M., del Carmen Vidal-Carou M. Histamine and Other Biogenic Amines in Food. From Scombroid Poisoning to Histamine Intolerance. In: Proestos C., editor. Biogenic Amines. IntechOpen; London, UK: 2019. [Google Scholar]
4. Dale H.H., Laidlaw P.P. The physiological action of β-iminazolylethylamine. J. Physiol. 1910;41:318–344. doi: 10.1113/jphysiol.1910.sp001406. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
5. Tansey E.M. The wellcome physiological research laboratories 1894–1904: The home office, pharmaceutical firms, and animal experiments. Med. Hist. 1989;33:1–41. doi: 10.1017/S0025727300048894. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
6. Riley J.F. Histamine and Sir Henry Dale. Br. Med. J. 1965;1:1488–1490. doi: 10.1136/bmj.1.5448.1488. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
7. Panula P., Chazot P.L., Cowart M., Gutzmer R., Leurs R., Liu W.L.S., Stark H., Thurmond R.L., Haas H.L. International union of basic and clinical pharmacology. XCVIII. histamine receptors. Pharmacol. Rev. 2015;67:601–655. doi: 10.1124/pr.114.010249. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
8. Vlieg-Boerstra B.J., van der Heide S., Oude Elberink J.N.G., Kluin-Nelemans J.C., Dubois A.E.J. Mastocytosis and adverse reactions to biogenic amines and histamine-releasing foods: What is the evidence? Neth. J. Med. 2005;63:244–249. [PubMed] [Google Scholar]
9. Russo P., Spano G., Arena M.P., Capozzi V., Fiocco D., Grieco F., Beneduce L. Are consumers aware of the risks related to biogenic amines in food. Curr Res. Technol. Edu. Top. Appl. Microbiol. Microb. Biotechnol. 2010:1087–1095. [Google Scholar]
10. Maintz L., Novak N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007;85:1185–1196. doi: 10.1093/ajcn/85.5.1185. [PubMed] [CrossRef] [Google Scholar]
11. Comas-Basté O., Latorre-Moratalla M.L., Bernacchia R., Veciana-Nogués M.T., Vidal-Carou M.C. New approach for the diagnosis of histamine intolerance based on the determination of histamine and methylhistamine in urine. J. Pharm. Biomed. Anal. 2017;145:379–385. doi: 10.1016/j.jpba.2017.06.029. [PubMed] [CrossRef] [Google Scholar]
12. Worm J., Falkenberg K., Olesen J. Histamine and migraine revisited: Mechanisms and possible drug targets. J. Headache Pain. 2019;20:30. doi: 10.1186/s10194-019-0984-1. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
13. Kovacova-Hanuskova E., Buday T., Gavliakova S., Plevkova J. Histamine, histamine intoxication and intolerance. Allergol. Immunopathol. (Madr.) 2015;43:498–506. doi: 10.1016/j.aller.2015.05.001. [PubMed] [CrossRef] [Google Scholar]
14. Elmore B.O., Bollinger J.A., Dooley D.M. Human kidney diamine oxidase: Heterologous expression, purification, and characterization. J. Biol. Inorg. Chem. 2002;7:565–579. doi: 10.1007/s00775-001-0331-1. [PubMed] [CrossRef] [Google Scholar]
15. Schwelberger H.G., Feurle J., Houen G. Mapping of the binding sites of human diamine oxidase (DAO) monoclonal antibodies. Inflamm. Res. 2018;67:245–253. doi: 10.1007/s00011-017-1118-3. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
16. Finney J., Moon H.J., Ronnebaum T., Lantz M., Mure M. Human copper-dependent amine oxidases. Arch. Biochem. Biophys. 2014;546:19–32. doi: 10.1016/j.abb.2013.12.022. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
17. Boehm T., Pils S., Gludovacz E., Szoelloesi H., Petroczi K., Majdic O., Quaroni A., Borth N., Valent P., Jilma B. Quantification of human diamine oxidase. Clin. Biochem. 2017;50:444–451. doi: 10.1016/j.clinbiochem.2016.12.011. [PubMed] [CrossRef] [Google Scholar]
18. Schwelberger H.G., Feurle J., Houen G. Mapping of the binding sites of human histamine N-methyltransferase (HNMT) monoclonal antibodies. Inflamm. Res. 2017;66:1021–1029. doi: 10.1007/s00011-017-1086-7. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
19. Schwelberger H.G. Histamine intolerance: Overestimated or underestimated? Inflamm. Res. 2009;58:51–52. doi: 10.1007/s00011-009-2004-4. [PubMed] [CrossRef] [Google Scholar]
20. Jarisch R., Wantke F., Raithel M., Hemmer W. Histamine Intolerance: Histamine and Seasickness. Springer; Berlin/Heidelberg, Germany: 2015. Histamine and biogenic amines; pp. 3–43. [Google Scholar]
21. Gludovacz E., Maresch D., De Carvalho L.L., Puxbaum V., Baier L.J., Sützl L., Guédez G., Grünwald-Gruber C., Ulm B., Pils S., et al. Oligomannosidic glycans at asn-110 are essential for secretion of human diamine oxidase. J. Biol. Chem. 2018;293:1070–1087. doi: 10.1074/jbc.M117.814244. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
22. Elsenhans B., Hunder G., Strugala G., Schümann K. Longitudinal pattern of enzymatic and absorptive functions in the small intestine of rats after short-term exposure to dietary cadmium chloride. Arch. Environ. Contam. Toxicol. 1999;36:341–346. doi: 10.1007/s002449900480. [PubMed] [CrossRef] [Google Scholar]
23. McGrath A.P., Hilmer K.M., Collyer C.A., Shepard E.M., Elmore B.O., Brown D.E., Dooley D.M., Guss J.M. Structure and inhibition of human diamine oxidase. Biochemistry. 2009;48:9810–9822. doi: 10.1021/bi9014192. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
24. Boehm T., Reiter B., Ristl R., Petroczi K., Sperr W., Stimpfl T., Valent P., Jilma B. Massive release of the histamine-degrading enzyme diamine oxidase during severe anaphylaxis in mastocytosis patients. Allergy Eur. J. Allergy Clin. Immunol. 2019;74:583–593. doi: 10.1111/all.13663. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
25. Maršavelski A., Petrović D., Bauer P., Vianello R., Kamerlin S.C.L. Empirical Valence Bond Simulations Suggest a Direct Hydride Transfer Mechanism for Human Diamine Oxidase. ACS Omega. 2018;3:3665–3674. doi: 10.1021/acsomega.8b00346. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
26. Smolinska S., Jutel M., Crameri R., O’Mahony L. Histamine and gut mucosal immune regulation. Allergy. 2014;69:273–281. doi: 10.1111/all.12330. [PubMed] [CrossRef] [Google Scholar]
27. Sjaastad Ö.V. Potentiation by aminoguanidine of the sensitivity of sheep to histamine given by mouth. Effect of aminoguanidine on the urinary excretion of endogenous histamine. Q. J. Exp. Physiol. Cogn. Med. Sci. 1967;52:319–330. doi: 10.1113/expphysiol.1967.sp001918. [PubMed] [CrossRef] [Google Scholar]
28. Sattler J., Häfner D., Klotter H.J., Lorenz W., Wagner P.K. Food-induced histaminosis as an epidemiological problem: Plasma histamine elevation and haemodynamic alterations after oral histamine administration and blockade of diamine oxidase (DAO) Agents Actions. 1988;23:361–365. doi: 10.1007/BF02142588. [PubMed] [CrossRef] [Google Scholar]
29. Klocker J., Mätzler S.A., Huetz G.-N., Drasche A., Kolbitsch C., Schwelberger H.G. Expression of histamine degrading enzymes in porcine tissues. Inflamm. Res. 2005;54(Suppl. 1):S54–S57. doi: 10.1007/s00011-004-0425-7. [PubMed] [CrossRef] [Google Scholar]
30. FAO (Food and Agriculture Organization of the United Nations) WHO (World Health Organization) Public Health Risks of Histamine and other Biogenic Amines from Fish and Fishery Products. Meeting Report. World Health Organization; Geneva, Switzerland: 2013. [Google Scholar]
31. Bover-Cid S., Latorre-Moratalla M.L., Veciana-Nogués M.T., Vidal-Carou M.C. Encyclopedia of Food Safety. Volume 2. Elsevier; Amsterdam, The Netherlands: 2014. Processing Contaminants: Biogenic Amines; pp. 381–391. [Google Scholar]
Biomolecules. 2020 Aug; 10(8): 1181.
Published online 2020 Aug 14. doi: 10.3390/biom10081181
PMCID: PMC7463562
PMID: 32824107
Histamine Intolerance: The Current State of the Art
Oriol Comas-Basté,1,2,3 Sònia Sánchez-Pérez,1,2,3 Maria Teresa Veciana-Nogués,1,2,3 Mariluz Latorre-Moratalla,1,2,3 and María del Carmen Vidal-Carou1,2,3,*
Author information Article notes Copyright and License information PMC Disclaimer
Abstract
Histamine intolerance, also referred to as enteral histaminosis or sensitivity to dietary histamine, is a disorder associated with an impaired ability to metabolize ingested histamine that was described at the beginning of the 21st century. Although interest in histamine intolerance has considerably grown in recent years, more scientific evidence is still required to help define, diagnose and clinically manage this condition. This article will provide an updated review on histamine intolerance, mainly focusing on its etiology and the existing diagnostic and treatment strategies. In this work, a glance on histamine intoxication will also be provided, as well as the analysis of some uncertainties historically associated to histamine intoxication outbreaks that may be better explained by the existence of interindividual susceptibility to ingested histamine.
Keywords: histamine, food intolerance, histamine intolerance, histaminosis, histamine intoxication, diamine oxidase (DAO), low-histamine diet, food supplement
1. Introduction
In 2011, the European Food Safety Authority (EFSA) issued a scientific report warning that the levels of biogenic amines found in foods marketed in European Union countries may still entail a consumer health risk [1]. Among them, histamine has the highest toxic potential, along with tyramine, and is therefore of great interest in terms of food safety. First described more than 60 years ago, the deleterious effects of excessive histamine ingestion were initially referred to as scombroid fish poisoning or scombrotoxicosis, as they were associated with the consumption of fish in this family, but the condition is now known as histamine intoxication or histamine poisoning. In recent years, another disorder associated with histamine intake, arising from an enzymatic deficiency, has been described. The inability of certain individuals to metabolize histamine in the intestine, resulting in sensitivity to normal or even low histamine levels in food, may help to explain some of the uncertainties historically associated with histamine intoxication.
During the last decade, histamine intolerance has gained social and scientific recognition, with a significant increase in the interest of researchers to investigate this disorder. This review aims to analyze the pathophysiological relevance of dietary histamine, giving special focus to the adverse effects derived from histamine intake and, in particular, to the state of the art concerning the etiology, diagnosis and treatment of histamine intolerance.
2. Histamine
Histamine (2-[4-imidazolyl]ethylamine) is a bioactive amine that is synthesized by decarboxylation of its precursor amino acid, histidine, in an enzymatic reaction first described by Windaus and Vogt in 1907 involving L-histidine decarboxylase (EC 4.1.1.22) (Figure 1) [2]. Due to its chemical structure and number of functional groups, histamine can be defined as a heterocyclic diamine with an imidazole ring and ethylamine (i.e., an organic compound that provides a functional group in the form of a primary amine) [1,3].

Synthesis of histamine by decarboxylation of its precursor amino acid.
The physiological and pathophysiological effects of histamine on the body were first described in 1910 by Dale and Laidlaw, two pioneering researchers who studied the functions of this organic compound at the Wellcome Physiological Research Laboratories [4,5,6]. Specifically, histamine is synthesized and stored in high concentrations in secretory granules, mainly in basophils and mast cells, and also in gastric enterochromaffin cells, lymph nodes and the thymus [1,7]. Functionally, this amine is involved in various immune and physiological mechanisms, stimulating gastric acid secretion, inflammation, smooth muscle cell contraction, vasodilation and cytokine production, among other processes [8,9,10,11]. In addition, histamine functions as a neurotransmitter, being synthesized by neurons located in the posterior region of the hypothalamus whose axons extend through the brain [12]. These wide-ranging physiological effects occur by interaction with four G-protein-coupled receptors with seven transmembrane domains (H1, H2, H3 and H4), which activate signal transduction pathways upon perceiving their ligand, histamine [7,12].
Two main histamine metabolic pathways are known in humans, involving the enzymes diamine oxidase (DAO) and histamine-N-methyltransferase (HNMT) (Figure 2) [10,11,13]. DAO (EC 1.4.3.22), also called histaminase or amiloride-binding protein, is a copper-dependent amino oxidase encoded by the AOC1 gene located on chromosome 7 (7q34-36) [14,15,16]. This functional enzyme, a homodimer with two isoforms, catalyzes the oxidative deamination of the primary amine group of histamine [14,16,17]. On the other hand, histamine can be metabolized to 1-methylhistamine by the enzyme HNMT (EC 2.1.1.8), a small monomeric protein encoded by a gene located on chromosome 2q22.1 [18]. HNMT catalyzes the methylation of the secondary amine group of the histamine imidazole aromatic heterocycle by a reaction requiring the S-adenosyl methionine cosubstrate as a methyl group donor [11,13,19].

Histamine metabolism in humans. DAO: diamine oxidase; HNMT: histamine-N-methyltransferase; ALDH: aldehyde dehydrogenase; MAO: monoamine oxidase.
Thus, depending on its location, the histamine present in the body is deaminated or methylated by the action of the enzymes DAO and HNMT, respectively [1,10,20]. DAO is a secretory protein stored in vesicular structures of the plasma membrane and is responsible for the degradation of extracellular histamine [1,15]. In mammals, the expression of DAO is restricted to certain tissues, mainly the small intestine, ascending colon, placenta and kidneys [14,21]. In the intestine, DAO activity increases progressively from the duodenum to the ileum and is located mainly in the intestinal villi [22]. In contrast, the enzyme HNMT is expressed in a wide range of human tissues, above all in the kidneys and liver, and also the spleen, colon, prostate, ovaries, spinal cord cells and the trachea and respiratory tract [10,13]. HNMT is a cytosolic protein responsible for the inactivation of intracellular histamine and can be synthesized in the cell itself or incorporated from the extracellular space by binding to a receptor or by membrane transporters [7,18]. Regarding substrates, HNMT is highly selective for histamine, whereas DAO can also metabolize other biogenic amines such as putrescine and cadaverine, although it shows a preference for histamine [14,16,23]. The affinity of DAO and HNMT for histamine is very similar, although the latter shows a slightly lower Michaelis–Menten enzymatic constant (KM: 6–13 μmol/L) than DAO (KM: 20 μmol/L) [10].
The gateway for dietary histamine in the body is the intestinal epithelium. Therefore, although HNMT is also present in the gastrointestinal tract, the more highly expressed DAO plays the major role in protecting the body against exogenous histamine, whether originating from ingested food or generated by the intestinal microbiota [24,25,26]. The protective effect of DAO has been demonstrated in animal experimentation models that were administered aminoguanidine for irreversible and selective DAO inhibition, followed by a dose of histamine [24,27,28]. The development of anaphylaxis symptoms in DAO-inhibited pigs and sheep compared to control groups indicates that the enzyme exerts a significant barrier effect against the absorption of exogenous histamine into the systemic circulation [1,13,19,24,29]. The HNMT enzyme ranks second to DAO in protecting against the absorption of dietary histamine from the intestinal lumen, but appears to be more effective against intravenously or intradermally supplied histamine [13,30].
3. Histamine in Foods
Histamine is present in a wide range of foods in highly variable concentrations, which are the main exogenous source of this compound [31]. The main route for histamine formation in food is the decarboxylation of histidine through the action of L-histidine decarboxylase, an enzyme of bacterial origin [32,33]. Apart from histamine, food can also contain other biogenic amines, mainly tyramine (4-hydroxy-phenethylamine), putrescine (1,4-diaminobutane) and cadaverine (1,5-diaminopentane), which are formed through enzymatic deamination of the amino acids tyrosine, ornithine (and/or agmatine) and lysine, respectively [31,34]. The accumulation of these compounds in food is the result of the transformation of amino acids by microorganisms and depends on various factors, such as the availability of the precursor amino acids and environmental conditions favorable for growth and/or the bacterial decarboxylase activity [31,34,35].
These decarboxylation reactions have been described as a survival strategy for microorganisms in acidic environments, as well as an alternative source of metabolic energy in situations of suboptimal substrate availability [1,9]. This enzymatic activity in bacteria is a species- and strain-dependent property [32]. Several Gram-positive and Gram-negative bacteria responsible for microbial spoilage or fermentative processes in food are able to produce histamine [1,36]. Specifically, the Enterobacteriaceae species Hafnai aluei, Morganella morganii and Klebsiella pneumonia have been identified as some of the most prolific histamine-forming bacteria in fish [9,37]. On the other hand, in cheeses, fermented meat, vegetable derivatives and fermented beverages, various lactic acid bacteria have also been described as histamine-producing microorganisms (e.g., Lactobacillus hilgardii, Lactobacillus buchnerii, Lactobacillus curvatus and Oenococcus oeni) as well as certain strains of Enterobacteriaceae [1,38,39].
Foods that potentially contain high levels of histamine are: a) those microbiologically altered, such as fish and meat, or derived products that may have been preserved or processed in unsuitably hygienic conditions; and b) fermented products, in which the bacteria responsible for the fermentation process may also have aminogenic capacity [3,40]. Table 1 shows histamine content in the different food categories from the Spanish market [31].
Table 1
Histamine content in different food categories. Adapted from [31].
FoodHistamine Content (mg/kg)nMean (SD)MedianMinimumMaximum
| Fruits, vegetables and plant-based products | |||||
| Fruits | 136 | 0.07 (0.20) | ND | ND | 2.51 |
| Nuts | 41 | 0.45 (1.23) | ND | ND | 11.86 |
| Vegetables | 98 | 2.82 (7.43) | ND | ND | 69.72 |
| Legumes | 11 | ND | ND | ND | ND |
| Cereals | 28 | 0.12 (0.33) | ND | ND | 0.89 |
| Chocolate | 25 | 0.58 (0.44) | 0.17 | 0.16 | 0.56 |
| Spices | 12 | ND | ND | ND | ND |
| Alcoholic beverages | |||||
| Beer | 176 | 1.23 (2.47) | 0.70 | ND | 21.60 |
| White wine | 83 | 1.24 (1.69) | 0.45 | 0.10 | 13.00 |
| Red wine | 260 | 3.81 (3.51) | 1.90 | 0.09 | 55.00 |
| Fish and seafood products | |||||
| Fresh fish | 136 | 0.79 (0.71) | ND | ND | 36.55 |
| Canned fish | 96 | 14.42 (16.03) | 5.93 | ND | 657.05 |
| Semipreserved fish | 49 | 3.48 (3.37) | 2.18 | ND | 34.90 |
| Meat and meat products | |||||
| Fresh meat | 6 | ND | ND | ND | ND |
| Cooked meat | 48 | 0.30 (0.26) | ND | ND | 4.80 |
| Cured meat | 23 | 12.98 (37.64) | 0.80 | ND | 150.00 |
| Dry-fermented sausages | 209 | 32.15 (14.22) | 8.03 | ND | 357.70 |
| Dairy products | |||||
| Unripened cheese | 20 | ND | ND | ND | ND |
| Raw milk cheese | 20 | 59.37 (106.74) | 18.38 | ND | 389.86 |
| Pasteurized milk cheese | 20 | 18.05 (38.23) | 4.59 | ND | 162.03 |
ND: not detected.
4. Uncertainties Associated with Histamine Poisoning: A Paradigm Shift Towards Histamine Intolerance
Although histamine has important physiological functions in the body, it can pose a health risk when ingested in high levels [41]. The proper functioning of histamine degradation systems is key in preventing its accumulation. Histamine intoxication, a kind of food poisoning, may occur after the consumption of foods with an unusually high histamine content that overpowers the degradation mechanisms (generally higher than 500 mg/kg) [1,3,42].
Historically, histamine intoxication has also been termed scombroid fish poisoning or the mahi-mahi flush because of its repeated association with the consumption of fish in the Scombridae and Scomberesocidae families (e.g., tuna, herring and mackerel) [43]. Histamine was first identified in 1946 as the causative agent of the toxic effects of consuming poorly transported tuna, and for a long time histamine poisoning was associated almost exclusively with the consumption of spoiled fish [44,45]. Over the years, the World Health Organization (WHO) has recommended the use of the term histamine intoxication to better designate this pathology, as it can be caused by marine species from other families (e.g., Clupeidae, Engraulidae, Coriphaenidae and Pomatomidae) and even other foods, such as cheese [43]. A meta-analysis carried out in 2018 of the different scientific reports of histamine intoxication between 1959 and 2013 established that the causative food in 98% of cases was fish, the remaining percentage being attributed to cheese [46]. Currently, international health administrations consider histamine intoxication to be one of the main problems of global food security, both for its effects on human health and its impact on trade [47,48].
Histamine intoxication is characterized by occurring in outbreaks and having a short incubation period (i.e., 20–30 min post-ingestion), with symptoms that are generally of low/moderate severity and remit in a few hours [3]. The symptoms are closely linked to the various physiological functions of histamine in the body, affecting the skin (e.g., redness, rash, urticaria, pruritus, edema and local inflammation), the gastrointestinal tract (e.g., nausea, vomiting and diarrhea) and the hemodynamic (hypotension) and neurological (e.g., headache, palpitations and tingling) bodily functions [1,41]. The symptomatic similarity of histamine intoxication with allergy means it is likely to be underdiagnosed [43,48,49]. The diagnosis of histamine intoxication is based primarily on the determination of elevated plasma histamine levels and/or the identification of an ingested food with an unusually high histamine content [13]. In general, an outbreak of histamine poisoning tends to involve more than one individual, lasts a short period of time and a particular causative food is identified [38].
In terms of incidence, the data available for the European Union shows an increase in histamine intoxication outbreaks in the last ten years, unlike other types of food poisoning, and with an almost hegemonic predominance of fish as the causative agent (over 90% of cases) [42,50]. The most recent data from the EFSA and European Center for Disease Prevention and Control (ECDC) show that in 2017, there was a 22% increase in outbreaks compared to the previous year [50]. Specifically, in 2017, there were a total of 117 outbreaks of histamine intoxication involving 572 people, 9% of whom required hospitalization. Fortunately, no deaths have been attributed to histamine poisoning over the past decade [42]. The same trend is observed in the information provided by the European Union Food and Feed Warning System (RASFF), with a progressive rise in the number of cases of histamine poisoning linked to tuna consumption in 2014–2017 and a particularly high increase in 2017 [3].
Although histamine intoxication has been extensively studied in recent decades, unresolved questions remain, concerning, for example, the variable histamine concentrations in the foods triggering outbreaks, or the heterogeneity in the degree and type of adverse effects [46]. Furthermore, the fact that oral administration of histamine in doses equivalent to those normally found in foods causing illness does not produce the same range and/or severity of symptoms is a paradox that has led to multiple hypotheses [30].
Several authors have proposed that alcohol and certain food components, such as other biogenic amines, may have a potentiating effect on histamine toxicity [13,48]. Amines such as putrescine and cadaverine, which are usually found in foods along with histamine, can also act as DAO substrates. It has therefore been suggested that these amines could weaken the protective barrier against dietary histamine by competitively interacting with degradation enzymes in the intestine [3,49]. Other possible potentiators are alcohol and its metabolite acetaldehyde, as they compete with histamine for the enzyme aldehyde dehydrogenase (ALDH), which is simultaneously involved in histamine and alcohol metabolism [1,32]. The potentiation effect of these components could help explain the differences in absorption of the same dose of histamine when ingested in isolation or in a food matrix [48,49]. The FAO and WHO have acknowledged that the involvement of potentiators can alter the threshold dose for toxicity, and they recommend that future studies focus on clarifying the ambiguities in the pathogenesis of histamine intoxication [30].
Finally, several authors have reported considerable interindividual variability in histamine tolerance, which has been demonstrated in intervention studies [1,3,10,13]. After the oral administration of the same histamine dosage, not all participants showed symptoms, and those who did varied in symptom type and severity and even had different blood histamine levels [48,51,52]. These results indicate the existence of population subgroups with greater sensitivity and clinical responses to histamine, likely linked to a diminished histamine degradation capacity, which could explain some of the historical uncertainties associated with histamine intoxication outbreaks [1]. Without disputing the clinical entity of histamine intoxication, the paradigm shift lies precisely in moving the focus from food to the human body, maintaining histamine as the causative agent, but focusing on how each person is able to respond to the intake of variable levels of histamine from food. Thus, histamine intolerance is the clinical condition that describes the inability of certain individuals to degrade histamine and results in the onset of symptoms caused by its accumulation in the blood (Figure 3).

Intestinal degradation of histamine by the DAO enzyme in three different situations: in a healthy individual, with histamine intoxication and with histamine intolerance. Adapted from [13].
5. Histamine Intolerance
According to the 2003 review of allergy nomenclature by the World Allergy Organization, adverse reactions to food without an immunological basis should be referred to as nonallergic food hypersensitivity, in order to clearly differentiate them from food allergies initiated by a specific immune mechanism [53]. Nonallergic food hypersensitivity is commonly known as food intolerance, a response triggered by a food or any of its components at a dose normally tolerated by the healthy population [54]. While the prevalence of food allergies is estimated at 1–2% in adults, currently almost 20% of the Westernized world’s population suffers from some type of food intolerance, with lactose intolerance being the most common [54].
Histamine intolerance, also referred to as enteral histaminosis or sensitivity to dietary histamine, can be defined as a disorder arising from reduced histamine degradation capacity in the intestine due to impaired DAO activity, leading to its accumulation in plasma and the appearance of adverse effects [11,41,55].
The DAO enzyme was first identified back in 1929 by Charles H. Best in autolyzing lung tissue, which he called histaminase because of its ability to degrade histamine [56]. Years later, given its ability to also degrade other diamines, as described above, the more accurate designation of DAO was proposed [57,58]. Beyond its role in the intestinal degradation of histamine in humans, DAO is also present in microorganisms, plants and animals, where it also catalyzes the oxidative deamination of the primary amino group of histamine into its corresponding aldehyde, concomitantly producing stoichiometric amounts of ammonia and hydrogen peroxide (Figure 4) [14,59,60].

Oxidative deamination of histamine by the DAO enzyme.
Although the first scientific references to histamine intolerance date from more than 20 years ago, it is significant that almost 80% are from the last decade, reflecting the growing interest of researchers in this disorder (Figure 5). In 2011, EFSA already considered histamine intolerance as one of the risks associated with histamine intake, clinically differentiating it from histamine intoxication [1]. In a subsequent joint report, the WHO and FAO emphasized that the no observed adverse effect level (NOAEL) established for histamine was only valid for healthy people, and not for members of susceptible populations, such as those with histamine intolerance [30]. EFSA concluded that only foods with histamine levels below the detection limits are safe for individuals with histamine intolerance [1].

Count of scientific publications containing the keywords histamine intolerance or histaminosis, according to a search performed through the PubMed search engine at the MEDLINE bibliographic database (search performed in July 2020).
Clinical manifestations of histamine intolerance consist of a wide range of nonspecific gastrointestinal and extraintestinal symptoms, due to the ubiquitous distribution of the four histamine receptors in different organs and tissues of the body (Figure 6) [10,13,54,61]. In a very recently published study, a team of Austrian researchers comprehensively analyzed the symptoms experienced by 133 patients diagnosed with histamine intolerance [62]. The most frequent and severe manifestations were gastrointestinal, with abdominal distension observed in 92% of patients and postprandial fullness, diarrhea, abdominal pain and constipation in 55–73%. Impairments of the nervous and cardiovascular systems, such as dizziness, headaches and palpitations, were recorded in second place, followed by respiratory and dermatological symptoms. Highlighting the complexity of the clinical picture of histamine intolerance, combinations of three or more symptoms involving different organs were recorded in 97% of cases, with an average of 11 symptoms per patient. The low specificity and complex variability of symptoms undoubtedly contribute to the current difficulty in achieving consensus on the diagnostic criteria for histamine intolerance, as will be discussed in detail below [13]. A lack of data also makes it difficult to determine the current incidence of this condition, although some authors have estimated that it affects 1–3% of the population, a percentage that will possibly increase as more knowledge and diagnostic tools for histamine intolerance become available [10,13,63].

Main symptoms of histamine intolerance and possibly corresponding histamine receptors [10,64].
5.1. The Etiology of Histamine Intolerance
As mentioned in previous sections, the main barrier against exogenous histamine in the intestines is the DAO enzyme, which prevents its passage into the systemic circulation [10,13,65]. Numerous clinical studies have provided data on the prevalence of low plasma DAO levels in individuals showing symptoms of histamine intolerance, mainly headaches and gastrointestinal or dermatological disorders [66]. Although certain studies have limitations, either in the design or number of participants, the majority point to an association between symptoms and DAO deficiency, establishing a general trend that supports the key role of DAO in the etiology of these disorders. A DAO deficiency that predisposes a population subgroup to histamine intolerance may have a genetic, pathological or pharmacological origin [1,41].
Regarding the genetic background of histamine intolerance, several studies have analyzed in depth the polymorphisms in genes encoding the enzymes L-histidine decarboxylase, DAO and HNMT, as well as the different histamine receptors. More than 50 nonsynonymous single-nucleotide polymorphisms (SNPs) in the DAO-encoding gene have been identified, some of which can produce a protein with altered activity and lead to symptoms of histamine intolerance [67,68,69,70,71,72]. Specifically, the most relevant SNPs affecting DAO enzyme functionality in Caucasian individuals are rs10156191, rs1049742, rs2268999 and especially rs1049793 [69,71]. On the other hand, an SNP in the promoter region of the gene has also been identified that causes a lower transcriptional activity of the DAO-encoding gene (rs2052129), as well as several genetic variations responsible for enzyme deficiency in people of Asian or African origin (rs45558339 and rs35070995, respectively) [67,72]. In most cases, the effect of these genetic variations on DAO functionality is through changes in enzyme kinetics, the resulting increase in KM causing a reduction in the rate of histamine degradation [69]. In parallel, three SNPs have been identified as being responsible for enhanced DAO enzyme activity (rs2071514, rs1049748 and rs2071517) [72]. There is also evidence of DAO mutations in patients with certain cardiovascular, gastrointestinal and nervous system pathologies, although with contradictory results regarding positive/negative effects [68].
DAO deficiency can also be an acquired condition, caused by certain pathologies or interaction with drugs. Several inflammatory bowel pathologies affecting mucosal integrity are known to result in impaired DAO activity, the degree of which can be correlated with the severity of mucosal damage [73,74,75]. Thus, DAO activity has been proposed as a marker of integrity of the intestinal mucosa. Miyoshi et al. demonstrated that DAO activity can be a useful predictor of intestinal mucosal damage in patients receiving chemotherapy [76]. Additionally, DAO deficiency has also been linked to certain functional gastrointestinal disorders, such as carbohydrate malabsorption and nonceliac gluten sensitivity (NCGS) [63,73,77,78,79]. Enko et al. found that a concomitant reduction in DAO and lactase enzyme activities could be a consequence of mucosal damage in the small intestine due to gastrointestinal disorders (e.g., gastroenteritis, irritable bowel syndrome, short bowel syndrome and gastrointestinal surgery) [73]. Moreover, patients with lactose intolerance and plasma DAO deficit showed higher end-expiratory H2 levels and the appearance of more symptoms during the H2 breath test in comparison with lactose-intolerant individuals with normal DAO activity [79]. More recently, two works have suggested a potential relationship between a reduced DAO activity and the presence of NCGS. Schnedl et al. based this relationship on the broad parallelism between the symptomatology of NCGS and histamine intolerance, while the pilot study conducted by Griauzdaite et al. reported a strong association between reduced DAO activity and the presence of NCGS, although with a reduced number of patients [77,78]. In fact, Griauzdaite et al. found out that nine of 10 patients with NCGS had decreased serum DAO activity levels [78]. This recently indicated relationship between both disorders, NCGS and histamine intolerance, should be further explored as it may be of interest for the correct clinical management of affected patients.
Finally, DAO deficiency can be a temporary and reversible condition, caused by the inhibitory effect of substances such as biogenic amines and alcohol, as discussed above, as well as several widely used drugs (Table 2) [1,10]. It has been estimated that approximately 20% of the European population regularly take DAO-inhibiting drugs, which significantly increases the number of people susceptible to the adverse effects of dietary histamine [28]. In vitro experimental results show a potent inhibitory effect (greater than 90%) of chloroquine, a historical antimalarial active ingredient, and clavulanic acid, a β-lactam antibiotic widely used in combination with amoxicillin [80]. A significant inhibition of the enzymatic activity has also been observed with the antihypertensive drug verapamil and the histamine H2 receptor antagonist cimetidine, although the clinical use of the latter is currently anecdotal [23,80]. Other substances have also shown an inhibitory effect, albeit to a lesser extent (Table 2) [23,80,81]. In most cases, the structural similarity of the cited drugs with histamine could explain their potential to bind to the active site of DAO and reduce its enzymatic activity [23]. Along the same lines, substances with an inhibitory effect on other enzymes involved in any of the metabolic pathways of histamine in the body (i.e., HNMT, ALDH and MAO) may act as a trigger of histamine hypersensitivity [82].
Table 2
Active ingredients with an experimentally demonstrated inhibitory effect on the DAO enzyme [23,28,80,81].
Active IngredientIndication
| Chloroquine | Antimalarial |
| Clavulanic acid | Antibiotic |
| Colistimethate | Antibiotic |
| Cefuroxime | Antibiotic |
| Verapamil | Antihypertensive |
| Clonidine | Antihypertensive |
| Dihydralazine | Antihypertensive |
| Pentamidine | Antiprotozoal |
| Isoniazid | Antituberculous |
| Metamizole | Analgesic |
| Diclofenac | Analgesic and anti-inflammatory |
| Acetylcysteine | Mucoactive |
| Amitriptyline | Antidepressant |
| Metoclopramide | Antiemetic |
| Suxamethonium | Muscle relaxant |
| Cimetidine | Antihistamine (H2 antagonist) |
| Prometazina | Antihistamine (H1 antagonist) |
| Ascorbic acid | Vitamin C |
| Thiamine | Vitamin B1 |
5.2. Prevalence of DAO Deficit in Persons with Symptoms Related to Histamine Intolerance
Several studies have evaluated the prevalence of DAO deficit in plasma of individuals with symptoms of histamine intolerance and/or diagnosis with certain chronic disorders.
Mušič et al. found DAO deficiency in 80% of 316 adult patients showing various symptoms associated with histamine intolerance (e.g., urticaria, pruritus, diarrhea, abdominal pain, vomiting, constipation, cough, rhinitis and headache), as well as significantly lower plasma DAO activity compared to the control group [83]. Similarly, in a retrospective study, Manzotti et al. evaluated DAO activity in 14 patients with a confirmed diagnosis of histamine intolerance who showed mainly gastrointestinal and dermatological symptoms, but also headaches [84]. In this case, patients showed a high prevalence of DAO deficit (71%) and a significantly lower mean DAO activity compared to healthy volunteers. A lower percentage of DAO deficiency in histamine-intolerant patients (24%) was reported by Pinzer et al. [63]. Those patients featured elevated histamine levels and constantly reduced DAO activities throughout the day.
In a study focused only on headache symptoms, Steinbrecher and Jarisch reported DAO deficiency in 23 of 27 patients (85%) [85]. In parallel, the authors described a significant increase in DAO activity after patients followed a low-histamine diet for four weeks, along with a remission or reduction in frequency of headaches in almost 90% of individuals. More recently, Izquierdo et al. studied the prevalence of DAO deficit in 137 patients diagnosed with a confirmed migraine diagnosis and in a control group of 61 nonmigraine individuals [66]. In this study, a high prevalence of DAO deficiency was observed in the migraine group (87%) and with a mean DAO activity significantly lower in comparison with that obtained from control volunteers. However, the prevalence of DAO deficiency in the control population amounted up to 44%, which was attributed to the fact that certain individuals could present other symptoms associated with histamine intolerance or DAO deficiency other than migraines. Another study with 44 migraine patients reported a 60% prevalence of DAO deficiency and a significant copresence of certain gastrointestinal disorders, such as celiac disease and NCGS [78].
In the field of dermatological symptomatology, several studies have monitored plasma DAO activity in patients with eczema, chronic idiopathic urticaria and atopic dermatitis. Overall, the reported prevalence of DAO deficiency ranges from 19 to 57%, with the exception of the study by Worm et al., who did not detect statistically significant differences in plasma DAO activity between control patients and those with atopic dermatitis [86,87,88,89].
Finally, regarding gastrointestinal symptoms, Honzawa et al. assessed the clinical significance of plasma DAO activity levels in 98 patients suffering inflammatory bowel disease [90]. This study showed that DAO activity in blood was significantly lower in patients with Crohn’s disease and ulcerative colitis compared to the control population, suggesting its potential importance as a marker of intestinal permeability. In a pediatric population under 15 years of age, Rosell-Camps et al. determined DAO deficiency in 88% of patients with abdominal pain, diarrhea and vomiting [91]. In contrast, in a more recent study by a group of Austrian researchers, DAO deficiency was found in only 8% of 394 children with chronic abdominal pain [92].
To date, little data is available on the prevalence of this enzymatic deficiency related to gender, and it is inconclusive. Klockler et al. found no differences in plasma DAO activity between men and women, although the number of individuals considered was scarce (n = 28) [93]. Likewise, the study performed by Izquierdo et al. reported similar percentages of DAO deficiency in migraine-suffering women (83%) and men (90%) [66]. On the contrary, García-Martín et al. did describe differences in plasma DAO activity by gender, with the prevalence of this enzyme deficiency being higher in women [94]. Significant fluctuations in DAO activity values have also been reported in women associated with different stages of the menstrual cycle [94,95].
One factor that could explain the discordance among the prevalence data of DAO deficit in patients with disorders associated with histamine intolerance is that the parameter considered in all of them was serum DAO activity, which, a priori, would not reflect an enzymatic deficiency derived from certain intestinal pathologies. Overall, in spite of the varying percentages in DAO deficiency, the currently available studies seem to indicate an etiological relationship between DAO deficiency and certain symptoms or disorders related to histamine intolerance. Nevertheless, more studies are needed to assess the clinical significance of the determination of plasma DAO activity, as well as to develop new diagnostic methods aimed at identifying individuals with histamine intolerance due to DAO deficiency.
5.3. Diagnosis of Histamine Intolerance
Despite significant advances in the understanding of histamine intolerance, reaching a consensus on a diagnostic algorithm remains a pending challenge. The nonspecificity of symptoms and lack of validated diagnostic tools prompts many affected individuals to go “doctor shopping”; that is, to consult several medical specialists in search of an explanation and solution for their varied symptomatology [13,63]. In the absence of a consensual and clinically validated diagnosis, Figure 7 shows a schematic summary of the diagnostic algorithm for histamine intolerance based on the available scientific evidence reviewed below.

Summary of the described approaches to the diagnosis of histamine intolerance. SNPs: single-nucleotide polymorphisms.
The combination of diagnostic criteria currently in use includes the appearance of typical clinical manifestations and the exclusion of other related disorders [10,13,54]. All the authors who have proposed a diagnostic algorithm for histamine intolerance emphasize the need to initially rule out other potential causes of symptoms associated with an increase in plasma histamine [10,13,54]. For this purpose, it is advisable to carry out an intradermal skin allergy test (i.e., skin prick test) to discard IgE sensitization caused by food allergy, and to measure plasma tryptase to exclude an underlying systemic mastocytosis [10]. It is also important to know whether the patient is taking any medication with a possible inhibitory effect on DAO activity [55]. If these conditions are negative, the appearance of two or more typical symptoms of histamine intolerance and their improvement or remission after the following of a low-histamine diet (i.e., a diet excluding foods that, a priori, contain high histamine levels) will confirm the diagnosis of histamine intolerance [10,54,96,97]. In the diet follow-up, a thorough 24-h record of all the foods consumed and symptoms experienced is recommended in order to establish a relationship, if any, between a food and the onset of symptoms [10,13]. The duration of the low-histamine diet to confirm the diagnosis is not clearly stipulated, although some studies suggest a period of 4 to 8 weeks [54,97]. In addition to the diet, testing the effect of antihistamine treatment on symptoms has also been proposed, although its usefulness once dietary histamine is removed is unclear [10,54].
Once it has been established that dietary histamine is responsible for the intolerance-associated symptoms, the diagnosis of this disorder is virtually confirmed. A range of nonvalidated complementary tests have also been proposed by several authors with the aim of obtaining a marker to confirm the diagnosis [97]. However, it has to be taken into account that not all of the tests consider the different origins of DAO deficiency (i.e., genetic, pathological or pharmacological). Thus, a genetic origin would lead to a reduction of the DAO enzymatic activity in the whole organism. Likewise, the pharmacological blockade of DAO would take place in all tissues where the drug is distributed after entering the systemic circulation, although in a punctual manner upon the substance’s introduction. Lastly, the scope of a DAO deficit due to intestinal pathologies would be limited to the local intestinal environment.
Due to the genetic background of DAO deficiency, one of the strategies for the diagnosis could be the determination of genetic polymorphisms (SNPs) that characterize the population as genetically susceptible to histamine [54]. Currently, there is already the possibility of performing a noninvasive genetic analysis capable of identifying three of the SNPs associated with reduced DAO activity (i.e., rs10156191, rs1049742 and rs1049793) from a sample of the oral mucosa, although evidence-based studies on the diagnosis potential of this test are still needed. It is important to note that this test will only reflect the existence of a genetic DAO deficiency.
The most studied, and possibly also the most controversial, is the determination of plasma DAO activity. This analytical test consists of measuring the amount of histamine degraded in a blood sample in a given time interval. Two types of commercial testing kits are currently available on the market, one consisting of an ELISA-type immunoassay, and the other a radioimmunoassay using radioactively labeled putrescine [83,84]. The evidence for the validity of blood DAO activity measurements for the diagnosis of histamine intolerance is neither abundant nor conclusive. Some studies have proposed that determining blood DAO activity may be helpful in identifying subjects with symptoms associated with histamine intolerance [63,83,84]. In contrast, three studies did not find a significant relationship between the clinical history of patients with typical symptoms of histamine intolerance and blood DAO activity values, concluding that this technique cannot be recommended as a diagnostic tool in routine clinical practice until studies have validated its effectiveness [98,99,100]. Moreover, the work performed by Schnoor et al. also reported a high interassay variation in DAO activity values that made the proper classification of histamine-intolerant subjects impossible [100]. This controversy is described in a joint article published in 2017 by the German and Swiss allergology societies, which emphasizes the need for more research before giving plasma DAO activity a definitive diagnostic value for histamine intolerance [97].
A variant of the intradermal skin allergy test called the histamine 50-skin-prick test was also proposed by Kofler et al. to diagnose histamine intolerance [101]. In this technique, the results were read after 50 min (as opposed to the usual 20 min) and showed that, although the size of the wheal did not differ between the histamine intolerant and control groups, the time course was significantly different. Patients with symptoms of intolerance showed a delayed remission of the wheal induced by cutaneous administration of histamine, signaling a reduced degradation ability. The same results were obtained in a study recently published by Wagner et al., who re-evaluated this skin test as a diagnostic tool of histamine intolerance, also observing a correlation between the delay in wheal disappearance and a lower plasma DAO activity [102].
Both the determination of plasma DAO activity and the histamine 50-skin-prick test could be suitable tests to identify a DAO deficiency from genetic or pharmacological origin, but they would not be useful to determine a deficit secondary to certain intestinal diseases.
On the contrary, there are certain alternatives, such as the intestinal biopsy, the histamine provocation test or the histamine metabolomics in urine, that could make it possible to diagnose histamine intolerance due to DAO deficiency without excluding any of the possible etiological causes.
The measurement of intestinal DAO activity by a colon biopsy during endoscopic procedures has been studied as a possible diagnostic marker. The few available studies have shown a reduced intestinal DAO catabolic activity in patients with recurrent urticaria, food allergy and colon adenoma, accompanied by an increase in histamine levels [103,104,105,106]. Although this test has interesting diagnostic potential, more studies are needed to validate its clinical significance and its relationship with the symptoms of histamine intolerance [97]. If proven, this diagnostic test would be very adequate since this disorder originates from a reduced ability of the intestinal DAO enzyme to cope with dietary histamine.
Histamine challenge/provocation test has also been proposed by some authors as a diagnostic tool for intolerance, which would, at the same time, establish the individual tolerance threshold. This double-blind, placebo-controlled test involves oral administration of histamine and requires patient medical supervision and hospitalization. In the study by Wöhrl et al., half of the healthy volunteers developed symptoms after the administration of a solution containing 75 mg of histamine [107]. In contrast, the results of a multicenter study by Komericki et al. using the same oral dose of histamine indicated the challenge test was unreliable for diagnosing histamine intolerance due to a lack of intraindividual reproducibility of symptoms after two different provocation tests [108]. The application of this procedure is still limited because of the risk of serious adverse side effects and the absence of a standardized dose of histamine and properly established protocol [97].
Finally, in recent years, efforts have been made to identify a noninvasive marker to establish a solid and clinically irrefutable diagnostic criterion for histamine intolerance due to DAO deficiency. Currently, the application of metabolomics as a tool for the identification of biomarkers of histamine metabolism in urine is also being challenged as a possible new diagnostic strategy [11]. The hypothesis is that individuals with histamine intolerance could have a different excretion profile of histamine and its metabolites in urine than normal individuals. For this purpose, Comas-Basté et al. have recently proposed a chromatographic approach that allows for determining in a fast and unequivocal manner the urinary levels of histamine and its methylated metabolite, methylhistamine [11]. It is still necessary to validate the potential diagnostic utility of this approach in patients with histamine intolerance, as well as complementing the excretion profile with other histamine metabolites to obtain a more accurate image of the possible alterations produced in this intolerance.
5.4. Treatment Approaches to Histamine Intolerance
Currently, the main strategy to avoid the symptoms of histamine intolerance is to follow a low-histamine diet. Supplementation with exogenous DAO has recently been postulated as a complementary treatment to enhance dietary histamine degradation in intolerant individuals who have a deficiency of this enzyme in the intestine [109,110].
5.4.1. Low-Histamine Diet
A low-histamine or histamine-free diet has been proposed as the main strategy for the preventive treatment of histamine intolerance [10,54,82,111]. Conceptually, these diets exclude a number of foods that patients associate with the onset of symptoms, primarily those that may contain high levels of histamine [82]. However, there is no a single dietary recommendation of a low-histamine diet. As it may be seen in Table 3, there is no coincidence in all the foods excluded in the different low-histamine diets found in the literature [10,87,91,112,113,114,115,116,117,118].
Table 3
Foods excluded in the different low-histamine diets found in the literature [10,87,91,112,113,114,115,116,117,118].
Foods Excluded by Low-Histamine Diets
| <20% * | 20–60% * | >60% * |
| Milk | Shellfish | Cured and semicured cheese |
| Lentils | Eggs | Grated cheese |
| Chickpeas | Fermented soy derivatives | Oily fish |
| Soybeans | Eggplant | Canned and semipreserved oily fish derivatives |
| Mushrooms | Avocado | Dry-fermented meat products |
| Banana | Spinach | |
| Kiwi | Tomatoes | |
| Pineapple | Fermented cabbage | |
| Plum | Citrus | |
| Nuts | Strawberries | |
| Chocolate | Wine | |
| Beer |
* Percentage of low-histamine diets from the literature that exclude each foodstuff.
Histamine is widely distributed in different food categories and in highly variable concentrations, as its accumulation is influenced by multiple factors [3,119]. In fresh foods such as fish and meat, and in some derived products, the presence of histamine is due to a lack of freshness or an inadequately hygienic quality of raw materials and/or production processes [31]. For this reason, meat and fish can be consumed in the framework of a low-histamine diet, as long as their freshness is ensured. In contrast, fermented products are systematically excluded, due to a high probability of containing histamine [31]. Other foods such as spinach, eggplant and tomatoes should also be avoided for the same reason. In general, all these abovementioned foods are unanimously eliminated in most published low-histamine diets (Table 3).
On the other hand, there are certain foods that a priori do not contain histamine, but that patients associate with the appearance of symptoms. For these foods, there is much more variability when it comes to their exclusion from low-histamine diets (Table 3). The exclusion of foods could be based on their content of other biogenic amines, such as putrescine and cadaverine, which act as competitive substrates for DAO and may therefore inhibit intestinal degradation of histamine if present in significant quantities [1,82]. Thus, the onset of symptoms after the consumption of citrus fruits, mushrooms, soybeans, bananas and nuts may be due to high levels of other amines, specially putrescine [82]. These diets may also exclude certain foods free of histamine and with low enough concentrations of other amines to justify their exclusion. This is the case, for example, for papayas, kiwis, strawberries, pineapples and plums, which have been reported to trigger the release of endogenous histamine, although the mechanism responsible has not yet been elucidated [8,13].
The effectiveness of a low-histamine diet has been demonstrated in clinical studies, which report favorable results in terms of improvement or total remission of symptoms frequently associated with histamine intolerance and DAO deficiency (Table 4). As shown in Table 4, over the past three decades, various clinical studies have assessed the effect of a low-histamine diet on the evolution of various symptoms, mainly dermatological, gastrointestinal and neurological, including cases with more than one type. Although most studies have involved only a small group of patients (a mean of 38 per study, with a minimum of 10 and maximum of 157), they report an efficacy rate for the diet ranging from 33% to 100%. Specifically, 10 of the 13 studies reviewed found an improvement in symptoms in more than 50% of patients who followed the diet; two studies show success rates of less than 50% (33% and 46%), and only one did not observe any beneficial effects (Table 4). Most of the studies involved patients with dermatological symptoms, primarily chronic idiopathic urticaria, atopic dermatitis and eczema. In this field, a recent systematic literature review included a total of 1668 patients with chronic urticaria undergoing different exclusion diets, including low-histamine, pseudoallergen-free (i.e., without preservatives and artificial colors present in processed foods or aromatic compounds from certain natural products) and fish exclusion diets [120]. Overall, following any of the exclusion diets resulted in the total or partial remission of symptoms in 4.9% and 37.5% of patients, respectively. A low-histamine diet for an average of 3 weeks resulted in one of the highest remission rates. Despite the promising results of a low-histamine diet for the treatment of dermatological conditions, scientific societies of dermatology still consider this exclusion diet of unproven utility pending randomized, double-blind, placebo-controlled clinical trials to confirm its effectiveness [121].
Table 4
Clinical studies on the efficacy of a low-histamine diet for the treatment of symptoms of histamine intolerance.
Design and Outcomes of the StudyNumber of Patients and SymptomsDurationPercentage of Patients with Improvement in the Study OutcomesReference
| Prospective study with evaluation of the evolution of the symptomatology | 28 patients with chronic headache and 17 with other dermatological and respiratory symptoms | 4 weeks | 68% reduction in chronic headache and 82% reduction in other symptoms | [124] |
| Prospective study with evaluation of the evolution of symptoms, plasma histamine levels and DAO activity | 10 patients with chronic idiopathic urticaria and 19 control individuals | 3 weeks | 100% reduction in symptoms, 100% reduction in plasma histamine and no changes in DAO activity | [122] |
| Prospective study with evaluation of the evolution of symptoms, plasma histamine levels and DAO activity | 35 patients with headache and other symptoms (urticaria, arrhythmia, diarrhea and asthma) | 4 weeks | 77% reduction in symptoms, 73% increase in DAO activity and no changes in plasma histamine levels | [85] |
| Prospective study with evaluation of the evolution of symptoms and DAO activity (in five of the patients) | 17 patients with DAO deficiency, atopic eczema and other symptoms (headache, flushing and gastrointestinal symptoms) | 2 weeks | 100% reduction in symptoms and 60% (three out of five) increase in DAO activity | [86] |
| Prospective study with evaluation of the evolution of symptoms and the use of antihistamine drugs | 13 patients with chronic idiopathic urticaria and 35 control patients (without diet) | 4 weeks | Lack of improvement in symptoms and no changes in the use of antihistamines | [125] |
| Prospective study with evaluation of the evolution of the symptomatology | 36 patients with atopic dermatitis and 19 control individuals | 2 weeks | 33% reduction in symptoms | [88] |
| Prospective study with evaluation of the evolution of the symptomatology and DAO activity | 20 patients with DAO deficiency and dermatological, gastrointestinal and respiratory symptoms | 6–12 months | 100% reduction in symptoms and 100% increase in DAO activity | [83] |
| Retrospective study with evaluation of the evolution of the symptomatology | 16 pediatric patients with diffuse abdominal pain, diarrhea, headache, vomiting and rash | 4 weeks | 100% reduction of symptoms | [91] |
| Prospective study with evaluation of the evolution of the symptomatology | 16 pediatric patients with chronic abdominal pain and DAO deficiency | 4 weeks | 88% reduction of symptoms | [92] |
| Retrospective study with evaluation of the evolution of the symptomatology | 157 patients with chronic idiopathic urticaria | 4 weeks | 46% reduction of symptoms | [126] |
| Prospective study with evaluation of the evolution of the symptomatology and DAO activity | 56 patients with chronic idiopathic urticaria and gastrointestinal symptoms | 3 weeks | 75% reduction in symptoms and no changes in DAO activity | [87] |
| Prospective study with evaluation of the evolution of symptoms, plasma histamine levels and DAO activity | 22 patients with chronic idiopathic urticaria | 4 weeks | 100% reduction in symptoms, 100% reduction in plasma histamine levels and no changes in DAO activity | [112] |
| Retrospective study with evaluation of the evolution of the symptomatology and DAO activity | 63 patients with gastrointestinal symptoms | 7–18 months | 79% reduction in symptoms and 52% increase in DAO activity | [123] |
In general, the duration of the dietary treatment considered in the different clinical studies ranges from 3 to 4 weeks, and no positive relationship could be established between a longer duration and the success rate in symptom remission (Table 4). As may also be seen in this table, some studies have also assessed the effect of diet on other variables, such as plasma histamine levels or plasma DAO activity [83,85,86,87,112,122,123]. Regarding DAO activity, the studies published by Steinbrecher et al., Maintz et al., Mušič et al. and Lackner et al. all point out an increase in plasma enzymatic activity in more than 50% of patients after the dietary intervention, although no explanatory hypothesis has been yet suggested [83,85,86,123]. In contrast, Guida et al., Wagner et al. and Son et al. reported no changes in serum DAO activity [87,112,122]. The inconsistency of these data highlights the need to develop more research in this specific field before conclusions can be drawn.
5.4.2. Exogenous DAO Supplementation
Similar to the current treatment for lactose intolerance, the possibility of oral supplementation with exogenous DAO has been proposed by several authors to facilitate dietary histamine degradation [13,127]. Improving intestinal DAO activity would allow a less restrictive diet, which could include foods with a tolerable dose of histamine [10,61]. In this context, in the update of the official list of novel foods in 2017, the European Commission gave the green light to the marketing of a DAO supplement as food supplement or as food for special medical purposes [128]. Specifically, European regulations authorize the formulation of porcine kidney protein extract with an enteric coating to ensure its integrity during its passage through the gastric environment [128]. In this specific regulation, the minimum DAO enzymatic capacity required for the supplement is determined through a radio extraction assay (REA). This technique, based on the radioactive labeling of putrescine and the scintillation counting of its consumption, is advantageous in terms of rapidity and sensitivity, but it was mainly conceived to be applicable to serum samples. Comas-Basté et al. have recently developed a rapid and reliable methodology through ultra-high performance liquid chromatography and fluorimetric detection (UHPLC-FL) for the in vitro determination of DAO activity specifically for the analysis of unpurified complex matrixes, such as porcine kidney extract and DAO supplements [129]. This methodological approach is based in the direct determination of histamine degradation and overcomes certain drawbacks in terms of matrix interferences and handling of radioactive materials.
Porcine kidneys are the main source of DAO enzyme, according to the literature. Several studies have demonstrated the capacity of this product to degrade histamine and other biogenic amines in vitro [57,129,130,131,132,133]. A wide variability of the DAO capacity of porcine kidney extracts has been reported (with values ranging from 0.1 to more than 100 mU/mg), depending on the purification grade applied to the matrix and/or the amine compound used as the reaction substrate [57,129,130,131,132,133]. In fact, many of these works sought the selective purification of the DAO enzyme to design biosensors for the biorecognition of biogenic amines as indicators of freshness in foods [134]. More recently, two studies have been published specifically focused in investigating the in vitro DAO activity of porcine kidney protein extract expressly used as active ingredient to formulate food supplements for the preventive treatment of histamine intolerance [129,130]. Comas-Basté et al. studied the in vitro enzymatic activity of 13 different production batches of porcine kidney protein extract used in the elaboration of food supplements, reporting a low influence of the raw material (porcine kidney) on the DAO activity of the extract, with a mean value of 0.23 ± 0.01 mU/mg [129]. Later, Kettner et al. obtained a porcine kidney crude extract with an in vitro DAO activity of 0.5 ± 0.06 mU/mg, and described a 10-fold increase of this enzymatic activity through the application of several consecutive purification steps [130].
Regarding the food supplement, divergent results have also been reported, since while certain authors discard its enzymatic capacity, DAO activity values ranging from 0.04 to 0.20 mU/mg have also been described for different commercial products available in the market [129,130]. Overall, these works coincide in the need to identify alternative sources to porcine kidney DAO for exogenous supplementation in histamine-intolerant individuals.
A higher catalytic capacity of DAO enzymes of plant origin in degrading certain amino substrates has been described by some authors in comparison with those of animal origin [129,135,136,137]. Specifically, the germinated sprouts of certain edible legumes have been pointed out as interesting sources of DAO enzyme. Germination is a physiological process that has been described as capable of increasing the DAO enzymatic capacity of sprouts by up to 250 times compared to ungerminated seeds [61,138]. The increased presence of DAO enzyme in legume sprouts could be associated with the importance of hydrogen peroxide, a byproduct of the deamination reaction, in the cell wall structuring, lignification and mobilization of seed reserves during germination [139,140,141,142]. In fact, it has been demonstrated that the germination of legume seeds for a period of 6–8 days in darkness provides the optimal environment to maximize the DAO activity of this plant-origin matrix [61,138,143]. From a commercial point of view, having a plant source of this enzyme would expand the target of this novel food for the vegetarian/vegan population, as well as those with religious restrictions on the consumption of pork products. In addition, obtaining DAO enzyme from legumes would be a practice in accordance with the current call for action of the Sustainable Development Goals.
Nowadays, only five published intervention studies (four from the last five years) have tested the clinical efficacy of exogenous DAO supplementation in patients with symptoms of histamine intolerance (Table 5). Although there is some variability, the available research points to the effectiveness of DAO supplements in reducing the appearance and intensity of symptoms. However, it is difficult to compare the different studies, since they differ in the design, the enzyme dosage, the intervention time and the measurement of efficacy outcomes. Komericki et al., Manzotti et al. and Schnedl et al. assayed the efficacy of DAO supplementation in patients with diverse symptoms associated with histamine intolerance (gastrointestinal, cardiovascular, respiratory and dermatological and/or neurological complaints) [84,108,109]. All three studies reported an important improvement in the intensity or frequency of symptoms, although they involved a small study population (14, 28 and 39 patients) and/or a reduced intervention time (from two to four weeks). Moreover, Schnedl et al. also evaluated the changes in plasmatic DAO activity, reporting a slight increase in 61% of patients during the intervention, which the authors linked to a possible improvement in the integrity of the intestinal mucosa due to the supplementation [109].
Table 5
Studies on the efficacy of DAO enzyme supplementation for the treatment of symptoms of histamine intolerance.
DesignNumber of Patients and SymptomsDuration of DAO SupplementationEfficacy OutcomesReference
| Randomized, double-blind, placebo-controlled, crossover provocation study using histamine-containing and histamine-free tea in combination with DAO capsules or placebo | 39 patients with histamine intolerance (headache and gastrointestinal and skin complaints) | - | Statistically significant reduction of histamine-associated symptoms compared to placebo | [108] |
| Retrospective study with evaluation of the clinical response to DAO supplementation | 14 patients with diagnosis of histamine intolerance (headache and gastrointestinal, cardiovascular, respiratory and skin complaints) | 2 weeks | Reduction of at least one of the reported symptoms in 93% of patients | [84] |
| Double-blind, placebo-controlled, crossover study | 20 patients with chronic spontaneous urticaria | 1 month | Significant reduction of 7-Day Urticaria Activity Score (UAS-7) and slight significant reduction of daily antihistamine dose | [144] |
| Randomized, double-blind, placebo-controlled clinical trial | 100 patients with episodic migraine and serum DAO deficit | 1 month | Significant decrease in the duration of migraine attacks and decrease in triptans intake | [110] |
| Open-label interventional pilot study | 28 patients with histamine intolerance (gastrointestinal, cardiovascular, respiratory and skin complaints) and reduced serum DAO values | 1 month of intervention and 1 month of follow-up | Significant improvement in frequency and intensity of all symptoms. 61% of patients showed slightly increase in serum DAO values. During the follow-up period (without DAO supplementation), the symptoms sum scores increased and DAO levels decreased. | [109] |
The clinical trials developed by Yacoub et al. and Izquierdo-Casas et al. were focused on a single disorder related to histamine intolerance (chronic spontaneous urticaria and migraine, respectively) [110,144]. Yacoub et al. considered 20 patients with chronic spontaneous urticaria who showed a significant reduction in the severity of the complaint according to the Urticaria Activity Score (UAS-7) [144]. Regarding migraines, the randomized double-blind clinical trial conducted by Izquierdo et al. considered a larger number of patients (100 patients) and obtained a statistically significant decrease in the duration of pain attacks with no recorded adverse side effects [110]. However, the authors did not find statistical differences when considering other research outputs, such as the frequency and intensity of pain.
Overall, despite the promising results, more ambitious clinical studies with a rigorous experimental design, longer treatment periods and properly sized samples are essential to establish the clinical efficacy of this treatment.
6. Conclusions and Perspectives
Histamine intolerance is currently a clinical entity of increasing interest, which can appear due to the intake of histamine from foods, mainly caused by a deficiency of the DAO enzyme at the intestinal level. Novel knowledge and studies of histamine intolerance have helped clarify many of the uncertainties that were classically associated with histamine intoxication. Etiologically, various SNPs have been identified in the gene encoding the DAO enzyme related to lower enzyme activity. Moreover, certain inflammatory bowel diseases that limit enzyme secretion or some DAO-inhibiting drugs have also been identified as possible causes of DAO deficiency. This intolerance manifests through a plethora of nonspecific gastrointestinal and extraintestinal symptoms.
The diagnosis of histamine intolerance is usually performed after ruling out allergic symptoms and by the presence of at least two clinical manifestations and their improvement or remission after following a low-histamine diet. Various complementary tests are currently being proposed to improve the diagnosis of this intolerance based, among others, on determining the DAO activity in blood or intestinal biopsy samples or on identifying genetic or metabolic urinary markers by noninvasive techniques.
The clinical management is carried out mainly through the follow-up of a low-histamine diet, although there is no consensus on the list of foods to be excluded. Even so, there are different clinical studies that show the efficacy of this dietary intervention in improving the quality of life of patients with symptoms of histamine intolerance. Oral supplementation with exogenous DAO enzyme from porcine kidney is also being used to enhance the intestinal capacity to degrade dietary histamine. Although few works have assayed the clinical efficacy of this preventive treatment, promising results have been obtained so far. Research is currently also being made to identify new sources of DAO enzyme, especially of plant origin, due to its higher catalytic capacity and other potential productive and commercial advantages.
In this context, it is necessary to keep promoting the multidisciplinary study of this disorder, both from basic (i.e., analytical chemistry, food science, physiology and biochemistry) and clinically applied research, meant to increase the scientific base and the currently available diagnostic and treatment strategies for histamine intolerance.
Acknowledgments
Sònia Sánchez-Pérez is a recipient of a doctoral fellowship from the University of Barcelona (APIF2018).
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
1. EFSA Panel on Biological Hazards (BIOHAZ) Scientific Opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011;9:1–93. doi: 10.2903/j.efsa.2011.2393. [CrossRef] [Google Scholar]
2. Windaus A., Vogt W. Synthese des Imidazolyl-äthylamins. Berichte der Dtsch. Chem. Gesellschaft. 1907;40:3691–3695. doi: 10.1002/cber.190704003164. [CrossRef] [Google Scholar]
3. Comas-Basté O., Luz Latorre-Moratalla M., Sánchez-Pérez S., Teresa Veciana-Nogués M., del Carmen Vidal-Carou M. Histamine and Other Biogenic Amines in Food. From Scombroid Poisoning to Histamine Intolerance. In: Proestos C., editor. Biogenic Amines. IntechOpen; London, UK: 2019. [Google Scholar]
4. Dale H.H., Laidlaw P.P. The physiological action of β-iminazolylethylamine. J. Physiol. 1910;41:318–344. doi: 10.1113/jphysiol.1910.sp001406. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
5. Tansey E.M. The wellcome physiological research laboratories 1894–1904: The home office, pharmaceutical firms, and animal experiments. Med. Hist. 1989;33:1–41. doi: 10.1017/S0025727300048894. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
6. Riley J.F. Histamine and Sir Henry Dale. Br. Med. J. 1965;1:1488–1490. doi: 10.1136/bmj.1.5448.1488. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
7. Panula P., Chazot P.L., Cowart M., Gutzmer R., Leurs R., Liu W.L.S., Stark H., Thurmond R.L., Haas H.L. International union of basic and clinical pharmacology. XCVIII. histamine receptors. Pharmacol. Rev. 2015;67:601–655. doi: 10.1124/pr.114.010249. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
8. Vlieg-Boerstra B.J., van der Heide S., Oude Elberink J.N.G., Kluin-Nelemans J.C., Dubois A.E.J. Mastocytosis and adverse reactions to biogenic amines and histamine-releasing foods: What is the evidence? Neth. J. Med. 2005;63:244–249. [PubMed] [Google Scholar]
9. Russo P., Spano G., Arena M.P., Capozzi V., Fiocco D., Grieco F., Beneduce L. Are consumers aware of the risks related to biogenic amines in food. Curr Res. Technol. Edu. Top. Appl. Microbiol. Microb. Biotechnol. 2010:1087–1095. [Google Scholar]
10. Maintz L., Novak N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007;85:1185–1196. doi: 10.1093/ajcn/85.5.1185. [PubMed] [CrossRef] [Google Scholar]
11. Comas-Basté O., Latorre-Moratalla M.L., Bernacchia R., Veciana-Nogués M.T., Vidal-Carou M.C. New approach for the diagnosis of histamine intolerance based on the determination of histamine and methylhistamine in urine. J. Pharm. Biomed. Anal. 2017;145:379–385. doi: 10.1016/j.jpba.2017.06.029. [PubMed] [CrossRef] [Google Scholar]
12. Worm J., Falkenberg K., Olesen J. Histamine and migraine revisited: Mechanisms and possible drug targets. J. Headache Pain. 2019;20:30. doi: 10.1186/s10194-019-0984-1. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
13. Kovacova-Hanuskova E., Buday T., Gavliakova S., Plevkova J. Histamine, histamine intoxication and intolerance. Allergol. Immunopathol. (Madr.) 2015;43:498–506. doi: 10.1016/j.aller.2015.05.001. [PubMed] [CrossRef] [Google Scholar]
14. Elmore B.O., Bollinger J.A., Dooley D.M. Human kidney diamine oxidase: Heterologous expression, purification, and characterization. J. Biol. Inorg. Chem. 2002;7:565–579. doi: 10.1007/s00775-001-0331-1. [PubMed] [CrossRef] [Google Scholar]
15. Schwelberger H.G., Feurle J., Houen G. Mapping of the binding sites of human diamine oxidase (DAO) monoclonal antibodies. Inflamm. Res. 2018;67:245–253. doi: 10.1007/s00011-017-1118-3. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
16. Finney J., Moon H.J., Ronnebaum T., Lantz M., Mure M. Human copper-dependent amine oxidases. Arch. Biochem. Biophys. 2014;546:19–32. doi: 10.1016/j.abb.2013.12.022. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
17. Boehm T., Pils S., Gludovacz E., Szoelloesi H., Petroczi K., Majdic O., Quaroni A., Borth N., Valent P., Jilma B. Quantification of human diamine oxidase. Clin. Biochem. 2017;50:444–451. doi: 10.1016/j.clinbiochem.2016.12.011. [PubMed] [CrossRef] [Google Scholar]
18. Schwelberger H.G., Feurle J., Houen G. Mapping of the binding sites of human histamine N-methyltransferase (HNMT) monoclonal antibodies. Inflamm. Res. 2017;66:1021–1029. doi: 10.1007/s00011-017-1086-7. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
19. Schwelberger H.G. Histamine intolerance: Overestimated or underestimated? Inflamm. Res. 2009;58:51–52. doi: 10.1007/s00011-009-2004-4. [PubMed] [CrossRef] [Google Scholar]
20. Jarisch R., Wantke F., Raithel M., Hemmer W. Histamine Intolerance: Histamine and Seasickness. Springer; Berlin/Heidelberg, Germany: 2015. Histamine and biogenic amines; pp. 3–43. [Google Scholar]
21. Gludovacz E., Maresch D., De Carvalho L.L., Puxbaum V., Baier L.J., Sützl L., Guédez G., Grünwald-Gruber C., Ulm B., Pils S., et al. Oligomannosidic glycans at asn-110 are essential for secretion of human diamine oxidase. J. Biol. Chem. 2018;293:1070–1087. doi: 10.1074/jbc.M117.814244. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
22. Elsenhans B., Hunder G., Strugala G., Schümann K. Longitudinal pattern of enzymatic and absorptive functions in the small intestine of rats after short-term exposure to dietary cadmium chloride. Arch. Environ. Contam. Toxicol. 1999;36:341–346. doi: 10.1007/s002449900480. [PubMed] [CrossRef] [Google Scholar]
23. McGrath A.P., Hilmer K.M., Collyer C.A., Shepard E.M., Elmore B.O., Brown D.E., Dooley D.M., Guss J.M. Structure and inhibition of human diamine oxidase. Biochemistry. 2009;48:9810–9822. doi: 10.1021/bi9014192. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
24. Boehm T., Reiter B., Ristl R., Petroczi K., Sperr W., Stimpfl T., Valent P., Jilma B. Massive release of the histamine-degrading enzyme diamine oxidase during severe anaphylaxis in mastocytosis patients. Allergy Eur. J. Allergy Clin. Immunol. 2019;74:583–593. doi: 10.1111/all.13663. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
25. Maršavelski A., Petrović D., Bauer P., Vianello R., Kamerlin S.C.L. Empirical Valence Bond Simulations Suggest a Direct Hydride Transfer Mechanism for Human Diamine Oxidase. ACS Omega. 2018;3:3665–3674. doi: 10.1021/acsomega.8b00346. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
26. Smolinska S., Jutel M., Crameri R., O’Mahony L. Histamine and gut mucosal immune regulation. Allergy. 2014;69:273–281. doi: 10.1111/all.12330. [PubMed] [CrossRef] [Google Scholar]
27. Sjaastad Ö.V. Potentiation by aminoguanidine of the sensitivity of sheep to histamine given by mouth. Effect of aminoguanidine on the urinary excretion of endogenous histamine. Q. J. Exp. Physiol. Cogn. Med. Sci. 1967;52:319–330. doi: 10.1113/expphysiol.1967.sp001918. [PubMed] [CrossRef] [Google Scholar]
28. Sattler J., Häfner D., Klotter H.J., Lorenz W., Wagner P.K. Food-induced histaminosis as an epidemiological problem: Plasma histamine elevation and haemodynamic alterations after oral histamine administration and blockade of diamine oxidase (DAO) Agents Actions. 1988;23:361–365. doi: 10.1007/BF02142588. [PubMed] [CrossRef] [Google Scholar]
29. Klocker J., Mätzler S.A., Huetz G.-N., Drasche A., Kolbitsch C., Schwelberger H.G. Expression of histamine degrading enzymes in porcine tissues. Inflamm. Res. 2005;54(Suppl. 1):S54–S57. doi: 10.1007/s00011-004-0425-7. [PubMed] [CrossRef] [Google Scholar]
30. FAO (Food and Agriculture Organization of the United Nations) WHO (World Health Organization) Public Health Risks of Histamine and other Biogenic Amines from Fish and Fishery Products. Meeting Report. World Health Organization; Geneva, Switzerland: 2013. [Google Scholar]
31. Bover-Cid S., Latorre-Moratalla M.L., Veciana-Nogués M.T., Vidal-Carou M.C. Encyclopedia of Food Safety. Volume 2. Elsevier; Amsterdam, The Netherlands: 2014. Processing Contaminants: Biogenic Amines; pp. 381–391. [Google Scholar]
Biomolecules. 2020 Aug; 10(8): 1181.
Published online 2020 Aug 14. doi: 10.3390/biom10081181
PMCID: PMC7463562
PMID: 32824107
Histamine Intolerance: The Current State of the Art
Oriol Comas-Basté,1,2,3 Sònia Sánchez-Pérez,1,2,3 Maria Teresa Veciana-Nogués,1,2,3 Mariluz Latorre-Moratalla,1,2,3 and María del Carmen Vidal-Carou1,2,3,*
Author information Article notes Copyright and License information PMC Disclaimer
Abstract
Histamine intolerance, also referred to as enteral histaminosis or sensitivity to dietary histamine, is a disorder associated with an impaired ability to metabolize ingested histamine that was described at the beginning of the 21st century. Although interest in histamine intolerance has considerably grown in recent years, more scientific evidence is still required to help define, diagnose and clinically manage this condition. This article will provide an updated review on histamine intolerance, mainly focusing on its etiology and the existing diagnostic and treatment strategies. In this work, a glance on histamine intoxication will also be provided, as well as the analysis of some uncertainties historically associated to histamine intoxication outbreaks that may be better explained by the existence of interindividual susceptibility to ingested histamine.
Keywords: histamine, food intolerance, histamine intolerance, histaminosis, histamine intoxication, diamine oxidase (DAO), low-histamine diet, food supplement
1. Introduction
In 2011, the European Food Safety Authority (EFSA) issued a scientific report warning that the levels of biogenic amines found in foods marketed in European Union countries may still entail a consumer health risk [1]. Among them, histamine has the highest toxic potential, along with tyramine, and is therefore of great interest in terms of food safety. First described more than 60 years ago, the deleterious effects of excessive histamine ingestion were initially referred to as scombroid fish poisoning or scombrotoxicosis, as they were associated with the consumption of fish in this family, but the condition is now known as histamine intoxication or histamine poisoning. In recent years, another disorder associated with histamine intake, arising from an enzymatic deficiency, has been described. The inability of certain individuals to metabolize histamine in the intestine, resulting in sensitivity to normal or even low histamine levels in food, may help to explain some of the uncertainties historically associated with histamine intoxication.
During the last decade, histamine intolerance has gained social and scientific recognition, with a significant increase in the interest of researchers to investigate this disorder. This review aims to analyze the pathophysiological relevance of dietary histamine, giving special focus to the adverse effects derived from histamine intake and, in particular, to the state of the art concerning the etiology, diagnosis and treatment of histamine intolerance.
2. Histamine
Histamine (2-[4-imidazolyl]ethylamine) is a bioactive amine that is synthesized by decarboxylation of its precursor amino acid, histidine, in an enzymatic reaction first described by Windaus and Vogt in 1907 involving L-histidine decarboxylase (EC 4.1.1.22) (Figure 1) [2]. Due to its chemical structure and number of functional groups, histamine can be defined as a heterocyclic diamine with an imidazole ring and ethylamine (i.e., an organic compound that provides a functional group in the form of a primary amine) [1,3].

Synthesis of histamine by decarboxylation of its precursor amino acid.
The physiological and pathophysiological effects of histamine on the body were first described in 1910 by Dale and Laidlaw, two pioneering researchers who studied the functions of this organic compound at the Wellcome Physiological Research Laboratories [4,5,6]. Specifically, histamine is synthesized and stored in high concentrations in secretory granules, mainly in basophils and mast cells, and also in gastric enterochromaffin cells, lymph nodes and the thymus [1,7]. Functionally, this amine is involved in various immune and physiological mechanisms, stimulating gastric acid secretion, inflammation, smooth muscle cell contraction, vasodilation and cytokine production, among other processes [8,9,10,11]. In addition, histamine functions as a neurotransmitter, being synthesized by neurons located in the posterior region of the hypothalamus whose axons extend through the brain [12]. These wide-ranging physiological effects occur by interaction with four G-protein-coupled receptors with seven transmembrane domains (H1, H2, H3 and H4), which activate signal transduction pathways upon perceiving their ligand, histamine [7,12].
Two main histamine metabolic pathways are known in humans, involving the enzymes diamine oxidase (DAO) and histamine-N-methyltransferase (HNMT) (Figure 2) [10,11,13]. DAO (EC 1.4.3.22), also called histaminase or amiloride-binding protein, is a copper-dependent amino oxidase encoded by the AOC1 gene located on chromosome 7 (7q34-36) [14,15,16]. This functional enzyme, a homodimer with two isoforms, catalyzes the oxidative deamination of the primary amine group of histamine [14,16,17]. On the other hand, histamine can be metabolized to 1-methylhistamine by the enzyme HNMT (EC 2.1.1.8), a small monomeric protein encoded by a gene located on chromosome 2q22.1 [18]. HNMT catalyzes the methylation of the secondary amine group of the histamine imidazole aromatic heterocycle by a reaction requiring the S-adenosyl methionine cosubstrate as a methyl group donor [11,13,19].

Histamine metabolism in humans. DAO: diamine oxidase; HNMT: histamine-N-methyltransferase; ALDH: aldehyde dehydrogenase; MAO: monoamine oxidase.
Thus, depending on its location, the histamine present in the body is deaminated or methylated by the action of the enzymes DAO and HNMT, respectively [1,10,20]. DAO is a secretory protein stored in vesicular structures of the plasma membrane and is responsible for the degradation of extracellular histamine [1,15]. In mammals, the expression of DAO is restricted to certain tissues, mainly the small intestine, ascending colon, placenta and kidneys [14,21]. In the intestine, DAO activity increases progressively from the duodenum to the ileum and is located mainly in the intestinal villi [22]. In contrast, the enzyme HNMT is expressed in a wide range of human tissues, above all in the kidneys and liver, and also the spleen, colon, prostate, ovaries, spinal cord cells and the trachea and respiratory tract [10,13]. HNMT is a cytosolic protein responsible for the inactivation of intracellular histamine and can be synthesized in the cell itself or incorporated from the extracellular space by binding to a receptor or by membrane transporters [7,18]. Regarding substrates, HNMT is highly selective for histamine, whereas DAO can also metabolize other biogenic amines such as putrescine and cadaverine, although it shows a preference for histamine [14,16,23]. The affinity of DAO and HNMT for histamine is very similar, although the latter shows a slightly lower Michaelis–Menten enzymatic constant (KM: 6–13 μmol/L) than DAO (KM: 20 μmol/L) [10].
The gateway for dietary histamine in the body is the intestinal epithelium. Therefore, although HNMT is also present in the gastrointestinal tract, the more highly expressed DAO plays the major role in protecting the body against exogenous histamine, whether originating from ingested food or generated by the intestinal microbiota [24,25,26]. The protective effect of DAO has been demonstrated in animal experimentation models that were administered aminoguanidine for irreversible and selective DAO inhibition, followed by a dose of histamine [24,27,28]. The development of anaphylaxis symptoms in DAO-inhibited pigs and sheep compared to control groups indicates that the enzyme exerts a significant barrier effect against the absorption of exogenous histamine into the systemic circulation [1,13,19,24,29]. The HNMT enzyme ranks second to DAO in protecting against the absorption of dietary histamine from the intestinal lumen, but appears to be more effective against intravenously or intradermally supplied histamine [13,30].
3. Histamine in Foods
Histamine is present in a wide range of foods in highly variable concentrations, which are the main exogenous source of this compound [31]. The main route for histamine formation in food is the decarboxylation of histidine through the action of L-histidine decarboxylase, an enzyme of bacterial origin [32,33]. Apart from histamine, food can also contain other biogenic amines, mainly tyramine (4-hydroxy-phenethylamine), putrescine (1,4-diaminobutane) and cadaverine (1,5-diaminopentane), which are formed through enzymatic deamination of the amino acids tyrosine, ornithine (and/or agmatine) and lysine, respectively [31,34]. The accumulation of these compounds in food is the result of the transformation of amino acids by microorganisms and depends on various factors, such as the availability of the precursor amino acids and environmental conditions favorable for growth and/or the bacterial decarboxylase activity [31,34,35].
These decarboxylation reactions have been described as a survival strategy for microorganisms in acidic environments, as well as an alternative source of metabolic energy in situations of suboptimal substrate availability [1,9]. This enzymatic activity in bacteria is a species- and strain-dependent property [32]. Several Gram-positive and Gram-negative bacteria responsible for microbial spoilage or fermentative processes in food are able to produce histamine [1,36]. Specifically, the Enterobacteriaceae species Hafnai aluei, Morganella morganii and Klebsiella pneumonia have been identified as some of the most prolific histamine-forming bacteria in fish [9,37]. On the other hand, in cheeses, fermented meat, vegetable derivatives and fermented beverages, various lactic acid bacteria have also been described as histamine-producing microorganisms (e.g., Lactobacillus hilgardii, Lactobacillus buchnerii, Lactobacillus curvatus and Oenococcus oeni) as well as certain strains of Enterobacteriaceae [1,38,39].
Foods that potentially contain high levels of histamine are: a) those microbiologically altered, such as fish and meat, or derived products that may have been preserved or processed in unsuitably hygienic conditions; and b) fermented products, in which the bacteria responsible for the fermentation process may also have aminogenic capacity [3,40]. Table 1 shows histamine content in the different food categories from the Spanish market [31].
Table 1
Histamine content in different food categories. Adapted from [31].
FoodHistamine Content (mg/kg)nMean (SD)MedianMinimumMaximum
| Fruits, vegetables and plant-based products | |||||
| Fruits | 136 | 0.07 (0.20) | ND | ND | 2.51 |
| Nuts | 41 | 0.45 (1.23) | ND | ND | 11.86 |
| Vegetables | 98 | 2.82 (7.43) | ND | ND | 69.72 |
| Legumes | 11 | ND | ND | ND | ND |
| Cereals | 28 | 0.12 (0.33) | ND | ND | 0.89 |
| Chocolate | 25 | 0.58 (0.44) | 0.17 | 0.16 | 0.56 |
| Spices | 12 | ND | ND | ND | ND |
| Alcoholic beverages | |||||
| Beer | 176 | 1.23 (2.47) | 0.70 | ND | 21.60 |
| White wine | 83 | 1.24 (1.69) | 0.45 | 0.10 | 13.00 |
| Red wine | 260 | 3.81 (3.51) | 1.90 | 0.09 | 55.00 |
| Fish and seafood products | |||||
| Fresh fish | 136 | 0.79 (0.71) | ND | ND | 36.55 |
| Canned fish | 96 | 14.42 (16.03) | 5.93 | ND | 657.05 |
| Semipreserved fish | 49 | 3.48 (3.37) | 2.18 | ND | 34.90 |
| Meat and meat products | |||||
| Fresh meat | 6 | ND | ND | ND | ND |
| Cooked meat | 48 | 0.30 (0.26) | ND | ND | 4.80 |
| Cured meat | 23 | 12.98 (37.64) | 0.80 | ND | 150.00 |
| Dry-fermented sausages | 209 | 32.15 (14.22) | 8.03 | ND | 357.70 |
| Dairy products | |||||
| Unripened cheese | 20 | ND | ND | ND | ND |
| Raw milk cheese | 20 | 59.37 (106.74) | 18.38 | ND | 389.86 |
| Pasteurized milk cheese | 20 | 18.05 (38.23) | 4.59 | ND | 162.03 |
ND: not detected.
4. Uncertainties Associated with Histamine Poisoning: A Paradigm Shift Towards Histamine Intolerance
Although histamine has important physiological functions in the body, it can pose a health risk when ingested in high levels [41]. The proper functioning of histamine degradation systems is key in preventing its accumulation. Histamine intoxication, a kind of food poisoning, may occur after the consumption of foods with an unusually high histamine content that overpowers the degradation mechanisms (generally higher than 500 mg/kg) [1,3,42].
Historically, histamine intoxication has also been termed scombroid fish poisoning or the mahi-mahi flush because of its repeated association with the consumption of fish in the Scombridae and Scomberesocidae families (e.g., tuna, herring and mackerel) [43]. Histamine was first identified in 1946 as the causative agent of the toxic effects of consuming poorly transported tuna, and for a long time histamine poisoning was associated almost exclusively with the consumption of spoiled fish [44,45]. Over the years, the World Health Organization (WHO) has recommended the use of the term histamine intoxication to better designate this pathology, as it can be caused by marine species from other families (e.g., Clupeidae, Engraulidae, Coriphaenidae and Pomatomidae) and even other foods, such as cheese [43]. A meta-analysis carried out in 2018 of the different scientific reports of histamine intoxication between 1959 and 2013 established that the causative food in 98% of cases was fish, the remaining percentage being attributed to cheese [46]. Currently, international health administrations consider histamine intoxication to be one of the main problems of global food security, both for its effects on human health and its impact on trade [47,48].
Histamine intoxication is characterized by occurring in outbreaks and having a short incubation period (i.e., 20–30 min post-ingestion), with symptoms that are generally of low/moderate severity and remit in a few hours [3]. The symptoms are closely linked to the various physiological functions of histamine in the body, affecting the skin (e.g., redness, rash, urticaria, pruritus, edema and local inflammation), the gastrointestinal tract (e.g., nausea, vomiting and diarrhea) and the hemodynamic (hypotension) and neurological (e.g., headache, palpitations and tingling) bodily functions [1,41]. The symptomatic similarity of histamine intoxication with allergy means it is likely to be underdiagnosed [43,48,49]. The diagnosis of histamine intoxication is based primarily on the determination of elevated plasma histamine levels and/or the identification of an ingested food with an unusually high histamine content [13]. In general, an outbreak of histamine poisoning tends to involve more than one individual, lasts a short period of time and a particular causative food is identified [38].
In terms of incidence, the data available for the European Union shows an increase in histamine intoxication outbreaks in the last ten years, unlike other types of food poisoning, and with an almost hegemonic predominance of fish as the causative agent (over 90% of cases) [42,50]. The most recent data from the EFSA and European Center for Disease Prevention and Control (ECDC) show that in 2017, there was a 22% increase in outbreaks compared to the previous year [50]. Specifically, in 2017, there were a total of 117 outbreaks of histamine intoxication involving 572 people, 9% of whom required hospitalization. Fortunately, no deaths have been attributed to histamine poisoning over the past decade [42]. The same trend is observed in the information provided by the European Union Food and Feed Warning System (RASFF), with a progressive rise in the number of cases of histamine poisoning linked to tuna consumption in 2014–2017 and a particularly high increase in 2017 [3].
Although histamine intoxication has been extensively studied in recent decades, unresolved questions remain, concerning, for example, the variable histamine concentrations in the foods triggering outbreaks, or the heterogeneity in the degree and type of adverse effects [46]. Furthermore, the fact that oral administration of histamine in doses equivalent to those normally found in foods causing illness does not produce the same range and/or severity of symptoms is a paradox that has led to multiple hypotheses [30].
Several authors have proposed that alcohol and certain food components, such as other biogenic amines, may have a potentiating effect on histamine toxicity [13,48]. Amines such as putrescine and cadaverine, which are usually found in foods along with histamine, can also act as DAO substrates. It has therefore been suggested that these amines could weaken the protective barrier against dietary histamine by competitively interacting with degradation enzymes in the intestine [3,49]. Other possible potentiators are alcohol and its metabolite acetaldehyde, as they compete with histamine for the enzyme aldehyde dehydrogenase (ALDH), which is simultaneously involved in histamine and alcohol metabolism [1,32]. The potentiation effect of these components could help explain the differences in absorption of the same dose of histamine when ingested in isolation or in a food matrix [48,49]. The FAO and WHO have acknowledged that the involvement of potentiators can alter the threshold dose for toxicity, and they recommend that future studies focus on clarifying the ambiguities in the pathogenesis of histamine intoxication [30].
Finally, several authors have reported considerable interindividual variability in histamine tolerance, which has been demonstrated in intervention studies [1,3,10,13]. After the oral administration of the same histamine dosage, not all participants showed symptoms, and those who did varied in symptom type and severity and even had different blood histamine levels [48,51,52]. These results indicate the existence of population subgroups with greater sensitivity and clinical responses to histamine, likely linked to a diminished histamine degradation capacity, which could explain some of the historical uncertainties associated with histamine intoxication outbreaks [1]. Without disputing the clinical entity of histamine intoxication, the paradigm shift lies precisely in moving the focus from food to the human body, maintaining histamine as the causative agent, but focusing on how each person is able to respond to the intake of variable levels of histamine from food. Thus, histamine intolerance is the clinical condition that describes the inability of certain individuals to degrade histamine and results in the onset of symptoms caused by its accumulation in the blood (Figure 3).

Intestinal degradation of histamine by the DAO enzyme in three different situations: in a healthy individual, with histamine intoxication and with histamine intolerance. Adapted from [13].
5. Histamine Intolerance
According to the 2003 review of allergy nomenclature by the World Allergy Organization, adverse reactions to food without an immunological basis should be referred to as nonallergic food hypersensitivity, in order to clearly differentiate them from food allergies initiated by a specific immune mechanism [53]. Nonallergic food hypersensitivity is commonly known as food intolerance, a response triggered by a food or any of its components at a dose normally tolerated by the healthy population [54]. While the prevalence of food allergies is estimated at 1–2% in adults, currently almost 20% of the Westernized world’s population suffers from some type of food intolerance, with lactose intolerance being the most common [54].
Histamine intolerance, also referred to as enteral histaminosis or sensitivity to dietary histamine, can be defined as a disorder arising from reduced histamine degradation capacity in the intestine due to impaired DAO activity, leading to its accumulation in plasma and the appearance of adverse effects [11,41,55].
The DAO enzyme was first identified back in 1929 by Charles H. Best in autolyzing lung tissue, which he called histaminase because of its ability to degrade histamine [56]. Years later, given its ability to also degrade other diamines, as described above, the more accurate designation of DAO was proposed [57,58]. Beyond its role in the intestinal degradation of histamine in humans, DAO is also present in microorganisms, plants and animals, where it also catalyzes the oxidative deamination of the primary amino group of histamine into its corresponding aldehyde, concomitantly producing stoichiometric amounts of ammonia and hydrogen peroxide (Figure 4) [14,59,60].

Oxidative deamination of histamine by the DAO enzyme.
Although the first scientific references to histamine intolerance date from more than 20 years ago, it is significant that almost 80% are from the last decade, reflecting the growing interest of researchers in this disorder (Figure 5). In 2011, EFSA already considered histamine intolerance as one of the risks associated with histamine intake, clinically differentiating it from histamine intoxication [1]. In a subsequent joint report, the WHO and FAO emphasized that the no observed adverse effect level (NOAEL) established for histamine was only valid for healthy people, and not for members of susceptible populations, such as those with histamine intolerance [30]. EFSA concluded that only foods with histamine levels below the detection limits are safe for individuals with histamine intolerance [1].

Count of scientific publications containing the keywords histamine intolerance or histaminosis, according to a search performed through the PubMed search engine at the MEDLINE bibliographic database (search performed in July 2020).
Clinical manifestations of histamine intolerance consist of a wide range of nonspecific gastrointestinal and extraintestinal symptoms, due to the ubiquitous distribution of the four histamine receptors in different organs and tissues of the body (Figure 6) [10,13,54,61]. In a very recently published study, a team of Austrian researchers comprehensively analyzed the symptoms experienced by 133 patients diagnosed with histamine intolerance [62]. The most frequent and severe manifestations were gastrointestinal, with abdominal distension observed in 92% of patients and postprandial fullness, diarrhea, abdominal pain and constipation in 55–73%. Impairments of the nervous and cardiovascular systems, such as dizziness, headaches and palpitations, were recorded in second place, followed by respiratory and dermatological symptoms. Highlighting the complexity of the clinical picture of histamine intolerance, combinations of three or more symptoms involving different organs were recorded in 97% of cases, with an average of 11 symptoms per patient. The low specificity and complex variability of symptoms undoubtedly contribute to the current difficulty in achieving consensus on the diagnostic criteria for histamine intolerance, as will be discussed in detail below [13]. A lack of data also makes it difficult to determine the current incidence of this condition, although some authors have estimated that it affects 1–3% of the population, a percentage that will possibly increase as more knowledge and diagnostic tools for histamine intolerance become available [10,13,63].

Main symptoms of histamine intolerance and possibly corresponding histamine receptors [10,64].
5.1. The Etiology of Histamine Intolerance
As mentioned in previous sections, the main barrier against exogenous histamine in the intestines is the DAO enzyme, which prevents its passage into the systemic circulation [10,13,65]. Numerous clinical studies have provided data on the prevalence of low plasma DAO levels in individuals showing symptoms of histamine intolerance, mainly headaches and gastrointestinal or dermatological disorders [66]. Although certain studies have limitations, either in the design or number of participants, the majority point to an association between symptoms and DAO deficiency, establishing a general trend that supports the key role of DAO in the etiology of these disorders. A DAO deficiency that predisposes a population subgroup to histamine intolerance may have a genetic, pathological or pharmacological origin [1,41].
Regarding the genetic background of histamine intolerance, several studies have analyzed in depth the polymorphisms in genes encoding the enzymes L-histidine decarboxylase, DAO and HNMT, as well as the different histamine receptors. More than 50 nonsynonymous single-nucleotide polymorphisms (SNPs) in the DAO-encoding gene have been identified, some of which can produce a protein with altered activity and lead to symptoms of histamine intolerance [67,68,69,70,71,72]. Specifically, the most relevant SNPs affecting DAO enzyme functionality in Caucasian individuals are rs10156191, rs1049742, rs2268999 and especially rs1049793 [69,71]. On the other hand, an SNP in the promoter region of the gene has also been identified that causes a lower transcriptional activity of the DAO-encoding gene (rs2052129), as well as several genetic variations responsible for enzyme deficiency in people of Asian or African origin (rs45558339 and rs35070995, respectively) [67,72]. In most cases, the effect of these genetic variations on DAO functionality is through changes in enzyme kinetics, the resulting increase in KM causing a reduction in the rate of histamine degradation [69]. In parallel, three SNPs have been identified as being responsible for enhanced DAO enzyme activity (rs2071514, rs1049748 and rs2071517) [72]. There is also evidence of DAO mutations in patients with certain cardiovascular, gastrointestinal and nervous system pathologies, although with contradictory results regarding positive/negative effects [68].
DAO deficiency can also be an acquired condition, caused by certain pathologies or interaction with drugs. Several inflammatory bowel pathologies affecting mucosal integrity are known to result in impaired DAO activity, the degree of which can be correlated with the severity of mucosal damage [73,74,75]. Thus, DAO activity has been proposed as a marker of integrity of the intestinal mucosa. Miyoshi et al. demonstrated that DAO activity can be a useful predictor of intestinal mucosal damage in patients receiving chemotherapy [76]. Additionally, DAO deficiency has also been linked to certain functional gastrointestinal disorders, such as carbohydrate malabsorption and nonceliac gluten sensitivity (NCGS) [63,73,77,78,79]. Enko et al. found that a concomitant reduction in DAO and lactase enzyme activities could be a consequence of mucosal damage in the small intestine due to gastrointestinal disorders (e.g., gastroenteritis, irritable bowel syndrome, short bowel syndrome and gastrointestinal surgery) [73]. Moreover, patients with lactose intolerance and plasma DAO deficit showed higher end-expiratory H2 levels and the appearance of more symptoms during the H2 breath test in comparison with lactose-intolerant individuals with normal DAO activity [79]. More recently, two works have suggested a potential relationship between a reduced DAO activity and the presence of NCGS. Schnedl et al. based this relationship on the broad parallelism between the symptomatology of NCGS and histamine intolerance, while the pilot study conducted by Griauzdaite et al. reported a strong association between reduced DAO activity and the presence of NCGS, although with a reduced number of patients [77,78]. In fact, Griauzdaite et al. found out that nine of 10 patients with NCGS had decreased serum DAO activity levels [78]. This recently indicated relationship between both disorders, NCGS and histamine intolerance, should be further explored as it may be of interest for the correct clinical management of affected patients.
Finally, DAO deficiency can be a temporary and reversible condition, caused by the inhibitory effect of substances such as biogenic amines and alcohol, as discussed above, as well as several widely used drugs (Table 2) [1,10]. It has been estimated that approximately 20% of the European population regularly take DAO-inhibiting drugs, which significantly increases the number of people susceptible to the adverse effects of dietary histamine [28]. In vitro experimental results show a potent inhibitory effect (greater than 90%) of chloroquine, a historical antimalarial active ingredient, and clavulanic acid, a β-lactam antibiotic widely used in combination with amoxicillin [80]. A significant inhibition of the enzymatic activity has also been observed with the antihypertensive drug verapamil and the histamine H2 receptor antagonist cimetidine, although the clinical use of the latter is currently anecdotal [23,80]. Other substances have also shown an inhibitory effect, albeit to a lesser extent (Table 2) [23,80,81]. In most cases, the structural similarity of the cited drugs with histamine could explain their potential to bind to the active site of DAO and reduce its enzymatic activity [23]. Along the same lines, substances with an inhibitory effect on other enzymes involved in any of the metabolic pathways of histamine in the body (i.e., HNMT, ALDH and MAO) may act as a trigger of histamine hypersensitivity [82].
Table 2
Active ingredients with an experimentally demonstrated inhibitory effect on the DAO enzyme [23,28,80,81].
Active IngredientIndication
| Chloroquine | Antimalarial |
| Clavulanic acid | Antibiotic |
| Colistimethate | Antibiotic |
| Cefuroxime | Antibiotic |
| Verapamil | Antihypertensive |
| Clonidine | Antihypertensive |
| Dihydralazine | Antihypertensive |
| Pentamidine | Antiprotozoal |
| Isoniazid | Antituberculous |
| Metamizole | Analgesic |
| Diclofenac | Analgesic and anti-inflammatory |
| Acetylcysteine | Mucoactive |
| Amitriptyline | Antidepressant |
| Metoclopramide | Antiemetic |
| Suxamethonium | Muscle relaxant |
| Cimetidine | Antihistamine (H2 antagonist) |
| Prometazina | Antihistamine (H1 antagonist) |
| Ascorbic acid | Vitamin C |
| Thiamine | Vitamin B1 |
5.2. Prevalence of DAO Deficit in Persons with Symptoms Related to Histamine Intolerance
Several studies have evaluated the prevalence of DAO deficit in plasma of individuals with symptoms of histamine intolerance and/or diagnosis with certain chronic disorders.
Mušič et al. found DAO deficiency in 80% of 316 adult patients showing various symptoms associated with histamine intolerance (e.g., urticaria, pruritus, diarrhea, abdominal pain, vomiting, constipation, cough, rhinitis and headache), as well as significantly lower plasma DAO activity compared to the control group [83]. Similarly, in a retrospective study, Manzotti et al. evaluated DAO activity in 14 patients with a confirmed diagnosis of histamine intolerance who showed mainly gastrointestinal and dermatological symptoms, but also headaches [84]. In this case, patients showed a high prevalence of DAO deficit (71%) and a significantly lower mean DAO activity compared to healthy volunteers. A lower percentage of DAO deficiency in histamine-intolerant patients (24%) was reported by Pinzer et al. [63]. Those patients featured elevated histamine levels and constantly reduced DAO activities throughout the day.
In a study focused only on headache symptoms, Steinbrecher and Jarisch reported DAO deficiency in 23 of 27 patients (85%) [85]. In parallel, the authors described a significant increase in DAO activity after patients followed a low-histamine diet for four weeks, along with a remission or reduction in frequency of headaches in almost 90% of individuals. More recently, Izquierdo et al. studied the prevalence of DAO deficit in 137 patients diagnosed with a confirmed migraine diagnosis and in a control group of 61 nonmigraine individuals [66]. In this study, a high prevalence of DAO deficiency was observed in the migraine group (87%) and with a mean DAO activity significantly lower in comparison with that obtained from control volunteers. However, the prevalence of DAO deficiency in the control population amounted up to 44%, which was attributed to the fact that certain individuals could present other symptoms associated with histamine intolerance or DAO deficiency other than migraines. Another study with 44 migraine patients reported a 60% prevalence of DAO deficiency and a significant copresence of certain gastrointestinal disorders, such as celiac disease and NCGS [78].
In the field of dermatological symptomatology, several studies have monitored plasma DAO activity in patients with eczema, chronic idiopathic urticaria and atopic dermatitis. Overall, the reported prevalence of DAO deficiency ranges from 19 to 57%, with the exception of the study by Worm et al., who did not detect statistically significant differences in plasma DAO activity between control patients and those with atopic dermatitis [86,87,88,89].
Finally, regarding gastrointestinal symptoms, Honzawa et al. assessed the clinical significance of plasma DAO activity levels in 98 patients suffering inflammatory bowel disease [90]. This study showed that DAO activity in blood was significantly lower in patients with Crohn’s disease and ulcerative colitis compared to the control population, suggesting its potential importance as a marker of intestinal permeability. In a pediatric population under 15 years of age, Rosell-Camps et al. determined DAO deficiency in 88% of patients with abdominal pain, diarrhea and vomiting [91]. In contrast, in a more recent study by a group of Austrian researchers, DAO deficiency was found in only 8% of 394 children with chronic abdominal pain [92].
To date, little data is available on the prevalence of this enzymatic deficiency related to gender, and it is inconclusive. Klockler et al. found no differences in plasma DAO activity between men and women, although the number of individuals considered was scarce (n = 28) [93]. Likewise, the study performed by Izquierdo et al. reported similar percentages of DAO deficiency in migraine-suffering women (83%) and men (90%) [66]. On the contrary, García-Martín et al. did describe differences in plasma DAO activity by gender, with the prevalence of this enzyme deficiency being higher in women [94]. Significant fluctuations in DAO activity values have also been reported in women associated with different stages of the menstrual cycle [94,95].
One factor that could explain the discordance among the prevalence data of DAO deficit in patients with disorders associated with histamine intolerance is that the parameter considered in all of them was serum DAO activity, which, a priori, would not reflect an enzymatic deficiency derived from certain intestinal pathologies. Overall, in spite of the varying percentages in DAO deficiency, the currently available studies seem to indicate an etiological relationship between DAO deficiency and certain symptoms or disorders related to histamine intolerance. Nevertheless, more studies are needed to assess the clinical significance of the determination of plasma DAO activity, as well as to develop new diagnostic methods aimed at identifying individuals with histamine intolerance due to DAO deficiency.
5.3. Diagnosis of Histamine Intolerance
Despite significant advances in the understanding of histamine intolerance, reaching a consensus on a diagnostic algorithm remains a pending challenge. The nonspecificity of symptoms and lack of validated diagnostic tools prompts many affected individuals to go “doctor shopping”; that is, to consult several medical specialists in search of an explanation and solution for their varied symptomatology [13,63]. In the absence of a consensual and clinically validated diagnosis, Figure 7 shows a schematic summary of the diagnostic algorithm for histamine intolerance based on the available scientific evidence reviewed below.

Summary of the described approaches to the diagnosis of histamine intolerance. SNPs: single-nucleotide polymorphisms.
The combination of diagnostic criteria currently in use includes the appearance of typical clinical manifestations and the exclusion of other related disorders [10,13,54]. All the authors who have proposed a diagnostic algorithm for histamine intolerance emphasize the need to initially rule out other potential causes of symptoms associated with an increase in plasma histamine [10,13,54]. For this purpose, it is advisable to carry out an intradermal skin allergy test (i.e., skin prick test) to discard IgE sensitization caused by food allergy, and to measure plasma tryptase to exclude an underlying systemic mastocytosis [10]. It is also important to know whether the patient is taking any medication with a possible inhibitory effect on DAO activity [55]. If these conditions are negative, the appearance of two or more typical symptoms of histamine intolerance and their improvement or remission after the following of a low-histamine diet (i.e., a diet excluding foods that, a priori, contain high histamine levels) will confirm the diagnosis of histamine intolerance [10,54,96,97]. In the diet follow-up, a thorough 24-h record of all the foods consumed and symptoms experienced is recommended in order to establish a relationship, if any, between a food and the onset of symptoms [10,13]. The duration of the low-histamine diet to confirm the diagnosis is not clearly stipulated, although some studies suggest a period of 4 to 8 weeks [54,97]. In addition to the diet, testing the effect of antihistamine treatment on symptoms has also been proposed, although its usefulness once dietary histamine is removed is unclear [10,54].
Once it has been established that dietary histamine is responsible for the intolerance-associated symptoms, the diagnosis of this disorder is virtually confirmed. A range of nonvalidated complementary tests have also been proposed by several authors with the aim of obtaining a marker to confirm the diagnosis [97]. However, it has to be taken into account that not all of the tests consider the different origins of DAO deficiency (i.e., genetic, pathological or pharmacological). Thus, a genetic origin would lead to a reduction of the DAO enzymatic activity in the whole organism. Likewise, the pharmacological blockade of DAO would take place in all tissues where the drug is distributed after entering the systemic circulation, although in a punctual manner upon the substance’s introduction. Lastly, the scope of a DAO deficit due to intestinal pathologies would be limited to the local intestinal environment.
Due to the genetic background of DAO deficiency, one of the strategies for the diagnosis could be the determination of genetic polymorphisms (SNPs) that characterize the population as genetically susceptible to histamine [54]. Currently, there is already the possibility of performing a noninvasive genetic analysis capable of identifying three of the SNPs associated with reduced DAO activity (i.e., rs10156191, rs1049742 and rs1049793) from a sample of the oral mucosa, although evidence-based studies on the diagnosis potential of this test are still needed. It is important to note that this test will only reflect the existence of a genetic DAO deficiency.
The most studied, and possibly also the most controversial, is the determination of plasma DAO activity. This analytical test consists of measuring the amount of histamine degraded in a blood sample in a given time interval. Two types of commercial testing kits are currently available on the market, one consisting of an ELISA-type immunoassay, and the other a radioimmunoassay using radioactively labeled putrescine [83,84]. The evidence for the validity of blood DAO activity measurements for the diagnosis of histamine intolerance is neither abundant nor conclusive. Some studies have proposed that determining blood DAO activity may be helpful in identifying subjects with symptoms associated with histamine intolerance [63,83,84]. In contrast, three studies did not find a significant relationship between the clinical history of patients with typical symptoms of histamine intolerance and blood DAO activity values, concluding that this technique cannot be recommended as a diagnostic tool in routine clinical practice until studies have validated its effectiveness [98,99,100]. Moreover, the work performed by Schnoor et al. also reported a high interassay variation in DAO activity values that made the proper classification of histamine-intolerant subjects impossible [100]. This controversy is described in a joint article published in 2017 by the German and Swiss allergology societies, which emphasizes the need for more research before giving plasma DAO activity a definitive diagnostic value for histamine intolerance [97].
A variant of the intradermal skin allergy test called the histamine 50-skin-prick test was also proposed by Kofler et al. to diagnose histamine intolerance [101]. In this technique, the results were read after 50 min (as opposed to the usual 20 min) and showed that, although the size of the wheal did not differ between the histamine intolerant and control groups, the time course was significantly different. Patients with symptoms of intolerance showed a delayed remission of the wheal induced by cutaneous administration of histamine, signaling a reduced degradation ability. The same results were obtained in a study recently published by Wagner et al., who re-evaluated this skin test as a diagnostic tool of histamine intolerance, also observing a correlation between the delay in wheal disappearance and a lower plasma DAO activity [102].
Both the determination of plasma DAO activity and the histamine 50-skin-prick test could be suitable tests to identify a DAO deficiency from genetic or pharmacological origin, but they would not be useful to determine a deficit secondary to certain intestinal diseases.
On the contrary, there are certain alternatives, such as the intestinal biopsy, the histamine provocation test or the histamine metabolomics in urine, that could make it possible to diagnose histamine intolerance due to DAO deficiency without excluding any of the possible etiological causes.
The measurement of intestinal DAO activity by a colon biopsy during endoscopic procedures has been studied as a possible diagnostic marker. The few available studies have shown a reduced intestinal DAO catabolic activity in patients with recurrent urticaria, food allergy and colon adenoma, accompanied by an increase in histamine levels [103,104,105,106]. Although this test has interesting diagnostic potential, more studies are needed to validate its clinical significance and its relationship with the symptoms of histamine intolerance [97]. If proven, this diagnostic test would be very adequate since this disorder originates from a reduced ability of the intestinal DAO enzyme to cope with dietary histamine.
Histamine challenge/provocation test has also been proposed by some authors as a diagnostic tool for intolerance, which would, at the same time, establish the individual tolerance threshold. This double-blind, placebo-controlled test involves oral administration of histamine and requires patient medical supervision and hospitalization. In the study by Wöhrl et al., half of the healthy volunteers developed symptoms after the administration of a solution containing 75 mg of histamine [107]. In contrast, the results of a multicenter study by Komericki et al. using the same oral dose of histamine indicated the challenge test was unreliable for diagnosing histamine intolerance due to a lack of intraindividual reproducibility of symptoms after two different provocation tests [108]. The application of this procedure is still limited because of the risk of serious adverse side effects and the absence of a standardized dose of histamine and properly established protocol [97].
Finally, in recent years, efforts have been made to identify a noninvasive marker to establish a solid and clinically irrefutable diagnostic criterion for histamine intolerance due to DAO deficiency. Currently, the application of metabolomics as a tool for the identification of biomarkers of histamine metabolism in urine is also being challenged as a possible new diagnostic strategy [11]. The hypothesis is that individuals with histamine intolerance could have a different excretion profile of histamine and its metabolites in urine than normal individuals. For this purpose, Comas-Basté et al. have recently proposed a chromatographic approach that allows for determining in a fast and unequivocal manner the urinary levels of histamine and its methylated metabolite, methylhistamine [11]. It is still necessary to validate the potential diagnostic utility of this approach in patients with histamine intolerance, as well as complementing the excretion profile with other histamine metabolites to obtain a more accurate image of the possible alterations produced in this intolerance.
5.4. Treatment Approaches to Histamine Intolerance
Currently, the main strategy to avoid the symptoms of histamine intolerance is to follow a low-histamine diet. Supplementation with exogenous DAO has recently been postulated as a complementary treatment to enhance dietary histamine degradation in intolerant individuals who have a deficiency of this enzyme in the intestine [109,110].
5.4.1. Low-Histamine Diet
A low-histamine or histamine-free diet has been proposed as the main strategy for the preventive treatment of histamine intolerance [10,54,82,111]. Conceptually, these diets exclude a number of foods that patients associate with the onset of symptoms, primarily those that may contain high levels of histamine [82]. However, there is no a single dietary recommendation of a low-histamine diet. As it may be seen in Table 3, there is no coincidence in all the foods excluded in the different low-histamine diets found in the literature [10,87,91,112,113,114,115,116,117,118].
Table 3
Foods excluded in the different low-histamine diets found in the literature [10,87,91,112,113,114,115,116,117,118].
Foods Excluded by Low-Histamine Diets
| <20% * | 20–60% * | >60% * |
| Milk | Shellfish | Cured and semicured cheese |
| Lentils | Eggs | Grated cheese |
| Chickpeas | Fermented soy derivatives | Oily fish |
| Soybeans | Eggplant | Canned and semipreserved oily fish derivatives |
| Mushrooms | Avocado | Dry-fermented meat products |
| Banana | Spinach | |
| Kiwi | Tomatoes | |
| Pineapple | Fermented cabbage | |
| Plum | Citrus | |
| Nuts | Strawberries | |
| Chocolate | Wine | |
| Beer |
* Percentage of low-histamine diets from the literature that exclude each foodstuff.
Histamine is widely distributed in different food categories and in highly variable concentrations, as its accumulation is influenced by multiple factors [3,119]. In fresh foods such as fish and meat, and in some derived products, the presence of histamine is due to a lack of freshness or an inadequately hygienic quality of raw materials and/or production processes [31]. For this reason, meat and fish can be consumed in the framework of a low-histamine diet, as long as their freshness is ensured. In contrast, fermented products are systematically excluded, due to a high probability of containing histamine [31]. Other foods such as spinach, eggplant and tomatoes should also be avoided for the same reason. In general, all these abovementioned foods are unanimously eliminated in most published low-histamine diets (Table 3).
On the other hand, there are certain foods that a priori do not contain histamine, but that patients associate with the appearance of symptoms. For these foods, there is much more variability when it comes to their exclusion from low-histamine diets (Table 3). The exclusion of foods could be based on their content of other biogenic amines, such as putrescine and cadaverine, which act as competitive substrates for DAO and may therefore inhibit intestinal degradation of histamine if present in significant quantities [1,82]. Thus, the onset of symptoms after the consumption of citrus fruits, mushrooms, soybeans, bananas and nuts may be due to high levels of other amines, specially putrescine [82]. These diets may also exclude certain foods free of histamine and with low enough concentrations of other amines to justify their exclusion. This is the case, for example, for papayas, kiwis, strawberries, pineapples and plums, which have been reported to trigger the release of endogenous histamine, although the mechanism responsible has not yet been elucidated [8,13].
The effectiveness of a low-histamine diet has been demonstrated in clinical studies, which report favorable results in terms of improvement or total remission of symptoms frequently associated with histamine intolerance and DAO deficiency (Table 4). As shown in Table 4, over the past three decades, various clinical studies have assessed the effect of a low-histamine diet on the evolution of various symptoms, mainly dermatological, gastrointestinal and neurological, including cases with more than one type. Although most studies have involved only a small group of patients (a mean of 38 per study, with a minimum of 10 and maximum of 157), they report an efficacy rate for the diet ranging from 33% to 100%. Specifically, 10 of the 13 studies reviewed found an improvement in symptoms in more than 50% of patients who followed the diet; two studies show success rates of less than 50% (33% and 46%), and only one did not observe any beneficial effects (Table 4). Most of the studies involved patients with dermatological symptoms, primarily chronic idiopathic urticaria, atopic dermatitis and eczema. In this field, a recent systematic literature review included a total of 1668 patients with chronic urticaria undergoing different exclusion diets, including low-histamine, pseudoallergen-free (i.e., without preservatives and artificial colors present in processed foods or aromatic compounds from certain natural products) and fish exclusion diets [120]. Overall, following any of the exclusion diets resulted in the total or partial remission of symptoms in 4.9% and 37.5% of patients, respectively. A low-histamine diet for an average of 3 weeks resulted in one of the highest remission rates. Despite the promising results of a low-histamine diet for the treatment of dermatological conditions, scientific societies of dermatology still consider this exclusion diet of unproven utility pending randomized, double-blind, placebo-controlled clinical trials to confirm its effectiveness [121].
Table 4
Clinical studies on the efficacy of a low-histamine diet for the treatment of symptoms of histamine intolerance.
Design and Outcomes of the StudyNumber of Patients and SymptomsDurationPercentage of Patients with Improvement in the Study OutcomesReference
| Prospective study with evaluation of the evolution of the symptomatology | 28 patients with chronic headache and 17 with other dermatological and respiratory symptoms | 4 weeks | 68% reduction in chronic headache and 82% reduction in other symptoms | [124] |
| Prospective study with evaluation of the evolution of symptoms, plasma histamine levels and DAO activity | 10 patients with chronic idiopathic urticaria and 19 control individuals | 3 weeks | 100% reduction in symptoms, 100% reduction in plasma histamine and no changes in DAO activity | [122] |
| Prospective study with evaluation of the evolution of symptoms, plasma histamine levels and DAO activity | 35 patients with headache and other symptoms (urticaria, arrhythmia, diarrhea and asthma) | 4 weeks | 77% reduction in symptoms, 73% increase in DAO activity and no changes in plasma histamine levels | [85] |
| Prospective study with evaluation of the evolution of symptoms and DAO activity (in five of the patients) | 17 patients with DAO deficiency, atopic eczema and other symptoms (headache, flushing and gastrointestinal symptoms) | 2 weeks | 100% reduction in symptoms and 60% (three out of five) increase in DAO activity | [86] |
| Prospective study with evaluation of the evolution of symptoms and the use of antihistamine drugs | 13 patients with chronic idiopathic urticaria and 35 control patients (without diet) | 4 weeks | Lack of improvement in symptoms and no changes in the use of antihistamines | [125] |
| Prospective study with evaluation of the evolution of the symptomatology | 36 patients with atopic dermatitis and 19 control individuals | 2 weeks | 33% reduction in symptoms | [88] |
| Prospective study with evaluation of the evolution of the symptomatology and DAO activity | 20 patients with DAO deficiency and dermatological, gastrointestinal and respiratory symptoms | 6–12 months | 100% reduction in symptoms and 100% increase in DAO activity | [83] |
| Retrospective study with evaluation of the evolution of the symptomatology | 16 pediatric patients with diffuse abdominal pain, diarrhea, headache, vomiting and rash | 4 weeks | 100% reduction of symptoms | [91] |
| Prospective study with evaluation of the evolution of the symptomatology | 16 pediatric patients with chronic abdominal pain and DAO deficiency | 4 weeks | 88% reduction of symptoms | [92] |
| Retrospective study with evaluation of the evolution of the symptomatology | 157 patients with chronic idiopathic urticaria | 4 weeks | 46% reduction of symptoms | [126] |
| Prospective study with evaluation of the evolution of the symptomatology and DAO activity | 56 patients with chronic idiopathic urticaria and gastrointestinal symptoms | 3 weeks | 75% reduction in symptoms and no changes in DAO activity | [87] |
| Prospective study with evaluation of the evolution of symptoms, plasma histamine levels and DAO activity | 22 patients with chronic idiopathic urticaria | 4 weeks | 100% reduction in symptoms, 100% reduction in plasma histamine levels and no changes in DAO activity | [112] |
| Retrospective study with evaluation of the evolution of the symptomatology and DAO activity | 63 patients with gastrointestinal symptoms | 7–18 months | 79% reduction in symptoms and 52% increase in DAO activity | [123] |
In general, the duration of the dietary treatment considered in the different clinical studies ranges from 3 to 4 weeks, and no positive relationship could be established between a longer duration and the success rate in symptom remission (Table 4). As may also be seen in this table, some studies have also assessed the effect of diet on other variables, such as plasma histamine levels or plasma DAO activity [83,85,86,87,112,122,123]. Regarding DAO activity, the studies published by Steinbrecher et al., Maintz et al., Mušič et al. and Lackner et al. all point out an increase in plasma enzymatic activity in more than 50% of patients after the dietary intervention, although no explanatory hypothesis has been yet suggested [83,85,86,123]. In contrast, Guida et al., Wagner et al. and Son et al. reported no changes in serum DAO activity [87,112,122]. The inconsistency of these data highlights the need to develop more research in this specific field before conclusions can be drawn.
5.4.2. Exogenous DAO Supplementation
Similar to the current treatment for lactose intolerance, the possibility of oral supplementation with exogenous DAO has been proposed by several authors to facilitate dietary histamine degradation [13,127]. Improving intestinal DAO activity would allow a less restrictive diet, which could include foods with a tolerable dose of histamine [10,61]. In this context, in the update of the official list of novel foods in 2017, the European Commission gave the green light to the marketing of a DAO supplement as food supplement or as food for special medical purposes [128]. Specifically, European regulations authorize the formulation of porcine kidney protein extract with an enteric coating to ensure its integrity during its passage through the gastric environment [128]. In this specific regulation, the minimum DAO enzymatic capacity required for the supplement is determined through a radio extraction assay (REA). This technique, based on the radioactive labeling of putrescine and the scintillation counting of its consumption, is advantageous in terms of rapidity and sensitivity, but it was mainly conceived to be applicable to serum samples. Comas-Basté et al. have recently developed a rapid and reliable methodology through ultra-high performance liquid chromatography and fluorimetric detection (UHPLC-FL) for the in vitro determination of DAO activity specifically for the analysis of unpurified complex matrixes, such as porcine kidney extract and DAO supplements [129]. This methodological approach is based in the direct determination of histamine degradation and overcomes certain drawbacks in terms of matrix interferences and handling of radioactive materials.
Porcine kidneys are the main source of DAO enzyme, according to the literature. Several studies have demonstrated the capacity of this product to degrade histamine and other biogenic amines in vitro [57,129,130,131,132,133]. A wide variability of the DAO capacity of porcine kidney extracts has been reported (with values ranging from 0.1 to more than 100 mU/mg), depending on the purification grade applied to the matrix and/or the amine compound used as the reaction substrate [57,129,130,131,132,133]. In fact, many of these works sought the selective purification of the DAO enzyme to design biosensors for the biorecognition of biogenic amines as indicators of freshness in foods [134]. More recently, two studies have been published specifically focused in investigating the in vitro DAO activity of porcine kidney protein extract expressly used as active ingredient to formulate food supplements for the preventive treatment of histamine intolerance [129,130]. Comas-Basté et al. studied the in vitro enzymatic activity of 13 different production batches of porcine kidney protein extract used in the elaboration of food supplements, reporting a low influence of the raw material (porcine kidney) on the DAO activity of the extract, with a mean value of 0.23 ± 0.01 mU/mg [129]. Later, Kettner et al. obtained a porcine kidney crude extract with an in vitro DAO activity of 0.5 ± 0.06 mU/mg, and described a 10-fold increase of this enzymatic activity through the application of several consecutive purification steps [130].
Regarding the food supplement, divergent results have also been reported, since while certain authors discard its enzymatic capacity, DAO activity values ranging from 0.04 to 0.20 mU/mg have also been described for different commercial products available in the market [129,130]. Overall, these works coincide in the need to identify alternative sources to porcine kidney DAO for exogenous supplementation in histamine-intolerant individuals.
A higher catalytic capacity of DAO enzymes of plant origin in degrading certain amino substrates has been described by some authors in comparison with those of animal origin [129,135,136,137]. Specifically, the germinated sprouts of certain edible legumes have been pointed out as interesting sources of DAO enzyme. Germination is a physiological process that has been described as capable of increasing the DAO enzymatic capacity of sprouts by up to 250 times compared to ungerminated seeds [61,138]. The increased presence of DAO enzyme in legume sprouts could be associated with the importance of hydrogen peroxide, a byproduct of the deamination reaction, in the cell wall structuring, lignification and mobilization of seed reserves during germination [139,140,141,142]. In fact, it has been demonstrated that the germination of legume seeds for a period of 6–8 days in darkness provides the optimal environment to maximize the DAO activity of this plant-origin matrix [61,138,143]. From a commercial point of view, having a plant source of this enzyme would expand the target of this novel food for the vegetarian/vegan population, as well as those with religious restrictions on the consumption of pork products. In addition, obtaining DAO enzyme from legumes would be a practice in accordance with the current call for action of the Sustainable Development Goals.
Nowadays, only five published intervention studies (four from the last five years) have tested the clinical efficacy of exogenous DAO supplementation in patients with symptoms of histamine intolerance (Table 5). Although there is some variability, the available research points to the effectiveness of DAO supplements in reducing the appearance and intensity of symptoms. However, it is difficult to compare the different studies, since they differ in the design, the enzyme dosage, the intervention time and the measurement of efficacy outcomes. Komericki et al., Manzotti et al. and Schnedl et al. assayed the efficacy of DAO supplementation in patients with diverse symptoms associated with histamine intolerance (gastrointestinal, cardiovascular, respiratory and dermatological and/or neurological complaints) [84,108,109]. All three studies reported an important improvement in the intensity or frequency of symptoms, although they involved a small study population (14, 28 and 39 patients) and/or a reduced intervention time (from two to four weeks). Moreover, Schnedl et al. also evaluated the changes in plasmatic DAO activity, reporting a slight increase in 61% of patients during the intervention, which the authors linked to a possible improvement in the integrity of the intestinal mucosa due to the supplementation [109].
Table 5
Studies on the efficacy of DAO enzyme supplementation for the treatment of symptoms of histamine intolerance.
DesignNumber of Patients and SymptomsDuration of DAO SupplementationEfficacy OutcomesReference
| Randomized, double-blind, placebo-controlled, crossover provocation study using histamine-containing and histamine-free tea in combination with DAO capsules or placebo | 39 patients with histamine intolerance (headache and gastrointestinal and skin complaints) | - | Statistically significant reduction of histamine-associated symptoms compared to placebo | [108] |
| Retrospective study with evaluation of the clinical response to DAO supplementation | 14 patients with diagnosis of histamine intolerance (headache and gastrointestinal, cardiovascular, respiratory and skin complaints) | 2 weeks | Reduction of at least one of the reported symptoms in 93% of patients | [84] |
| Double-blind, placebo-controlled, crossover study | 20 patients with chronic spontaneous urticaria | 1 month | Significant reduction of 7-Day Urticaria Activity Score (UAS-7) and slight significant reduction of daily antihistamine dose | [144] |
| Randomized, double-blind, placebo-controlled clinical trial | 100 patients with episodic migraine and serum DAO deficit | 1 month | Significant decrease in the duration of migraine attacks and decrease in triptans intake | [110] |
| Open-label interventional pilot study | 28 patients with histamine intolerance (gastrointestinal, cardiovascular, respiratory and skin complaints) and reduced serum DAO values | 1 month of intervention and 1 month of follow-up | Significant improvement in frequency and intensity of all symptoms. 61% of patients showed slightly increase in serum DAO values. During the follow-up period (without DAO supplementation), the symptoms sum scores increased and DAO levels decreased. | [109] |
The clinical trials developed by Yacoub et al. and Izquierdo-Casas et al. were focused on a single disorder related to histamine intolerance (chronic spontaneous urticaria and migraine, respectively) [110,144]. Yacoub et al. considered 20 patients with chronic spontaneous urticaria who showed a significant reduction in the severity of the complaint according to the Urticaria Activity Score (UAS-7) [144]. Regarding migraines, the randomized double-blind clinical trial conducted by Izquierdo et al. considered a larger number of patients (100 patients) and obtained a statistically significant decrease in the duration of pain attacks with no recorded adverse side effects [110]. However, the authors did not find statistical differences when considering other research outputs, such as the frequency and intensity of pain.
Overall, despite the promising results, more ambitious clinical studies with a rigorous experimental design, longer treatment periods and properly sized samples are essential to establish the clinical efficacy of this treatment.
6. Conclusions and Perspectives
Histamine intolerance is currently a clinical entity of increasing interest, which can appear due to the intake of histamine from foods, mainly caused by a deficiency of the DAO enzyme at the intestinal level. Novel knowledge and studies of histamine intolerance have helped clarify many of the uncertainties that were classically associated with histamine intoxication. Etiologically, various SNPs have been identified in the gene encoding the DAO enzyme related to lower enzyme activity. Moreover, certain inflammatory bowel diseases that limit enzyme secretion or some DAO-inhibiting drugs have also been identified as possible causes of DAO deficiency. This intolerance manifests through a plethora of nonspecific gastrointestinal and extraintestinal symptoms.
The diagnosis of histamine intolerance is usually performed after ruling out allergic symptoms and by the presence of at least two clinical manifestations and their improvement or remission after following a low-histamine diet. Various complementary tests are currently being proposed to improve the diagnosis of this intolerance based, among others, on determining the DAO activity in blood or intestinal biopsy samples or on identifying genetic or metabolic urinary markers by noninvasive techniques.
The clinical management is carried out mainly through the follow-up of a low-histamine diet, although there is no consensus on the list of foods to be excluded. Even so, there are different clinical studies that show the efficacy of this dietary intervention in improving the quality of life of patients with symptoms of histamine intolerance. Oral supplementation with exogenous DAO enzyme from porcine kidney is also being used to enhance the intestinal capacity to degrade dietary histamine. Although few works have assayed the clinical efficacy of this preventive treatment, promising results have been obtained so far. Research is currently also being made to identify new sources of DAO enzyme, especially of plant origin, due to its higher catalytic capacity and other potential productive and commercial advantages.
In this context, it is necessary to keep promoting the multidisciplinary study of this disorder, both from basic (i.e., analytical chemistry, food science, physiology and biochemistry) and clinically applied research, meant to increase the scientific base and the currently available diagnostic and treatment strategies for histamine intolerance.
Acknowledgments
Sònia Sánchez-Pérez is a recipient of a doctoral fellowship from the University of Barcelona (APIF2018).
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
1. EFSA Panel on Biological Hazards (BIOHAZ) Scientific Opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011;9:1–93. doi: 10.2903/j.efsa.2011.2393. [CrossRef] [Google Scholar]
2. Windaus A., Vogt W. Synthese des Imidazolyl-äthylamins. Berichte der Dtsch. Chem. Gesellschaft. 1907;40:3691–3695. doi: 10.1002/cber.190704003164. [CrossRef] [Google Scholar]
3. Comas-Basté O., Luz Latorre-Moratalla M., Sánchez-Pérez S., Teresa Veciana-Nogués M., del Carmen Vidal-Carou M. Histamine and Other Biogenic Amines in Food. From Scombroid Poisoning to Histamine Intolerance. In: Proestos C., editor. Biogenic Amines. IntechOpen; London, UK: 2019. [Google Scholar]
4. Dale H.H., Laidlaw P.P. The physiological action of β-iminazolylethylamine. J. Physiol. 1910;41:318–344. doi: 10.1113/jphysiol.1910.sp001406. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
5. Tansey E.M. The wellcome physiological research laboratories 1894–1904: The home office, pharmaceutical firms, and animal experiments. Med. Hist. 1989;33:1–41. doi: 10.1017/S0025727300048894. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
6. Riley J.F. Histamine and Sir Henry Dale. Br. Med. J. 1965;1:1488–1490. doi: 10.1136/bmj.1.5448.1488. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
7. Panula P., Chazot P.L., Cowart M., Gutzmer R., Leurs R., Liu W.L.S., Stark H., Thurmond R.L., Haas H.L. International union of basic and clinical pharmacology. XCVIII. histamine receptors. Pharmacol. Rev. 2015;67:601–655. doi: 10.1124/pr.114.010249. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
8. Vlieg-Boerstra B.J., van der Heide S., Oude Elberink J.N.G., Kluin-Nelemans J.C., Dubois A.E.J. Mastocytosis and adverse reactions to biogenic amines and histamine-releasing foods: What is the evidence? Neth. J. Med. 2005;63:244–249. [PubMed] [Google Scholar]
9. Russo P., Spano G., Arena M.P., Capozzi V., Fiocco D., Grieco F., Beneduce L. Are consumers aware of the risks related to biogenic amines in food. Curr Res. Technol. Edu. Top. Appl. Microbiol. Microb. Biotechnol. 2010:1087–1095. [Google Scholar]
10. Maintz L., Novak N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007;85:1185–1196. doi: 10.1093/ajcn/85.5.1185. [PubMed] [CrossRef] [Google Scholar]
11. Comas-Basté O., Latorre-Moratalla M.L., Bernacchia R., Veciana-Nogués M.T., Vidal-Carou M.C. New approach for the diagnosis of histamine intolerance based on the determination of histamine and methylhistamine in urine. J. Pharm. Biomed. Anal. 2017;145:379–385. doi: 10.1016/j.jpba.2017.06.029. [PubMed] [CrossRef] [Google Scholar]
12. Worm J., Falkenberg K., Olesen J. Histamine and migraine revisited: Mechanisms and possible drug targets. J. Headache Pain. 2019;20:30. doi: 10.1186/s10194-019-0984-1. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
13. Kovacova-Hanuskova E., Buday T., Gavliakova S., Plevkova J. Histamine, histamine intoxication and intolerance. Allergol. Immunopathol. (Madr.) 2015;43:498–506. doi: 10.1016/j.aller.2015.05.001. [PubMed] [CrossRef] [Google Scholar]
14. Elmore B.O., Bollinger J.A., Dooley D.M. Human kidney diamine oxidase: Heterologous expression, purification, and characterization. J. Biol. Inorg. Chem. 2002;7:565–579. doi: 10.1007/s00775-001-0331-1. [PubMed] [CrossRef] [Google Scholar]
15. Schwelberger H.G., Feurle J., Houen G. Mapping of the binding sites of human diamine oxidase (DAO) monoclonal antibodies. Inflamm. Res. 2018;67:245–253. doi: 10.1007/s00011-017-1118-3. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
16. Finney J., Moon H.J., Ronnebaum T., Lantz M., Mure M. Human copper-dependent amine oxidases. Arch. Biochem. Biophys. 2014;546:19–32. doi: 10.1016/j.abb.2013.12.022. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
17. Boehm T., Pils S., Gludovacz E., Szoelloesi H., Petroczi K., Majdic O., Quaroni A., Borth N., Valent P., Jilma B. Quantification of human diamine oxidase. Clin. Biochem. 2017;50:444–451. doi: 10.1016/j.clinbiochem.2016.12.011. [PubMed] [CrossRef] [Google Scholar]
18. Schwelberger H.G., Feurle J., Houen G. Mapping of the binding sites of human histamine N-methyltransferase (HNMT) monoclonal antibodies. Inflamm. Res. 2017;66:1021–1029. doi: 10.1007/s00011-017-1086-7. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
19. Schwelberger H.G. Histamine intolerance: Overestimated or underestimated? Inflamm. Res. 2009;58:51–52. doi: 10.1007/s00011-009-2004-4. [PubMed] [CrossRef] [Google Scholar]
20. Jarisch R., Wantke F., Raithel M., Hemmer W. Histamine Intolerance: Histamine and Seasickness. Springer; Berlin/Heidelberg, Germany: 2015. Histamine and biogenic amines; pp. 3–43. [Google Scholar]
21. Gludovacz E., Maresch D., De Carvalho L.L., Puxbaum V., Baier L.J., Sützl L., Guédez G., Grünwald-Gruber C., Ulm B., Pils S., et al. Oligomannosidic glycans at asn-110 are essential for secretion of human diamine oxidase. J. Biol. Chem. 2018;293:1070–1087. doi: 10.1074/jbc.M117.814244. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
22. Elsenhans B., Hunder G., Strugala G., Schümann K. Longitudinal pattern of enzymatic and absorptive functions in the small intestine of rats after short-term exposure to dietary cadmium chloride. Arch. Environ. Contam. Toxicol. 1999;36:341–346. doi: 10.1007/s002449900480. [PubMed] [CrossRef] [Google Scholar]
23. McGrath A.P., Hilmer K.M., Collyer C.A., Shepard E.M., Elmore B.O., Brown D.E., Dooley D.M., Guss J.M. Structure and inhibition of human diamine oxidase. Biochemistry. 2009;48:9810–9822. doi: 10.1021/bi9014192. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
24. Boehm T., Reiter B., Ristl R., Petroczi K., Sperr W., Stimpfl T., Valent P., Jilma B. Massive release of the histamine-degrading enzyme diamine oxidase during severe anaphylaxis in mastocytosis patients. Allergy Eur. J. Allergy Clin. Immunol. 2019;74:583–593. doi: 10.1111/all.13663. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
25. Maršavelski A., Petrović D., Bauer P., Vianello R., Kamerlin S.C.L. Empirical Valence Bond Simulations Suggest a Direct Hydride Transfer Mechanism for Human Diamine Oxidase. ACS Omega. 2018;3:3665–3674. doi: 10.1021/acsomega.8b00346. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
26. Smolinska S., Jutel M., Crameri R., O’Mahony L. Histamine and gut mucosal immune regulation. Allergy. 2014;69:273–281. doi: 10.1111/all.12330. [PubMed] [CrossRef] [Google Scholar]
27. Sjaastad Ö.V. Potentiation by aminoguanidine of the sensitivity of sheep to histamine given by mouth. Effect of aminoguanidine on the urinary excretion of endogenous histamine. Q. J. Exp. Physiol. Cogn. Med. Sci. 1967;52:319–330. doi: 10.1113/expphysiol.1967.sp001918. [PubMed] [CrossRef] [Google Scholar]
28. Sattler J., Häfner D., Klotter H.J., Lorenz W., Wagner P.K. Food-induced histaminosis as an epidemiological problem: Plasma histamine elevation and haemodynamic alterations after oral histamine administration and blockade of diamine oxidase (DAO) Agents Actions. 1988;23:361–365. doi: 10.1007/BF02142588. [PubMed] [CrossRef] [Google Scholar]
29. Klocker J., Mätzler S.A., Huetz G.-N., Drasche A., Kolbitsch C., Schwelberger H.G. Expression of histamine degrading enzymes in porcine tissues. Inflamm. Res. 2005;54(Suppl. 1):S54–S57. doi: 10.1007/s00011-004-0425-7. [PubMed] [CrossRef] [Google Scholar]
30. FAO (Food and Agriculture Organization of the United Nations) WHO (World Health Organization) Public Health Risks of Histamine and other Biogenic Amines from Fish and Fishery Products. Meeting Report. World Health Organization; Geneva, Switzerland: 2013. [Google Scholar]
31. Bover-Cid S., Latorre-Moratalla M.L., Veciana-Nogués M.T., Vidal-Carou M.C. Encyclopedia of Food Safety. Volume 2. Elsevier; Amsterdam, The Netherlands: 2014. Processing Contaminants: Biogenic Amines; pp. 381–391. [Google Scholar]