https://www.youtube.com/watch?v=45qMEOAkpug
Biomolecules. 2022 May; 12(5): 656.
Published online 2022 Apr 30. doi: 10.3390/biom12050656
PMCID: PMC9138604
PMID: 35625584
What Is Parvalbumin for?
Eugene A. Permyakov1,* and Vladimir N. Uversky1,2,*
Luigi Vitagliano, Academic Editor
Author information Article notes Copyright and License information PMC Disclaimer
Associated DataData Availability Statement
Abstract
Parvalbumin (PA) is a small, acidic, mostly cytosolic Ca2+-binding protein of the EF-hand superfamily. Structural and physical properties of PA are well studied but recently two highly conserved structural motifs consisting of three amino acids each (clusters I and II), which contribute to the hydrophobic core of the EF-hand domains, have been revealed. Despite several decades of studies, physiological functions of PA are still poorly known. Since no target proteins have been revealed for PA so far, it is believed that PA acts as a slow calcium buffer. Numerous experiments on various muscle systems have shown that PA accelerates the relaxation of fast skeletal muscles. It has been found that oxidation of PA by reactive oxygen species (ROS) is conformation-dependent and one more physiological function of PA in fast muscles could be a protection of these cells from ROS. PA is thought to regulate calcium-dependent metabolic and electric processes within the population of gamma-aminobutyric acid (GABA) neurons. Genetic elimination of PA results in changes in GABAergic synaptic transmission. Mammalian oncomodulin (OM), the β isoform of PA, is expressed mostly in cochlear outer hair cells and in vestibular hair cells. OM knockout mice lose their hearing after 3–4 months. It was suggested that, in sensory cells, OM maintains auditory function, most likely affecting outer hair cells’ motility mechanisms.
파발부민(PA)은
EF-hand 수퍼패밀리에 속하는
작고 산성이며
대부분 세포질 Ca2+ 결합 단백질입니다.

PA의 구조적 및 물리적 특성은 잘 연구되어 왔지만
최근에는 EF-손 도메인의 소수성 핵심에 기여하는
각각 3개의 아미노산으로 구성된 고도로 보존된
두 개의 구조 모티브(클러스터 I 및 II)가 밝혀졌습니다.
수십 년에 걸친 연구에도 불구하고
PA의 생리적 기능은 여전히 잘 알려져 있지 않습니다.
현재까지
PA에 대한 표적 단백질이 밝혀지지 않았기 때문에
PA는 느린 칼슘 완충제 역할을 하는 것으로 추정되고 있습니다.
다양한 근육 시스템에 대한 수많은 실험에서
PA가 빠른 골격근의 이완을 촉진하는 것으로 나타났습니다.
활성산소종(ROS)에 의한 PA의 산화는
형태 의존적이며,
빠른 근육에서 PA의 또 다른 생리적 기능은 ROS로부터
이러한 세포를 보호하는 것일 수 있다는 것이 밝혀졌습니다.
PA는
감마 아미노부티르산(GABA) 뉴런 집단 내에서
칼슘 의존적 대사 및 전기적 과정을 조절하는 것으로 생각됩니다.
PA를 유전적으로 제거하면
GABA성 시냅스 전달에 변화가 생깁니다.
PA의 β 이소형인 포유류 온코모둘린(OM)은
주로 달팽이관 외유모세포와 전정유모세포에서 발현됩니다.
OM 녹아웃 마우스는
3-4개월 후에 청력을 상실합니다.
감각 세포에서 OM은
청각 기능을 유지하며
외유모세포의 운동 메커니즘에 영향을 미칠 가능성이 높다고 제안되었습니다.
Keywords: parvalbumin, oncomodulin, structure, stability, calcium binding, physiological functions
1. Introduction
Parvalbumin (PA) is a small, acidic, mostly cytosolic Ca2+-binding protein of the EF-hand superfamily. It was found in lower and higher vertebrates, including humans (for reviews, see [1,2,3,4]). PA was found in fast twitch muscle cells, heart tissue, nephrons, specific neurons of the central and peripheral nervous system, certain cells of several endocrine glands, and sensory cells of the mammalian auditory organ, the organ of Corti, and some other cells.
Intracellular PA was suggested and partially proved to serve as a soluble relaxing factor accelerating the Ca2+-mediated relaxation phase in fast muscles (reviewed in [1]). It is assumed that PA serves as a slow metal buffer in non-muscle tissues, modulating flows of metal ions. An extracellular form of PA was found in some biological systems [5,6,7]. Biological functions proposed for extracellular PA include antibacterial, chemoattractant, and immunomodulatory actions.
Despite many years of research on PAs, which have provided a good understanding of their structure and physicochemical properties, the physiological functions of PA are still far from being clear. This short review covers some new findings on structural and physicochemical properties of PAs and focuses on their potential physiological significance.
파발부민(PA)은 EF-손 수퍼패밀리에 속하는
작고 산성이며 대부분 세포질 Ca2+ 결합 단백질입니다.
인간을 포함한
하등 척추동물과 고등 척추동물에서 발견되었습니다(리뷰는 [1,2,3,4] 참조).
PA는
빠른 경련 근육 세포, 심장 조직,
네프론,
중추 및 말초 신경계의 특정 뉴런,
여러 내분비선의 특정 세포,
포유류 청각 기관인 코르티 기관의 감각 세포 및 기타 일부 세포에서 발견되었습니다.
세포 내 PA는
빠른 근육에서 Ca2+ 매개 이완 단계를 가속화하는
가용성 이완 인자로 작용하는 것으로 제안되었고
부분적으로 입증되었습니다([1]에서 검토됨).
PA는
비근육 조직에서
느린 금속 완충 역할을 하여
금속 이온의 흐름을 조절하는 것으로 추정됩니다.
일부 생물학적 시스템에서 세포 외 형태의 PA가 발견되었습니다[5,6,7].
세포 외 PA에 대해 제안된 생물학적 기능으로는 항균, 화학 유인, 면역 조절 작용 등이 있습니다.
PA에 대한 수년간의 연구로
PA의 구조와 물리화학적 특성에 대한 이해가 높아졌지만,
PA의 생리적 기능은 아직 명확하게 밝혀지지 않았습니다.
이 짧은 리뷰에서는
PA의 구조 및 물리화학적 특성에 대한
몇 가지 새로운 발견을 다루고
잠재적인 생리적 중요성에 초점을 맞춥니다.
2. Parvalbumin Structure
PA consists of 106–113 amino acid residues and its molecular mass is ~11–12 kDa. Isoelectric points of PAs are within the range of 3.9 to 6.6 (see Table 1). PAs are characterized by very low content of Trp (a single residue only in several fish species) and Tyr (absent or present as 1 or 2 residues), but high content of Phe residues (9–11 per molecule). These amino acid composition biases are reflected in the characteristic ultraviolet absorption spectra of PAs with clearly visible fine vibrational peaks of the Phe chromophores.
PA는
106~113개의 아미노산 잔기로 구성되며
분자량은 ~11~12kDa입니다.
PA의 등전점은 3.9~6.6 범위 내에 있습니다( 표 1 참조).
PA는
Trp(몇몇 어종에만 존재하는 단일 잔기)와
Tyr(1개 또는 2개의 잔기로 존재)의 함량이 매우 낮고,
Phe 잔기(분자당 9~11개)의 함량이 높은 것이 특징입니다.
이러한
아미노산 조성 편향은
PA의 특징적인 자외선 흡수 스펙트럼에 반영되며,
Phe 발색단의 미세한 진동 피크가 뚜렷하게 보입니다.
Table 1
Number of amino acid residues, pI values, and molecular masses of some parvalbumins (corresponding data are derived from the UniProt database).
Parvalbumin (UniProt ID)Number of Amino AcidspIMolecular Mass (Da)
| Pike α-PA (P02628) | 108 | 5.0 | 11,707 |
| Pike β-PA (P02619) | 107 | 4.2 | 11,390 |
| Cod β-PA (P02622) | 113 | 4.4 | 12,108 |
| Rat α-PA (P02625) | 110 | 5.5 | 11,926 |
| Rat β-PA oncomodulin (P02631) | 109 | 4.4 | 12,188 |
The PA family diverged into α and β sub-lineages [8,9,10]. β-PAs are also referred to as ‘oncomodulins’. The α and β isoforms differ in isoelectric point (α: pI > 5; β: pI < 5) and show differences in amino acid residues in at least 11 positions [10]. β-PAs are characterized by the presence of Cys in the loop of the AB domain and the absence of an additional C-terminal residue in the C-terminal helix. Despite the fact that the primary structures of α and β PAs differ significantly, their three-dimensional structures are very close.
PA molecule is a compact globule composed mostly of α-helices and a small amount of β-strands (reviewed in [1]) (Figure 1). Kretsinger and coworkers [11,12] determined the crystal structure of carp β-PA and revealed that a Ca2+-binding domain is composed of two α-helices linked by a Ca2+-binding loop creating a structural motif, which is currently known as ‘EF-hand’.
PA 계열은 α와 β 하위 계통으로 나뉩니다[8,9,10].
β-PA는 '온코모듈린'이라고도 합니다. α와 β 동형체는 등전점이 다르며(α: pI > 5, β: pI < 5), 최소 11개 위치에서 아미노산 잔기의 차이를 보입니다[10]. β-PA는 AB 도메인의 루프에 Cys가 존재하고 C-말단 나선에 추가적인 C-말단 잔기가 없는 것이 특징입니다. α와 β PA의 기본 구조는 크게 다르지만 3차원 구조는 매우 유사합니다.
PA 분자는 대부분 α-나선과 소량의 β-가닥으로 구성된 조밀한 소구입니다([1]에서 검토됨)(그림 1). Kretsinger와 동료들[11,12]은 잉어 β-PA의 결정 구조를 결정하고 Ca2+ 결합 도메인이 두 개의 α-헬기로 구성되어 있으며, 이 구조적 모티프가 현재 'EF-hand'로 알려져 있음을 밝혀냈습니다.

Ribbon model of the 3D-structure of rat parvalbumin (oncomodulin) (PDB code 1rro) with two bound calcium ions. Three EF-hand domains are shown by different colors. Helices are marked with letters from A to F. Ca2+ coordinating Asp and Glu residues are shown.
Ca2+-binding loop of the EF-hand usually contains the most commonly observed consensus sequence DxDxDG. The negatively charged Ca2+-liganding oxygen atoms donated by carboxyl and carbonyl groups are located at vertices of a distorted octahedron. The side chains of loop residues at positions 1, 3, 5, and 12 provide the +x, +y, +z, and −z oxygen ligands, respectively. Water molecule oxygen is located at the −x position, and a backbone carbonyl oxygen occupies the −y position. In some cases, the carboxylate at −z position coordinates calcium by both oxygens and the octahedral geometry of the Ca2+ binding site turns into a distorted pentagonal pyramid geometry.
PA molecule contains two active EF-hands, CD and EF domains. The AB domain of PA cannot bind Ca2+, since its AB-loop is too short and the DxDxDG motif is disturbed. Meanwhile, the AB domain shields the hydrophobic core of the protein and the hydrophobic parts of the functional EF-hands from solvent and influences their calcium affinities [13,14,15]. The CD and EF loops are connected by a short antiparallel β-sheet between them. Conservative Arg75 and Glu81 residues (numbering according to carp β-PA) form a salt bridge protected from solvent by the AB domain (see Figure 2).
두 개의 칼슘 이온이 결합된 쥐 파발부민(온코모둘린)의 3D 구조 리본 모델(PDB 코드 1rro). 세 개의 EF-손 도메인은 서로 다른 색상으로 표시되어 있습니다. 나선은 A부터 F까지 문자로 표시되어 있으며, Ca2+와 결합하는 Asp 및 Glu 잔기가 표시되어 있습니다.
EF-손의 Ca2+ 결합 루프는 일반적으로 가장 일반적으로 관찰되는 컨센서스 서열 DxDxDG를 포함합니다. 카르복실기와 카르보닐기에 의해 기증된 음전하를 띤 Ca2+-리간딩 산소 원자는 왜곡된 팔면체의 꼭짓점에 위치합니다. 위치 1, 3, 5, 12에 있는 고리 잔기의 측쇄는 각각 +x, +y, +z, -z 산소 리간드를 제공합니다. 물 분자 산소는 -x 위치에 위치하며, 백본 카르보닐 산소는 -y 위치를 차지합니다. 경우에 따라 -z 위치에 있는 카르복실레이트는 두 산소에 의해 칼슘을 조정하고 Ca2+ 결합 부위의 팔면체 형상이 왜곡된 오각형 피라미드 형상으로 바뀝니다.
PA 분자는 두 개의 활성 EF-손, 즉 CD 도메인과 EF 도메인을 포함합니다. PA의 AB 도메인은 AB-루프가 너무 짧고 DxDxDG 모티프가 방해받기 때문에 Ca2+와 결합할 수 없습니다. 한편, AB 도메인은 단백질의 소수성 코어와 기능성 EF-손의 소수성 부분을 용매로부터 보호하고 칼슘 친화도에 영향을 미칩니다 [13,14,15]. CD와 EF 루프는 그 사이에 짧은 반평행 β-시트로 연결됩니다. 보수적인 Arg75 및 Glu81 잔기(잉어 β-PA에 따른 번호
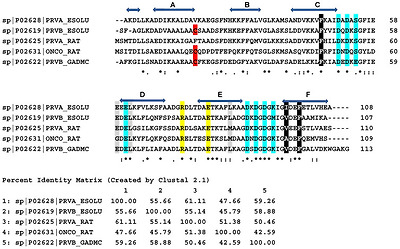
Amino acid sequences of pike α- and β-PAs, cod β-PA, and rat α- and β-PAs. Horizontal blue arrows show amino acids forming α-helices A through F in the native structure of pike α-PA. The consensus sequence characterizes identical residues as ‘*’, strongly similar residues as ‘:’, and weakly similar residues as ‘.’. Conservative Cys residue in β-PAs is shown in red. Amino acids taking part in calcium coordination are shown in cyan. Conservative Arg and Glu residues forming a salt bridge in the native structure of all PAs are shown in yellow. The data are taken from the UniProt database. Amino acids forming conservative clusters I and II are shown in black and gray, respectively. Percent identity matrix is also shown. Sequences were aligned by Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/; accessed on 23 April 2022).
Some of the PAs contain cysteine residues (up to four, as in β-PA of Atlantic salmon), but they do not form S–S links under the non-oxidizing conditions.
Comparative analysis of the spatial structures of proteins of the EF-hand superfamily, including PA, made it possible to discover two highly conserved structural motifs, which form a supporting scaffold for the Ca2+-binding loops [16]. Each conserved structural motif forms a cluster consisting of three amino acids: cluster I (‘black’ cluster) and cluster II (‘grey’ cluster) (Figure 2 and Figure 3). Cluster I (‘black’) is most conserved and consists of mostly aromatic amino acids. The cluster is stabilized by a set of linked CH-π and CH-O hydrogen bonds between the side chains of the amino acids. Cluster I was suggested to be important for structural stabilization of the paired EF-hand domain in its region, where the polypeptide chain enters and exits the domain [16]. In contrast, cluster II consists of a mix of aromatic, hydrophobic, and polar amino acids. It is much less conserved and lacks stabilizing interactions. It has been suggested that the higher variability of cluster II (‘gray’) could facilitate adaptation of the EF-hand domain to various conformational and dynamic changes [16].
파이크 α-PA와 β-PA, 대구 β-PA, 쥐 α-PA와 β-PA의 아미노산 서열. 가로 파란색 화살표는 파이크 α-PA의 기본 구조에서 α-나선 A부터 F까지를 형성하는 아미노산을 나타냅니다. 합의 서열은 동일한 잔기는 '*', 매우 유사한 잔기는 ':', 약하게 유사한 잔기는 '.'로 표시합니다. β-PA의 보수적인 Cys 잔기는 빨간색으로 표시되어 있습니다. 칼슘 조정에 참여하는 아미노산은 시안색으로 표시됩니다. 모든 PA의 기본 구조에서 염교를 형성하는 보수적인 Arg 및 Glu 잔기는 노란색으로 표시되어 있습니다. 데이터는 UniProt 데이터베이스에서 가져온 것입니다. 보수적 클러스터 I과 II를 형성하는 아미노산은 각각 검은색과 회색으로 표시되어 있습니다. 퍼센트 동일성 매트릭스도 표시됩니다. 서열은 클러스터 오메가(https://www.ebi.ac.uk/Tools/msa/clustalo/, 2022년 4월 23일에 액세스)에 의해 정렬되었습니다.
일부 PA에는 시스테인 잔기가 포함되어 있지만(대서양 연어의 β-PA에서와 같이 최대 4개까지), 비산화 조건에서는 S-S 링크를 형성하지 않습니다.
PA를 포함한 EF-손 수퍼패밀리 단백질의 공간 구조를 비교 분석하여 Ca2+ 결합 루프의 지지 발판을 형성하는 고도로 보존된 두 가지 구조 모티프를 발견할 수 있었습니다[16]. 보존된 각 구조 모티프는 세 개의 아미노산으로 구성된 클러스터 I('검은색' 클러스터)과 클러스터 II('회색' 클러스터)를 형성합니다(그림 2 및 그림 3). 클러스터 I('검은색')은 가장 많이 보존되어 있으며 대부분 방향족 아미노산으로 구성되어 있습니다. 이 클러스터는 아미노산의 측쇄 사이에 연결된 일련의 CH-π 및 CH-O 수소 결합에 의해 안정화됩니다. 클러스터 I은 폴리펩타이드 사슬이 도메인에 들어오고 나가는 영역에서 쌍을 이루는 EF-손 도메인의 구조적 안정화에 중요한 것으로 제안되었습니다 [16]. 이와 대조적으로 클러스터 II는 방향족, 소수성, 극성 아미노산이 혼합되어 있습니다. 보존성이 훨씬 떨어지고 안정화된 상호작용이 부족합니다. 클러스터 II('회색')의 높은 가변성은 다양한 형태 및 동적 변화에 대한 EF-손 도메인의 적응을 용이하게 할 수 있다고 제안되었습니다 [16].

Ribbon model of the 3D-structure of rat parvalbumin (oncomodulin) (PDB code 1rro) with two bound calcium ions. Clusters I (‘black’) and II (‘gray’) are shown.
Figure 3 shows three-dimensional (3D) structure of rat β-PA (oncomodulin) with its clusters I (‘black’) and II (‘gray’), consisting of F48, A100, and F103; and G61, L64, and M87, respectively. The amino acids of the clusters I and II were sequentially substituted by alanine and physical properties of the resulting mutant proteins were studied [17]. In spite of rather complicated patterns of effects of separate residue substitutions in the clusters I and II, the Ala substitutions in cluster I cause noticeably more pronounced changes in various structural parameters of proteins, such as hydrodynamic radius of apo-form, thermal stability of Ca2+- and Mg2+-loaded forms, and total energy of Ca2+ binding in comparison with the changes caused by similar amino acid substitutions in the cluster II. In agreement with these experimental results, it was found that local intrinsic disorder propensities and the overall levels of predicted disorder in rat β-PA are strongly affected by mutations in cluster I, whereas mutations in cluster II generate less pronounced effects [2]. These results demonstrated that the amino acids of the cluster I provide more essential contribution to the maintenance of structural and functional properties of the protein in comparison with the residues of the cluster II.
3. Binding of Metal Ions to Parvalbumin
The general Scheme 1 describing the binding of Ca2+ by a protein with two binding sites is as follows [1,18]:

The general scheme of binding of Ca2+ by a protein with two binding sites.
In the Scheme 1, P is a protein. It means that, in general cases, the binding of Ca2+ to PA occurs in two ways when the CD and EF binding sites are filled by Ca2+ simultaneously. At the same time, experiments carried out on mutant human and rat parvalbimins demonstrate that the substitution of one of the Ca2+ chelating Glu by Gln in the CD site completely abolishes the high metal affinity of parvalbumins, whereas similar mutation in the EF site abolishes the high affinity metal binding to the EF site and only causes a decrease in the metal affinity of the CD site by an order of magnitude [19]. This suggests that the binding of Ca2+/Mg2+ to parvalbumin is a sequential process (1) and the CD site is occupied first
P ↔ PCa ↔ CaPCa
(1)
The results of many other studies carried out by various methods also suggested the sequential mechanism of Ca2+ binding to PA (reviewed in [1]). At the same time, there is a disagreement in the literature regarding the sequence of the filling the CD and EF binding sites with calcium. According to our experimental data, the CD site seems to be filled by Ca2+ first, followed by the filling of the EF site [19]. Pauls et al. [20] found that inactivation of the EF site in rat PA, much more than of the CD site, impairs divalent cation binding. These discrepancies can be explained by the fact that Pauls et al. studied mutants with multiple substitutions, which were very different from those used in our work and under very different conditions.
PAs are characterized by extremely high affinity to Ca2+ ions. In fact, equilibrium Ca2+-association constants of PAs measured at low ionic strength lie in the range from 108 to 1010 M−1 (reviewed in [1]). Mg2+ ions compete with Ca2= ions for the same two binding sites. The equilibrium Mg2+-binding constants of PAs measured at low ionic strength are within the range from 104 to 105 M−1 (reviewed in [1]). PA also binds Na+ and K+ ions with equilibrium association constants in the range from 0.1 M−1 to 50 M−1, and Na+ binds an order of magnitude more strongly than K+ (reviewed in [1]).
Equilibrium metal association constant Ka is the ratio of the kinetic constants of association (kon, M−1s−1) and dissociation (koff, s−1) (2):
Ka = kon/koff
(2)
Fluorescent stopped flow method was used to study kinetics of Ca2+ and Mg2+ dissociation from cod PA after rapid mixing of the metal bound protein with a strong metal chelator, EDTA [21]. Analysis of the experimental kinetic curves showed that the koff values for Ca2+ and Mg2+ are rather close to each other in the temperature region from 10 to 30 °C. Two exponential terms in the kinetic curves were interpreted as Ca2+/Mg2+ dissociation from the two binding sites of PA. The values of dissociation rate constants obtained from these analyses at temperatures from 10 °C to 30 °C are within the range 0.03–0.8 s−1 and 0.2–5 s−1 for Ca2+-PA complex and 0.9–4.5 s−1 and 4–33 s−1 for Mg2+-PA complex. It means that the differences in the equilibrium binding constant values for Ca2+ and Mg2+ (Ka) are mostly due to the differences in the kon values. The kon values for Ca2+ calculated from these data are within the range from 106 to 109 M−1s−1 (temperature range 10 to 30 °C); i.e., they are close to the diffusion-controlled limit. The kon values for Mg2+ are 2 to 5 orders of magnitude lower. The drastically lower kon values for Mg2+ this can be explained by the fact that Mg2+ ion in aqueous solution is much more strongly hydrated compared to Ca2+ ion due to the fact that it has 3.5 times higher charge density.
Analysis of the 3D structures of the EF-hand proteins with and without bound calcium showed that the binding of calcium does not dramatically change interactions within cluster I (contact area with and without bound calcium averaging 89 ± 12 Å2 and 84 ± 11 Å2, respectively) [16]. In some EF-hand Ca2+ binding proteins, the interaction with calcium also slightly changes the contact surface area in the cluster II (68 ± 12 Å2). In the other group of Ca2+ binding proteins, calcium binding results in a dramatic increase in the interactions between the three residues of cluster II (contact surface area 11 ± 8 Å2). PA belongs to the first group of proteins: the binding of Ca2+ does little to change the interactions in both conservative clusters [16].
Removal of bound metal ions from PA (formation of apo-form) makes its structure looser and makes many of its groups more accessible to the solvent (reviewed in [1]). The removal of Ca2+ from PA results in a decrease in its α-helical content [22,23,24]. Interestingly, character of the Ca2+-induced changes in α-PAs and β-PAs is different [25,26]. Rat α-PA in the apo- and Ca2+-loaded state has approximately the same three-dimensional structure. Ca2+ binding to α-PA causes changes mainly in the structure of the loop regions. In contrast to this, Ca2+ binding to rat β-PA results in a conformational change in the AB domain; rotation of the C, D, and E helices; changes in interdomain contacts; and rearrangement of the hydrophobic core. However, all these changes do not affect the overall protein folding. In the Ca2+-loaded rat β-PA, Phe70 is buried in the protein core, but in the apo-protein is located near the protein surface. Similarly, the single Trp of cod or whiting β-PA is buried deep inside the Ca2+-loaded protein, but Ca2+ removal causes a movement of this Trp to the protein surface [27].
Thermal stability of apo-PA is low: mid-transition temperature, Tm, of apo-PAs under low ionic strength is within the range from 30 °C to 50 °C [28,29,30]. Calcium binding causes a pronounced shift of thermal transition to higher temperatures (up to 120 °C in some cases) [27]. Interestingly, the scanning calorimetry method revealed the lack of fixed tertiary structure in some apo-PAs, for example in pike apo-α-PA, which is reflected in the absence of ‘all-or-none’ thermal transitions for these proteins [24]. This is consistent with the facts that Ca2+ removal from such PAs decreases their α-helical content, and increases mobility of the aromatic residue environment, monitored by near-UV circular dichroism method. These results show that pike apo-α-PA is in the classical molten globule state [31,32,33]. At the same time, the apo-form of pike β-PA is characterized by a much less conserved secondary structure, and this does not allow us to attribute this state to the classical state of molten globule. Intrinsic disorder analysis of PAs with folded and unfolded apo-state using the predictor of naturally disordered regions PONDR® VSL2 showed that the N-terminal region of PA—including α-helix A, AB-loop, and N-terminal half of α-helix B—was predicted to be less ordered in PAs with disordered apo-state [34]. It has been shown that PAs with disordered apo-state comprise about 16–19% of all PAs.
Thermal denaturation of Ca2+- and Mg2+-loaded states of five PAs (cod PA, α and β isoforms of pike, and rat PAs) was studied by scanning calorimetry method in a temperature range from 15 °C to 125 °C [29]. The proteins were divided into three groups according to their different thermal behavior. The proteins of the first group have a single heat sorption peak in their Ca2+-loaded state (rat PAs). The second group is characterized by two distinct heat-sorption peaks in their Ca2+-loaded state (cod PA and pike α-PA). The unfolding behavior of the proteins of the last group (pike β-PA) above 100 °C is complicated by an oligomerization process. An analysis of the thermal denaturation curves of the Ca2+-bound PAs of the first group showed that their unfolding is not described by the simple ‘all-or-none’ scheme, and the process proceeds with a formation of an intermediate state. The multistage character of the thermal unfolding is evident in the melting curves of the PAs of the second group (pike α-PA and cod PA). They are characterized by two distinct heat-sorption peaks separated by 20–30 °C [29].
In contrast to Ca2+-bound state, Mg2+- and Na+-bound states of pike α-PA are characterized by a single heat sorption peak [29]. Mg2+ binding causes expectedly less pronounced stabilizing effect in comparison with that induced by Ca2+ binding (Tm is ca 77 °C in 1 mM MgCl2, compared with 90 °C in 1 mM CaCl2). Na+ binding results in even less pronounced stabilization (Tm is ca 33 °C in 300 mM NaCl). The thermal unfolding of both Mg2+- and Na+-loaded states of pike α-PA obeys the simple ‘all-or none’ mechanism.
These results show that the exchange of Mg2+ by Ca2+ ions in the PA binding sites seriously alters its thermodynamic properties: the simple ‘all-or-none’ transition converts into a more complex multistage transition. Moreover, depending on its interactions with metal ions, PA (for example, pike PA) can be an intrinsically disordered protein (apo-form) or an ordered mesophilic (Na+-bound state), thermophilic (Mg2+-bound state), or even hyperthermophilic (Ca2+-bound state) protein [29].
The additional peak in the heat-sorption curves of some Ca2+-loaded PAs seems to arise due to stabilization of an intermediate state, which is absent in PAs exhibiting single peak in their heat-sorption curves. It has been found [29] that the structure of pike α-PA, which demonstrates two heat sorption peaks during thermal unfolding, is notably different from the structure of three other PAs studied: it has nine interior cavities located mostly between the EF and CD subdomains versus four to six in other PAs. Moreover, the total area (S) and volume (V) of the cavities in pike α-PA exceeds the S and V values of other PAs by more than 40%. This indicates that the overall packing density of pike α-PA is essentially lower than that of other PAs. The PONDR® analysis showed the EF subdomain is included in the most stable thermodynamic domain in pike α-PA, whereas the CD and AB subdomains are contained in the less stable thermodynamic domain.
It is well known that Ca2+/Mg2+ exchange in the binding sites of PAs results in alternation in the metal ion coordination mode: the coordination number decreases from 7 in the Ca2+-bound state to 6 in the Mg2+-bound state [35,36,37]. The Glu residue at position 12 in the EF-hand loop sequence (gateway ‘Glu12′) acts as a bidentate ligand in the Ca2+-bound state and as a monodentate ligand in the Mg2+-bond state, which can change the protein conformation and seriously affect thermodynamic properties of the protein [29].
4. Interactions with Peptides and Membranes
So far, no target proteins have been found for rat or mouse PA. At the same time, it was shown that under certain conditions, PA is able to interact with short peptides. It was found that pike PAs pI 4.2 and 5.0 bind amphiphilic bee venom peptide melittin (26 amino acid residues) [38]. In apo-state, the PAs form a tight equimolar complex with melittin (binding constant 106 M−1 at 18 °C), while their Ca2+- and Mg2+-loaded states do not bind this peptide [38]. Heating of the apo-PAs up to the temperatures above their thermal unfolding transition does not change the stoichiometry of the complexes, but increases their association constants by an order of magnitude (binding constant 107 M−1 at 44 °C). Isolated Ca2+-binding fragment 38–108 of pike PA pI 5.0 (CD-EF) retains the ability for Ca2+-inhibited equimolar binding of melittin.
In contrast, the well-known Ca2+-sensor protein of the EF-hand family, calmodulin, binds melittin with a very high binding constant 1010 M−1 in the Ca2+loaded state [39,40]. In the absence of Ca2+, calmodulin binds melittin with a much lower affinity, with the binding constant 105 M−1 [40].
Since both intracellular and extracellular PA is always found in an environment with high concentrations of divalent calcium and magnesium cations, it almost never exists in the apo-state. For this reason, its interaction with the amphiphilic peptide, discovered in [38], is unlikely to have a physiological significance.
It has been found that cod and pike PAs interact with model synthetic (dipalmitoylphosphatidylcholine, DPPC) and natural (phosphatidylcholine and phospatidylethanolamine) vesicles (mean diameter 300–500 Å) [41]. It has been found that the binding of Ca2+ and Mg2+ ions to PA modulates its interaction with the DPPC vesicles. The interaction of the liposomes with Ca2+-bound PA shifts its thermal transition towards higher temperatures by 2 °C to 3 °C, while the interaction of the liposomes with the metal-free and Mg2+-bound protein results in similar shift of the thermal transition but towards lower temperatures. Moreover, the interaction of the protein with the liposomes causes changes in the calorimetric heat sorption peak of model synthetic liposomes. The denaturation curves measured by tryptophan intrinsic fluorescence method for free and liposome-bound cod PA are strikingly different. The thermally induced red spectral shift for the liposome-bound protein, which reflects an unfolding of the protein, begins at 10 °C lower temperatures than that for free PA, but the shift magnitude is less pronounced in this case. Tryptophan residue in the thermally denatured liposome-bound cod PA remains inaccessible to water, while the thermal unfolding of the free protein causes a translocation of its single Trp to the protein surface.
Interestingly, PA immunoreactivity was found in (or near) membranous systems, such as mitochondria and (or) microtubule in male gonads of Drosophila melanogaster [42].
지금까지 쥐 또는 마우스 PA에 대한 표적 단백질은 발견되지 않았습니다. 동시에 특정 조건에서 PA는 짧은 펩티드와 상호 작용할 수 있는 것으로 나타났습니다. 파이크 PA pI 4.2와 5.0은 양친매성 봉독 펩티드 멜리틴(26개의 아미노산 잔기)과 결합하는 것으로 밝혀졌습니다[38]. 아포 상태에서 PA는 멜리틴과 긴밀한 등몰 복합체를 형성하지만(18°C에서 결합 상수 106 M-1), Ca2+- 및 Mg2+- 로드 상태에서는 이 펩티드와 결합하지 않습니다[38]. apo-PA를 열적 전개 전이 온도 이상으로 가열해도 복합체의 화학량론은 변하지 않지만 결합 상수는 크게 증가합니다(44°C에서 결합 상수 107 M-1). 분리된 Ca2+-결합 단편 38-108의 파이크 PA pI 5.0(CD-EF)은 멜리틴의 Ca2+-억제 등몰 결합 능력을 유지합니다.
이와 대조적으로, EF-손 계열의 잘 알려진 Ca2+ 센서 단백질인 칼모둘린은 Ca2+가 로드된 상태에서 매우 높은 결합 상수 1010 M-1로 멜리틴과 결합합니다[39,40]. Ca2+가 없는 경우 칼모둘린은 훨씬 낮은 친화력으로 멜리틴과 결합하며, 결합 상수는 105 M-1입니다[40].
세포 내 및 세포 외 PA는 항상 고농도의 2가 칼슘 및 마그네슘 양이온이 있는 환경에서 발견되기 때문에 아포 상태에서는 거의 존재하지 않습니다. 이러한 이유로 [38]에서 발견된 양친매성 펩타이드와의 상호 작용은 생리적으로 중요한 의미를 갖지 않을 것으로 보입니다.
대구와 파이크 PA는 모델 합성(디팔미토일포스파티딜콜린, DPPC) 및 천연(포스파티딜콜린 및 포스파티딜에탄올아민) 소포(평균 직경 300-500 Å)와 상호 작용하는 것으로 밝혀졌습니다[41]. Ca2+ 및 Mg2+ 이온이 PA에 결합하면 DPPC 소포와의 상호 작용이 조절되는 것으로 밝혀졌습니다. 리포솜과 Ca2+ 결합 PA의 상호작용은 열 전이를 2°C에서 3°C까지 더 높은 온도로 이동시키는 반면, 리포솜과 무금속 및 Mg2+ 결합 단백질의 상호작용은 비슷한 열 전이를 일으키지만 더 낮은 온도로 이동시킵니다. 또한 단백질과 리포솜의 상호작용은 모델 합성 리포솜의 열량 측정 열 흡착 피크에 변화를 일으킵니다. 트립토판 고유 형광법으로 측정한 자유 및 리포솜에 결합된 대구 PA의 변성 곡선은 현저하게 다릅니다. 리포솜 결합 단백질의 열 유도 적색 스펙트럼 이동은 단백질의 폴딩을 반영하며, 유리 PA보다 10°C 낮은 온도에서 시작되지만 이 경우 이동 크기가 덜 두드러집니다. 열 변성된 리포솜 결합 대구 PA의 트립토판 잔류물은 물에 접근하지 못하는 반면, 유리 단백질의 열적 전개는 단일 Trp를 단백질 표면으로 전위시킵니다.
흥미롭게도, PA 면역 반응성은 미토콘드리아 및 (또는) 초파리 수
5. Possible Functions of Parvalbumin
5.1. Parvalbumin in Muscle Cells
Of the possible biological functions of PA, perhaps the one that has been explored the most is that in muscle cells. It has long been known that PA concentration in muscles correlates with the speed of muscle contraction and relaxation. PA concentration in sarcoplasm of fast skeletal muscles reaches a millimolar level, while slow-twitch muscles are characterized by about two orders of magnitude lower levels of PA [43,44,45]. The positive correlation between PA content and relaxation rate of muscles was found and this led to the assumption that PA could facilitate Ca2+ translocation within the sarcoplasm and therefore could be a relaxation factor in fast-twitch muscles [43,44].
Free Ca2+ concentration in sarcoplasm of a resting muscle is very low (<1 μM), while free Mg2+ concentration in the muscle is in the millimolar region. For this reason, PA in the sarcoplasm of a resting muscle seems to be loaded by Mg2+ ions. Ca2+ transiently released from the sarcoplasmic reticulum diffuses through the sarcoplasm and activates muscle contraction interacting with troponin complex located on the actin fibrils. PA is located in the sarcoplasm of muscle cells right on the way of the Ca2+ movement, but since the exchange of Mg2+ for Ca2+ in the binding sites of PA is governed by the relatively slow process of Mg2+ dissociation, Ca2+ ions can reach the Ca2+-specific sites of troponin C [46]. Later on, the strong Ca2+/Mg2+-sites of PA will compete for Ca2+ and promote its removal from troponin C, which will start muscle relaxation. Since the dissociation rate of Mg2+ in PA is low, it is reasonable to think that PA will not have time to remove calcium during a single contraction–relaxation cycle (twitch), especially in fast muscles. Instead, the Mg2+-Ca2+ exchange in PA can only happen after many successive twitches (after tetanus). Below are the results of some experiments indicating the important role of PA in accelerating the relaxation of fast muscles.
Cross-reinnervation of slow- and fast-twitch muscles results in a decrease in PA content in the fast-twitch muscle, and to an increase in PA content in the slow muscle [47,48]. PA concentrations in fast muscle fibers of small mammals are higher than in fast muscle fibers of big mammals because of the essential difference in their contraction and relaxation rates [49].
Direct gene transfer experiments were carried out to check whether PA really could work as a relaxing factor. PA cDNA was transferred in vivo to normal and regenerating rat soleus muscles, which do not normally synthesize PA [50]. Considerable concentrations of PA mRNA and PA appeared in uninjured and regenerating muscles two weeks after the transfection, which caused a shortening of half-relaxation time of these muscles. In another work, it was found that knock out of PA gene in mice fast-contracting/relaxing muscles results in slowing down the decay of Ca2+ level after a 20-ms stimulation of the isolated exterior digitorum longus (33% lower rate constant of Ca2+ decay), and in a reduction in the time to reach peak twitch tension [51].
It was found that the relaxation rate of extensor digitorum longus in PA-deficient mice at 20 °C is low and does not depend on tetanus duration (<3.2 s) [52]. In contrast, the relaxation rate of normal wild type muscles decreases when tetanus duration increases from 0.2 to 3.2 s and the fast relaxation is restored with increasing rest interval. The slowing of the relaxation stage caused by the tetanus duration increase was explained by a saturation of PA by Ca2+, while the fast relaxation recovery after the increase in rest interval by a formation of Ca2+-free PA. In flexor digitorum brevis muscles, the effects of tetanus duration on wild type and PA-deficient muscles were qualitatively similar to those observed in extensor digitorum longus muscles. The authors concluded that PA accelerates calcium movement from myofibrils to sarcoplasmic reticulum, mostly after tetanus.
The superfast toadfish swimbladder muscle, the fastest vertebrate muscle known [53], is characterized by the highest PA concentration (1.20–1.35 mM) [54,55]. Toadfish produce sounds by their swimbladder muscles which contract at frequencies exceeding 100 Hz. Toadfish produce a 400 ms high frequency call followed by a 5–15 s intercall interval [54]. It has been proposed that PA rapidly binds most of the Ca2+ released during the call and this Ca2+ moves back into the sarcoplasmic reticulum during the long intercall interval [55]. Midshipman (Porichthys notatus) is another fish which produces sounds with a frequency 80–100 Hz at a temperature of 12–15 °C with a 100% duty cycle without any intercall intervals. In this case, PA seems to be of little use as a relaxation factor since it would become saturated at an early stage of calling. Tikunov and Rome [55] found that the midshipman swimbladder muscle still has high PA concentration (0.18 mM). Interestingly, total PA content in calling male midshipman swimbladder and PA content in swimbladder of non-calling female and much slower locomotory muscles are practically identical. These results show that PA does not play the role of relaxation factor in the swimbladder muscle of midshipman fish. Moreover, these observations raise some doubts that the main function of parvalbumin in fast muscles is to accelerate their relaxation.
In addition to fast skeletal muscles, PA has also been found in cardiac muscle tissue of rat, mouse, chicken, rabbit, and pig [56,57], and it was revealed that PA takes part in the relaxation of cardiac myocytes [58,59,60,61,62,63]. It has been suggested [60] that at the end of systole, Ca2+ dissociates from troponin C and binds to PA, which causes cardiac myocyte relaxation. Now PA is tested as a pharmacological agent to treat heart dysfunctions. For example, it has been found that PA fully normalizes the relaxation rate in diseased cardiac myocytes taken from an animal model of human diastolic dysfunction [58]. PA gene transfer to the heart in vivo results in PA expression up to the levels similar to those in fast skeletal muscles. The synthesized PA accelerates heart relaxation in normal hearts and corrects heart relaxation in an animal model of slowed cardiac muscle relaxation [59]. Similar results were obtained by Michele et al. [61]: they found that PA gene transfer and expression in vivo increased relaxation rate in the aged myocardium. Asp et al. [64] used mutant PA E101D (monodentate instead of bidentate Ca2+ coordination in the CD binding site) to treat diastolic heart failure. E101D PA is characterized by 114-fold decreased Ca2+ affinity and 28-fold increased Mg2+ affinity compared to the wild type PA. E101D PA increased contraction amplitude of myocytes compared to both untreated myocytes and myocytes with E101Q PA, with slight improvements in relaxation. Moreover, E101D PA increased spontaneous contractions after pacing stress.
Summarizing, we can conclude that a lot of experimental data indicate that in many cases, PA can accelerate the relaxation stage of fast muscles, but this effect is not too pronounced, as the PA gene knock out results in 33% lower rate constant of Ca2+ decay [51].
Interestingly, fast muscles with high PA concentrations are characterized by the highly efficient oxygen consumption (oxygen uptake up to 57 mL/min [65]). The molecular oxygen is utilized for oxidative phosphorylation in mitochondria for production of ATP molecules. During the consumption by mitochondria, up to 0.5% of oxygen escape from the electron-transfer chain (complexes I and III) and is reduced with the formation of superoxide anion (O2●–). Later O2●– is converted to hydrogen peroxide (H2O2) (reviewed in [66]). H2O2 is one of the intracellular reactive oxygen species (ROS). It is most abundant (0.1 μM) and long living (half-life time of 10 μs) ROS, which acts both as an inducer of oxidative damage and a signaling molecule [65]. The major intracellular source of ROS is mitochondrial respiration. The PA-rich cells are usually characterized by high oxygen consumption and intense production of ATP and ROS, including O2●– and H2O2. It is of interest that PA immunoreactivity was found in (or near) mitochondria [42].
We have explored the possibility that PA could serve as a free radical scavenger. ORAC (oxygen radical absorbance capacity), TEAC (trolox equivalent antioxidant capacity), and hydrogen peroxide AOC assays were used to study antioxidant capacities (AOC) of various forms of intact rat α-PA [67]. We have found that the oxidation of PA depends on its conformation: AOC value for apo-PA (similar to AOC for the proteolized protein) 4–11-fold exceeds AOC for the Ca2+-loaded protein, while AOC for Mg2+-bound PA is similar to that of apo-PA. The interactions of PA with ROS causes oxidation of its Phe residues. It has been found that total antioxidant capacity of PA under in vivo conditions may reach the level of reduced glutathione. For this reason, one can suggest that one more physiological function of PA in fast muscles could be a protection of these cells from reactive oxygen species. Moreover, PA could change intracellular redox equilibria and signaling in a Ca2+-dependent manner.
PA의 가능한 생물학적 기능 중 가장 많이 연구된 것은 아마도
근육 세포에서의 기능일 것입니다.
근육의 PA 농도는
근육 수축 및 이완 속도와 상관관계가 있는 것으로 오랫동안 알려져 왔습니다.
빠른 골격근의 골질 내 PA 농도는 밀리몰 수준에 도달하는 반면,
느린 근육은 약 두 배 정도 낮은 수준의 PA가 특징입니다 [43,44,45].
PA 함량과 근육의 이완 속도 사이에 양의 상관관계가 발견되었으며, 이는 PA가 근질량 내에서 Ca2+ 전위를 촉진하여 빠른 근육의 이완 인자가 될 수 있다는 가정으로 이어졌습니다 [43,44].
휴식 중인 근육의 실질질 내 유리 Ca2+ 농도는 매우 낮은 반면(<1 μM), 근육의 유리 Mg2+ 농도는 밀리몰 영역에 있습니다. 이러한 이유로 휴식 중인 근육의 실질질에 있는 PA는 Mg2+ 이온에 의해 부하되는 것으로 보입니다. 일시적으로 소포체에서 방출된 Ca2+는 소포체를 통해 확산되어 액틴 원섬유에 위치한 트로포닌 복합체와 상호 작용하여 근육 수축을 활성화합니다. PA는 Ca2+가 이동하는 도중에 근육 세포의 실질질에 위치하지만, PA의 결합 부위에서 Mg2+와 Ca2+의 교환은 상대적으로 느린 Mg2+ 해리 과정에 의해 지배되기 때문에 Ca2+ 이온이 트로포닌 C의 Ca2+ 특이 부위에 도달할 수 있습니다 [46]. 나중에 PA의 강력한 Ca2+/Mg2+ 부위는 Ca2+를 놓고 경쟁하며 트로포닌 C로부터의 제거를 촉진하여 근육 이완을 시작합니다. PA에서 Mg2+의 해리 속도가 낮기 때문에 PA는 특히 빠른 근육에서 단일 수축-이완 주기(트위치) 동안 칼슘을 제거할 시간이 없을 것이라고 생각하는 것이 합리적입니다. 대신, PA에서 Mg2+-Ca2+ 교환은 여러 번의 연속적인 경련(파상풍 이후) 후에만 일어날 수 있습니다. 다음은 빠른 근육의 이완을 가속화하는 데 있어 PA의 중요한 역할을 나타내는 몇 가지 실험 결과입니다.
느린 근육과 빠른 근육의 교차 재신경화는 빠른 근육의 PA 함량을 감소시키고, 느린 근육의 PA 함량을 증가시킵니다 [47,48]. 작은 포유류의 빠른 근육 섬유의 PA 농도는 큰 포유류의 빠른 근육 섬유보다 높으며, 이는 수축 및 이완 속도의 본질적인 차이 때문입니다 [49].
PA가 실제로 이완 인자로 작용할 수 있는지 확인하기 위해 직접 유전자 전달 실험을 수행했습니다. 정상적으로 PA를 합성하지 않는 정상 및 재생 중인 쥐의 가자미근에 PA cDNA를 생체 내에서 전달했습니다 [50]. 감염 후 2 주 후에 손상되지 않은 근육과 재생중인 근육에서 상당한 농도의 PA mRNA와 PA가 나타나 이러한 근육의 절반 이완 시간이 단축되었습니다. 또 다른 연구에서는 쥐의 빠른 수축/이완 근육에서 PA 유전자를 녹아웃시키면 분리된 외지 장근의 20-ms 자극 후 Ca2+ 수준의 붕괴가 느려지고(Ca2+ 붕괴 속도 상수가 33% 낮아짐), 최대 경련 장력에 도달하는 시간이 감소하는 것으로 밝혀졌습니다[51].
20°C에서 PA 결핍 마우스의 장근 신근의 이완 속도는 낮고 파상풍 지속 시간(<3.2초)에 의존하지 않는 것으로 밝혀졌습니다[52]. 대조적으로, 파상풍 지속 시간이 0.2초에서 3.2초로 증가하면 정상 야생형 근육의 이완 속도가 감소하고 휴식 간격이 증가함에 따라 빠른 이완이 회복됩니다. 파상풍 지속 시간 증가로 인한 이완 단계의 둔화는 Ca2+에 의한 PA의 포화로 설명되었고, 휴식 간격 증가 후 빠른 이완 회복은 Ca2+가 없는 PA의 형성에 의해 설명되었습니다. 장요근 굴곡근에서 파상풍 지속시간이 야생형 및 PA 결핍 근육에 미치는 영향은 장요근 신근에서 관찰된 것과 질적으로 유사했습니다. 저자들은 PA가 파상풍 이후 근섬유에서 유육종으로 칼슘 이동을 촉진한다는 결론을 내렸습니다.
알려진 가장 빠른 척추동물 근육인 초고속 두꺼비 방광 근육[53]은 가장 높은 PA 농도(1.20-1.35 mM)가 특징입니다[54,55]. 두꺼비는 100Hz를 초과하는 주파수에서 수축하는 부낭 근육으로 소리를 냅니다. 두꺼비들은 400밀리초의 고주파 발신 후 5-15초의 인터콜 간격을 두고 소리를 냅니다 [54]. PA는 호출 중에 방출된 대부분의 Ca2+와 빠르게 결합하고, 이 Ca2+는 긴 인터콜 간격 동안 다시 소포체로 이동한다고 제안되었습니다 [55]. 미드십맨(Porichthys notatus)은 인터콜 간격 없이 100% 듀티사이클로 12-15°C의 온도에서 80-100Hz 주파수의 소리를 내는 또 다른 어류입니다. 이 경우 PA는 호출의 초기 단계에서 포화 상태가 되기 때문에 이완 요인으로 거의 사용되지 않는 것으로 보입니다. 티쿠노프와 로마[55]는 중간 선원 수영 방광 근육의 PA 농도가 여전히 높다는 것을 발견했습니다(0.18mM). 흥미롭게도, 호출하는 수컷 미드십맨 수영 방광의 총 PA 함량과 호출하지 않는 암컷 및 훨씬 느린 운동 근육의 수영 방광의 PA 함량은 거의 동일합니다. 이러한 결과는 PA가 중등도 어류의 수영 방광 근육에서 이완 인자의 역할을하지 않는다는 것을 보여줍니다. 또한 이러한 관찰은 빠른 근육에서 파발부민의 주요 기능이 이완을 가속화하는 것이라는 의구심을 불러일으킵니다.
빠른 골격근 외에도 PA는 쥐, 생쥐, 닭, 토끼, 돼지의 심장 근육 조직에서도 발견되었으며[56,57], PA가 심장 근육 세포의 이완에 관여한다는 것이 밝혀졌습니다 [58,59,60,61,62,63]. 수축기 말기에 Ca2+는 트로포닌 C에서 해리되어 PA와 결합하여 심장근육세포 이완을 유발한다고 제안되었습니다[60]. 이제 PA는 심장 기능 장애를 치료하기 위한 약리학적인 약제로 테스트되고 있습니다. 예를 들어, 인간 이완기 기능 장애의 동물 모델에서 채취한 병든 심장 근육 세포의 이완 속도를 완전히 정상화한다는 사실이 밝혀졌습니다 [58]. PA 유전자를 생체 내에서 심장으로 전달하면 빠른 골격근에서와 유사한 수준까지 PA가 발현됩니다. 합성된 PA는 정상 심장에서 심장 이완을 가속화하고 심장 근육 이완이 느려진 동물 모델에서 심장 이완을 교정합니다 [59]. 미셸 등[61]도 비슷한 결과를 얻었는데, 이들은 생체 내 PA 유전자 전달과 발현이 노화된 심근의 이완 속도를 증가시킨다는 사실을 발견했습니다. Asp 등[64]은 이완기 심부전 치료를 위해 돌연변이 PA E101D(CD 결합 부위에서 이염기성 Ca2+ 배위 대신 모노덴테이트)를 사용했습니다. E101D PA는 야생형 PA에 비해 Ca2+ 친화력이 114배 감소하고 Mg2+ 친화력이 28배 증가한 것이 특징입니다. E101D PA는 처리되지 않은 심근 세포와 E101Q PA를 처리한 심근 세포 모두에 비해 심근 세포의 수축 진폭을 증가시켰으며 이완은 약간 개선되었습니다. 또한, E101D PA는 페이싱 스트레스 후 자발적 수축을 증가시켰습니다.
요약하면, 많은 실험 데이터에 따르면 많은 경우 PA가 빠른 근육의 이완 단계를 가속화 할 수 있지만 PA 유전자가 녹아웃되면 Ca2+ 붕괴 속도 상수가 33 % 낮아지기 때문에이 효과가 너무 뚜렷하지는 않다는 결론을 내릴 수 있습니다 [51].
흥미롭게도 PA 농도가 높은 빠른 근육은 매우 효율적인 산소 소비(최대 57mL/min [65]의 산소 흡수)가 특징입니다. 분자 산소는 미토콘드리아에서 산화적 인산화를 통해 ATP 분자를 생성하는 데 사용됩니다. 미토콘드리아에서 소비되는 동안 최대 0.5%의 산소가 전자 전달 사슬(복합체 I 및 III)에서 빠져나와 슈퍼옥사이드 음이온(O2●-)의 형성과 함께 환원됩니다. 나중에 O2●-는 과산화수소(H2O2)로 전환됩니다([66]에서 검토됨). H2O2는 세포 내 활성 산소 종(ROS) 중 하나입니다. 가장 풍부하고(0.1 μM) 수명이 긴(반감기 10 μs) ROS로, 산화적 손상을 유발하는 동시에 신호 분자로도 작용합니다[65]. ROS의 주요 세포 내 공급원은 미토콘드리아 호흡입니다. PA가 풍부한 세포는 일반적으로 높은 산소 소비량과 O2●- 및 H2O2를 포함한 ATP 및 ROS의 집중적인 생성이 특징입니다. 미토콘드리아 (또는 그 근처)에서 PA 면역 반응성이 발견되었다는 점이 흥미롭습니다 [42].
저희는 PA가 활성산소 제거제 역할을 할 수 있다는 가능성을 탐구했습니다. 다양한 형태의 온전한 쥐 α-PA의 항산화 능력(AOC)을 연구하기 위해 ORAC(산소 라디칼 흡수 능력), TEAC(트록스 등가 항산화 능력) 및 과산화수소 AOC 분석을 사용했습니다[67]. PA의 산화는 그 형태에 따라 달라진다는 사실을 발견했습니다: 단백질화된 단백질에 대한 AOC 값(단백질화된 단백질의 AOC와 유사)은 Ca2+가 결합된 단백질에 대한 AOC보다 4-11배 높은 반면, Mg2+가 결합된 PA의 AOC는 apo-PA와 유사합니다. PA와 ROS의 상호 작용은 Phe 잔기의 산화를 유발합니다. 생체 내 조건에서 PA의 총 항산화 능력이 환원 글루타티온 수준에 도달할 수 있다는 사실이 밝혀졌습니다. 이러한 이유로 빠른 근육에서 PA의 또 다른 생리적 기능은 활성 산소 종으로부터 이러한 세포를 보호하는 것일 수 있다고 제안할 수 있습니다. 또한 PA는 Ca2+ 의존적인 방식으로 세포 내 산화 환원 평형과 신호를 변화시킬 수 있습니다.
면역 반응성은 미토콘드리아 및 (또는) 초파리 수컷 생식선의 미세소관과 같은 막 시스템에서 (또는 그 근처에서) 발견되었습니다 [42].
5.2. Parvalbumin in Neurons
Calcium binding proteins of the EF-hand superfamily, parvalbumin, calbindin, and calretinin, have been found in various classes of inhibitory interneurons as well as in some pyramidal neurons in the mammalian neocortex (see [68,69] for reviews). Their physiological role is assumed to be Ca2+ buffering, Ca2+ transport, regulation of activity of various enzymes, and a protection of neurons against calcium overload. It is assumed that neurons containing high concentrations of these Ca2+-binding proteins would be more resistant to degeneration due to their Ca2+ buffering capacity [70].
In the rat somatosensory cortex, PA is contained only in gamma-aminobutyric acid (GABA) neurons (in hippocampus, cerebellum, and neocortex) [71] (reviewed in [4]). GABA is one of the major inhibitory neurotransmitters in the central nervous system. PA-containing GABAergic interneurons regulate input–output functions in some brain regions. PA seems to take part in regulation of calcium-dependent metabolic and electric processes in such neurons. PA level in soma and neurites of the GABAergic neurons of the brain is high and reaches 50 μM in interneurons [72]. In the neurons located in the brain regions of zebra finch responsible for songs [73] and in neurons located in the optic cortex of cat [74], PA was found in amorphous material, dendrites, and axons; in most nuclei and in association with microtubules; postsynaptic densities; and intracellular membranes.
Caillard et al. [75] suggested that PA may change intracellular Ca2+ transients after an action potential. To test this hypothesis, they have applied paired-pulse stimulations (with 30- to 300-ms intervals) at GABAergic synapses between interneurons and Purkinje cells, both in wild-type (PA+/+) mice and in PA knockout (PA−/−) mice. They found paired-pulse depression in PA+/+ mice, but paired-pulse facilitation in PA−/− mice. 1 mM Ca2+ buffer EGTA rescued paired-pulse depression in PA−/− mice. The authors concluded that PA can effectively modulate short-term synaptic plasticity.
It has been found that genetic elimination of PA results in changes in GABAergic synaptic transmission, such as an enhancement of synaptic facilitation, a reduction in asynchronous transmitter release, and an increase in the power of gamma oscillations [75,76,77]. PA is thought to act as a slow Ca2+ buffer but affects such fast processes as transmitter release at hippocampal and cerebellar GABAergic synapses. Eggerman and Jonas tried to resolve this paradox [78]. They measured PA concentration and paired recordings in rodent hippocampus and cerebellum and found that PA only affects synaptic dynamics in high concentrations approaching its concentration in fast skeletal muscle (~1 mM). According to theoretical evaluations of the authors, although the fraction of free PA (apo-PA) present under physiological conditions is less than 10%, the absolute concentration of the free PA is sufficient to influence fast processes. Note that it is not clear from the text of the article which PA calcium binding constants were used by the authors for these estimates.
Both in neurons and muscle fibers, PA expression strongly depends on their activity. Decreased neuronal activity or oxidative stress in PA containing neurons causes a decrease in PA-immunoreactive neurons, PA messenger RNA, and PA levels [79,80,81].
The effects of PA and mitochondria on the shape of Ca2+ transients in neurons are quite similar (reviewed in [69]). It was found that PA and mitochondria demonstrate similarities in their kinetics of Ca2+ binding and Ca2+ uptake, respectively. Moreover, in all model systems they are regulated in an antagonistic way: an increase in one results in a decrease in the other. This was demonstrated in the case of fast-twitch muscles [82] and Purkinje cells [83] of PA−/− mice, as well as in several cell models, for example, in myotubes [84] and MDCK kidney cells [85].
PA containing neurons are involved in some neuropsychiatric diseases, in particular in schizophrenia and bipolar disorder and in neurodevelopmental disorders including autism spectrum disorder (ASD) [86]. The degradation of PA neurons in ASD is thought to be associated with PA downregulation (reviewed in [69]). This effect was explained by a homeostatic compensation to increase synaptic output of PA neurons [87].
Oxidative stress in PA-GABAergic interneurons and their dysfunction associated with a decrease in the levels of both PA and reduced glutathione (GSH) are hallmarks of the development of schizophrenia [81,88,89,90,91]. Since total antioxidant capacity (AOC) of PA may reach the AOC level of GSH, we suggested that PA might modulate intracellular redox equilibria in a calcium-dependent manner [67]. The loss of PA and GSH results in a decrease in the total cellular AOC, which results in an increase in sensitivity of the cells to oxidative stress. The elevation of cytosolic free Ca2+ levels induced by oxidation will result in loading of PA with Ca2+ ions, which, in its turn, will decrease its AOC [67]. For these reasons, the increase in ROS level should cause an amplification of the oxidative stress in PA-GABAergic interneurons, which causes their oxidation induced damage and finally their loss. Moreover, the oxidation-induced elevation in cytosolic free Ca2+ may initiate specific signaling cascades leading to cell death [92].
Mammalian oncomodulin (OM) is the β isoform of PA, which has at least 53% sequence identity with α-PA [93,94]. Initially, OM was found in cancerous tissue as an oncoprotein [95]. Later, OM was revealed in sensory cells of the guinea pig cochlea [96,97,98]. OM is expressed mostly in cochlear outer hair cells and in vestibular hair cells [99,100,101]. OM was also found in immune cells [102,103,104].
파발부민, 칼빈딘, 칼레티닌과 같은
EF-손 수퍼패밀리의 칼슘 결합 단백질은
포유류 신피질의 일부 피라미드 뉴런뿐만 아니라
다양한 종류의 억제성 신경세포에서 발견되었습니다(리뷰는 [68,69]를 참조하세요).
이들의 생리적 역할은
Ca2+ 완충, Ca2+ 수송, 다양한 효소의 활성 조절, 칼슘 과부하로부터
뉴런을 보호하는 것으로 추정됩니다.
이러한
Ca2+ 결합 단백질이
고농도로 함유된 뉴런은 Ca2+ 완충 능력으로 인해 퇴화에 더 저항력이 있을 것으로 추정됩니다 [70].
쥐 체성 감각 피질에서 PA는 감마 아미노부티르산(GABA) 뉴런(해마, 소뇌, 신피질)에만 포함되어 있습니다[71]([4]에서 검토됨). GABA는 중추신경계의 주요 억제성 신경전달물질 중 하나입니다. PA를 함유한 가바성 인터뉴런은 일부 뇌 영역에서 입력-출력 기능을 조절합니다. PA는 이러한 뉴런에서 칼슘 의존적 대사 및 전기적 과정의 조절에 관여하는 것으로 보입니다. 뇌의 GABAergic 뉴런의 체질과 뉴라이트의 PA 수준은 높고 뉴런에서 50 μM에 도달합니다 [72]. 노래를 담당하는 얼룩 핀치의 뇌 영역에 위치한 뉴런 [73]과 고양이의 시피질에 위치한 뉴런 [74]에서 PA는 무정형 물질, 수상 돌기 및 축삭, 대부분의 핵과 미세 소관, 시냅스 후 밀도 및 세포 내 막에서 발견되었습니다.
Caillard 등[75]은 PA가 활동 전위 후 세포 내 Ca2+ 과도 현상을 변화시킬 수 있다고 제안했습니다. 이 가설을 테스트하기 위해 야생형(PA+/+) 마우스와 PA 녹아웃(PA-/-) 마우스 모두에서 뉴런과 퍼킨예 세포 사이의 GABAergic 시냅스에 30~300ms 간격으로 짝을 이룬 펄스 자극을 적용했습니다. 연구진은 PA+/+ 마우스에서는 쌍-맥박 우울증이 발견되었지만, PA-/- 마우스에서는 쌍-맥박 촉진이 발견되었습니다. 1mM Ca2+ 버퍼 EGTA는 PA-/- 마우스의 쌍맥박 저하를 완화했습니다. 저자들은 PA가 단기 시냅스 가소성을 효과적으로 조절할 수 있다고 결론지었습니다.
PA의 유전적 제거는 시냅스 촉진의 향상, 비동기 전달 물질 방출의 감소, 감마 진동의 힘 증가와 같은 GABAergic 시냅스 전달의 변화를 초래하는 것으로 밝혀졌습니다 [75,76,77]. PA는 느린 Ca2+ 버퍼로 작용하는 것으로 생각되지만 해마 및 소뇌 GABAergic 시냅스에서 송신기 방출과 같은 빠른 과정에 영향을 미칩니다. 에거먼과 조나스는 이 역설을 해결하려고 노력했습니다[78]. 그들은 설치류 해마와 소뇌에서 PA 농도와 쌍을 이루는 기록을 측정한 결과, PA가 빠른 골격근의 농도(~1mM)에 근접하는 고농도에서만 시냅스 역학에 영향을 미친다는 사실을 발견했습니다. 저자들의 이론적 평가에 따르면, 생리적 조건에서 존재하는 유리 PA(apo-PA)의 비율은 10% 미만이지만, 유리 PA의 절대 농도는 빠른 과정에 영향을 미치기에 충분합니다. 논문 본문에서는 저자들이 이러한 추정치를 위해 어떤 PA 칼슘 결합 상수를 사용했는지 명확하지 않습니다.
뉴런과 근육 섬유 모두에서 PA 발현은 활동에 따라 크게 달라집니다. PA 함유 뉴런의 뉴런 활동 감소 또는 산화 스트레스는 PA 면역 반응 뉴런, PA 메신저 RNA 및 PA 수준을 감소시킵니다 [79,80,81].
뉴런에서 Ca2+ 과도현상의 형태에 대한 PA와 미토콘드리아의 영향은 매우 유사합니다([69]에서 검토됨). PA와 미토콘드리아는 각각 Ca2+ 결합과 Ca2+ 흡수의 동역학에서 유사성을 보이는 것으로 나타났습니다. 또한, 모든 모델 시스템에서 이들은 길항적인 방식으로 조절되는데, 한쪽이 증가하면 다른 쪽이 감소합니다. 이는 PA-/- 마우스의 빠른 경련 근육 [82]과 푸르킨예 세포 [83]의 경우뿐만 아니라 근육 튜브 [84] 및 MDCK 신장 세포 [85]와 같은 여러 세포 모델에서 입증되었습니다.
PA를 포함하는 뉴런은 일부 신경정신질환, 특히 조현병과 양극성 장애, 자폐 스펙트럼 장애(ASD)를 포함한 신경발달 장애에 관여합니다[86]. ASD에서 PA 뉴런의 퇴화는 PA의 하향 조절과 관련이 있는 것으로 생각됩니다([69]에서 검토됨). 이 효과는 PA 뉴런의 시냅스 출력을 증가시키기 위한 항상성 보상으로 설명되었습니다 [87].
PA-GABAergic 뉴런의 산화 스트레스와 PA 및 글루타치온(GSH) 수치 감소와 관련된 기능 장애는 조현병 발병의 특징입니다 [81,88,89,90,91]. PA의 총 항산화 능력(AOC)이 GSH의 AOC 수준에 도달할 수 있기 때문에 PA가 칼슘 의존적인 방식으로 세포 내 산화 환원 평형을 조절할 수 있다고 제안했습니다[67]. PA와 GSH의 손실은 총 세포 AOC의 감소를 초래하여 산화 스트레스에 대한 세포의 민감도를 증가시킵니다. 산화에 의해 유도된 세포질 유리 Ca2+ 수준의 상승은 PA에 Ca2+ 이온을 로딩하여 결과적으로 AOC를 감소시킵니다 [67]. 이러한 이유로 ROS 수준의 증가는 PA-GABAergic 인터뉴런의 산화 스트레스를 증폭시켜 산화로 인한 손상을 유발하고 최종적으로 손실을 초래합니다. 또한, 산화로 인한 세포질 유리 Ca2+의 상승은 세포 사멸로 이어지는 특정 신호 캐스케이드를 시작할 수 있습니다 [92].
포유류 온코모둘린(OM)은 α-PA와 최소 53%의 서열 동일성을 갖는 PA의 β 이소형입니다[93,94]. 처음에 OM은 암 조직에서 종양 단백질로 발견되었습니다 [95]. 나중에 OM은 기니피그 달팽이관의 감각 세포에서 밝혀졌습니다 [96,97,98]. OM은 주로 달팽이관 외유모세포와 전정유모세포에서 발현됩니다 [99,100,101]. OM은 면역 세포에서도 발견되었습니다 [102,103,104].
5.3. Oncomodulin
Essential features of mammalian OM are similar to those of most other vertebrate β-PAs, such as a length of 109 amino acid residues, isolectric point < 5.0, and a cysteine at position 19. In spite of this, it has been found that mammalian OM and lower vertebrate β-PAs belong to different phylogenetic lineages (reviewed in [96]). In vertebrates, millimolar concentration of OM in outer hair cells is comparable with the millimolar concentration of PA in fast-twitch muscle fibers [105], which means that OM is an effective Ca2+ buffer in these cells.
Solution structures of Ca2+-free and Ca2+-bound rat OM [26] show that removal of Ca2+ from OM results in structural alterations (substantial reorganization of the C, D, and E helices), which are more pronounced in comparison with those caused by Ca2+ removal from α-PAs. This is illustrated by Figure 4 comparing the 3D structures of Ca2+-bound and Ca2+-free forms of rat OM (Figure 4A,a, respectively) with the holo- and apo-forms of rat α-PA (Figure 4B,b), as well as Ca2+-bound forms of pike α-PA (Figure 4C,c) and pike β-PA (Figure 4D). Comparison of all of these 3D structures show remarkable structural similarity of these proteins. Therefore, although the presence of noticeable Ca2+-driven reorganization of OM is discussed, the actual structural changes are not easily detectable by the naked eye. This is further illustrated by Figure 4E representing aligned structures of all of these proteins.
포유류 OM의 필수적인 특징은
109개의 아미노산 잔기의 길이, 5.0 미만의 등전점, 19번 위치에 있는 시스테인 등 대부분의 다른 척추동물 β-PA의 특징과 유사합니다. 그럼에도 불구하고 포유류 OM과 척추동물 하부 β-PA는 서로 다른 계통학적 계통에 속하는 것으로 밝혀졌습니다([96]에서 검토됨). 척추동물에서 외부 유모세포에서 OM의 밀리몰 농도는 속발성 근육 섬유에서 PA의 밀리몰 농도와 비슷하며[105], 이는 OM이 이러한 세포에서 효과적인 Ca2+ 완충제임을 의미합니다.
Ca2+가 없는 쥐와 Ca2+가 결합된 쥐 OM의 용액 구조[26]는 OM에서 Ca2+를 제거하면 구조적 변화(C, D 및 E 나선의 상당한 재구성)가 발생하며, 이는 α-PA에서 Ca2+ 제거로 인한 것과 비교할 때 더 뚜렷하게 나타납니다. 이는 Ca2+ 결합 및 Ca2+ 비결합 형태의 쥐 OM(그림 4A,a)의 3D 구조를 쥐 α-PA의 홀로 및 아포 형태(그림 4B,b), Ca2+ 결합 형태의 파이크 α-PA(그림 4C,c) 및 파이크 β-PA(그림 4D)와 비교한 그림 4에서 확인할 수 있습니다. 이러한 모든 3D 구조를 비교하면 이들 단백질의 놀라운 구조적 유사성을 알 수 있습니다. 따라서 눈에 띄는 Ca2+에 의한 OM의 재구성이 논의되고 있지만, 실제 구조 변화는 육안으로 쉽게 감지할 수 없습니다. 이는 모든 단백질의 정렬된 구조를 나타내는 그림 4E에자세히 설명되어 있습니다.

Comparison of 3D structures of various PAs. (A) X-ray crystal structure of the holo-form of rat OM. (a) Solution NMR structure of the apo-form of rat OM. (B) X-ray crystal structure of the holo-form of rat α-PA. (b) Solution NMR structure of the apo-form of rat α-PA. (C) X-ray crystal structure of the holo-form of pike α-PA. (c) Solution NMR structure of the holo-form of pike α-PA. (D) X-ray crystal structure of the holo-form of pike β-PA. (E) Multiple structural alignment of all these proteins in their holo- and apo-forms. Structures of the holo- and apo-forms of rat OM are shown by blue and cyan colors. Red and pink colors represent structures of the the holo- and apo-forms of rat α-PA. Crystal and solution structures of pike α-PA are shown by orange and yellow color, whereas crystal structure of the holo-form of pike β-PA is shown in gray. Multiple structural alignment was conducted using MultiProt server (http://bioinfo3d.cs.tau.ac.il/MultiProt/; Accessed on 23 April 2022).
While α-PAs have two Ca2+ binding sites with approximately equal affinities (reviewed in [1,2,3]), the Ca2+ binding sites of OM are characterized by essentially different affinities [106] (reviewed in [94]). Thus, unlike PA, which has two Ca2+/Mg2+ sites, OM has one Ca2+/Mg2+ site and one that is more Ca2+ specific. Climer et al. [94] suggested that OM is not pure Ca2+ buffer, but it may act as a Ca2+ sensor under the right physiological conditions.
Analysis of recent experimental data showed that OM may play an ambiguous role that depends upon the cell type in which it is expressed [94]. Experiments on deletion of OM showed that it is not essential to cochlear development [101]. At the same time, it was found that OM knockout mice start to lose their hearing at 1–2 months and are essentially deaf after 3–4 months. Since progressive hearing loss at 2 months occurs prior to the loss of hair cells in the OM knockout mice, the authors suggested that OM could protect against hearing loss [101]. Triple knock out of three Ca2+ buffers, PA, calbindin 1, and calbindin 2, resulted in only minor impacts on hearing [107], which shows that these Ca2+ buffers cannot replace OM function. Climery et al. [94] suggested that, in sensory cells, OM maintains auditory function, most likely affecting outer hair cells’ motility mechanisms. They proposed that OM takes part in regulation of outer hair cells elongation (contraction) and shortening (relaxation) mechanisms associated with the cortical lattice, which determines outer hair cells stiffness and electromotility.
OM has been found in macrophages and neutrophils. Benowitz et al. have studied the role of OM in an inflammatory-mediated nerve regenerative model [102,108,109,110]. Analysis of these data shows that, in immune cells, OM seems to be secreted in response to inflammation and its function in this case is facilitation of axon regeneration [94]. Secreted OM either binds to a surface receptor or enters neurons using endocytosis, but only when intracellular cAMP is above basal levels.
Initially, OM was found in several types of mouse, rat, and human tumors, which made it attractive as a potential cancer marker [95]. Later on, it became clear that some normal tissues also contain OM and not all human tumor cell lines contain OM [111]. That is why interest in using this protein as a potential cancer has disappeared.
OM does not cause transformation of cultured cells [112], but neoplastic transformation of rodent cell lines elevates the expression of OM [95,113,114,115,116]. OM expression may be suppressed in non-transformed cells, but can be increased in transformed cells. In order to reveal OM function in tumors, some researchers investigated possible interaction of OM with glutathione reductase, cyclic nucleotide phosphodiesterase, and cell-cycle regulation. Palmer et al. found that purified OM inhibited glutathione reductase in the presence of Ca2+ [117]. Glutathione reductase is an enzyme responsible for maintaining reducing conditions within cells. The results of this early study are interesting in connection with a study by Permyakov et al. [67] that showed that PAs have strong antioxidant properties. Klee and Heppel [118] and Clayshulte et al. [119] did not find any modulatory effect of OM on cyclic nucleotide phosphodiesterases.
Two proteins were isolated from frog cutaneous mucus proteome, which take part in prey recognition by snakes of the genus Thamnophis [120]. These proteins, members of the PA family, act as Ca2+/Mg2+ dependent chemoattractants. They elicit the vomeronasal organ-mediated predatory behavior in Thamnophis marcianus. These results show that PAs’ locations are not strictly intracellular, but that they can also be found in exocrine secretions.
High PA immunoreactivity was found in the Leydig cells during testicular development at the stages of intensive testosterone synthesis [121]. PA levels become low at the stages of low Leydig cell activity. It suggests that PA may be involved in the production of testosterone in the Leydig cells. Furthermore, PA immunoreactivity was found in the seminiferous tubules in maturing spermatids and in several other endocrine glands—such as ovaries, pituitary, thyroid, parathyroid, and adrenal glands—which suggests that PA might play some role in endocrine secretions [122,123,124].
It has been found that PA is selectively expressed in the early distal convoluted tubule (DCT) of kidney [125]. PA is colocalized with the thiazide-sensitive Na+-Cl- cotransporter (NCC) in the early DCT. Genetically modified (PA−/−) mice showed increased diuresis and kaliuresis at baseline with higher aldosterone levels and lower lithium clearance. Endogenous NCC expression was found to be Ca2+-dependent and modulated by PA expression level. It has been concluded that, in this system, PA regulates the expression of NCC by modulating intracellular Ca2+ signaling in response to ATP in DCT cells [125,126].
6. Intrinsic Disorder, Structural Flexibility, and Multifunctionality of Parvalbumin
Although no target proteins have been described for the rat PA, human oncomodulin was reported to interact with mitochondrial creatine kinases 1A and 1B, syndecan binding protein (syntenin), ubiquitin-conjugating enzyme E2A, and WAP four-disulfide core domain 1 (https://thebiogrid.org/576309; accessed on 23 April 2022). Furthermore, analysis human oncomodulin by STRING platform [127] that utilizes seven types of evidence to build the protein–protein interaction (PPI) network for a query protein—such as neighborhood evidence, fusion evidence, experimental evidence, co-occurrence evidence, database evidence, text mining evidence, and co-expression evidence—revealed that this protein can interact with at least 32 partners.
Figure 5 shows that human PA is located at the center of tightly connected PPI network, members of which are linked via 137 interactions. Therefore, on average, each representative of this network interacts with 8.3 partners and together they form a highly interactive system characterized by the average local clustering coefficient of 0.713. Note that the local clustering coefficient of 1 characterizes a network, where every neighbor connected to a given node/protein is also connected to every other node/protein within the neighborhood.
쥐 PA에 대한 표적 단백질은 설명되어 있지 않지만, 인간 온코모둘린은 미토콘드리아 크레아틴 키나제 1A 및 1B, 신데칸 결합 단백질(신테닌), 유비퀴틴 결합 효소 E2A, WAP 4-이황화물 코어 도메인 1과 상호작용한다고 보고되었습니다(https://thebiogrid.org/576309, 2022년 4월 23일 액세스). 또한, 이웃 증거, 융합 증거, 실험 증거, 동시 발생 증거, 데이터베이스 증거, 텍스트 마이닝 증거, 공동 발현 증거 등 쿼리 단백질에 대한 단백질-단백질 상호작용(PPI) 네트워크를 구축하는 7가지 유형의 증거를 활용하는 STRING 플랫폼[127]으로 인간 온코모둘린을 분석한 결과 이 단백질이 최소 32개의 파트너와 상호 작용할 수 있다는 것이 밝혀졌습니다.
그림 5는 인간 PA가 137개의 상호작용을 통해 연결된 긴밀하게 연결된 PPI 네트워크의 중심에 위치하고 있음을 보여줍니다. 따라서 이 네트워크의 각 대표자는 평균적으로 8.3명의 파트너와 상호작용하며, 이들은 평균 로컬 클러스터링 계수 0.713을 특징으로 하는 매우 상호 작용적인 시스템을 형성합니다. 로컬 클러스터링 계수가 1이면 특정 노드/프로필에 연결된 모든 이웃이
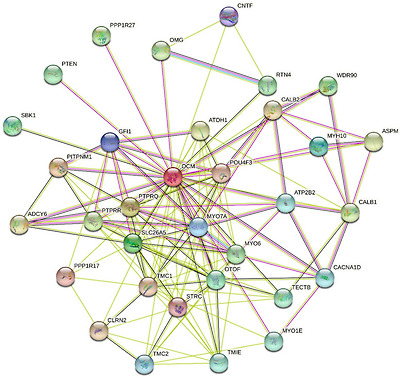
STRING-generated PPI network of human oncomodulin (UniProt ID: P0CE72) using the medium confidence level of 0.4. Seven types of evidence used to build the corresponding network are shown by the differently colored lines: a green line represents neighborhood evidence; a red line—the presence of fusion evidence; a purple line—experimental evidence; a blue line—co-occurrence evidence; a light blue line—database evidence; a yellow line—text mining evidence; and a black line—co-expression evidence [126].
Since multifunctionality and binding promiscuity are considered as specific features of intrinsically disordered proteins of hybrid proteins contacting disordered and ordered regions/domains, we looked at the disorder propensity of human oncomodulin (as an illustrative member of the oncomodulin family) using a set of commonly utilized disorder predictors—such as PONDR® VLXT [128], PONDR® VLS2 [129], PONDR® VL3 [130], PONDR® FIT [131], IUPred2 (Short), and IUPred2 (Long) [132,133]. Results of this analysis are summarized in Figure 6A, which shows that this protein contains significant levels of intrinsic disorder. Most noticeable is highly disordered status of 12 N-terminal and 35 C-terminal residues. Furthermore, as per the outputs of PONDR® VLS2B [129], which was recognized as predictor no. 3 among 43 disorder predicting tools participated in the recently conducted ‘Critical assessment of protein intrinsic disorder prediction’ (CAID) experiment [134], 73.4% residues in human oncomodulin are predicted as disordered, and the entire protein is characterized by the average disorder score (ADS) of 0.59 ± 0.12, which place oncomodulin in the category of highly disordered proteins.

Evaluating intrinsic disorder status of human oncomodulin. (A) Intrinsic disorder profile generated using outputs of PONDR® VLXT [128], PONDR® VLS2 [129], PONDR® VL3 [130], PONDR® FIT [131], IUPred2 (Short), and IUPred2 (Long) [132,133]. The outputs of the evaluation of the per-residue disorder propensity by these tools are represented as real numbers between 1 (ideal prediction of disorder) and 0 (ideal prediction of order). Mean disorder prediction (MDP) was calculated by averaging the outputs of individual predictors. Light pink shadow shows distribution of errors of mean. A threshold of ≥0.5 was used to identify disordered residues and regions in query proteins, whereas a threshold of ≥0.15 was implemented to identify flexible residues. (B) Functional disorder profile of human oncomodulin based on the D2P2 analysis [135]. Here, disorder propensity was evaluated based on the outputs of PONDR® VLXT [127], PONDR® VSL2 [129], IUPred [133,134], PV2 [136], PrDOS [137], and ESpritz [138] shown by nine colored bars representing the locations of disordered regions predicted by these tools. Agreement between these predictors is shown by the blue–green–white bar, where darker blue and green parts correspond to the stronger disorder consensus. Lines with colored and numbered bars show the positions of the predicted SCOP domains [139,140] using the SUPERFAMILY predictor [141]. Yellow zigzagged bar shows the location of the predicted disorder-based binding site (MoRF regions) identified by the ANCHOR algorithm [142]. Red circles represent location of phosphorylation sites based on the outputs of the PhosphoSitePlus platform [143].
This classification is based on the accepted practice of grouping proteins based on their levels of ADS and/or percent of predicted disordered residues (PPDR). Here, proteins are considered as highly ordered, moderately disordered, and highly disordered if they satisfy the following criteria: ADS < 0.15 (PPDR < 10%), 0.15 ≤ ADS < 0.5 (10% ≤ PPDR < 30%), and ADS ≥ 0.5 (PPDR ≥ 30%), respectively [135]. One might ask an important question as to how come a highly disordered protein such as a PA, putatively quite flexible, has been crystallized and shows a not so high B-factor? The answer to this question can be found in the remarkable dependence of the PA structures on the environmental conditions in general, and on the interaction of these proteins with metal ions in particular. This is well illustrated by the aforementioned conformational behavior of pike PA, which—being intrinsically disordered in its apo-form (similarly to the human OM)—folds in the presence of metal ions into a 3D structure, the stability of which is critically dependent on the nature of the bound metal ions. As far as human OM is concerned, no X-ray crystal structure is available for this protein, and solution NMR structure was solved for its Ca2+-bound form in the presence of 100 mM NaCl.
Figure 6A also shows that, according to the outputs of all predictors (with the exception to PONDR® VLXT), all residues in oncomodulin are characterized by the disorder scores exceeding 0.15, suggesting that the entire protein is expected to be either flexible or disordered. This important observation suggests that the unique 3D structures reported for PAs are stabilized by interaction of these proteins with metal ions.
To shed more light on the potential role of intrinsic disorder in functionality of PAs, we used D2P2 platform (http://d2p2.pro/, accessed on 23 April 2022) [136], which in addition to the information on the disorder status of a query protein generates outputs showing localization of sites of various posttranslational modifications (PTMs) and positions of the intrinsic disorder-based binding sites, molecular recognition features (MoRFs), which are disordered protein regions that undergo disorder-to-order transition at interaction with specific binding partners. Figure 6B represents the corresponding functional disorder profile and shows that human oncomodulin contains one MoRF (residues 64–70) and four phosphorylation sites (residues Ser73, Thr79, Ser81, and Thr83). Furthermore, PAs can be acetylated at N-terminal serine residue. Therefore, PAs can not only bind peptides as discussed in the previous sections, but they also can use their intrinsic disorder for interaction with target proteins. Further analysis is required for better understanding of the roles of intrinsic disorder and structural flexibility in function of these interesting proteins.
Taken together, data presented in this review indicate that PAs can serve as illustrative examples of the “protein structure-function continuum” model [144,145,146,147,148,149] based on the proteoform concept, which suggests that a single gene can encode for multiple structurally and functionally distinct protein molecules—proteoforms, originating as a result of alternative splicing and PTMs [150]—due to the presence of intrinsic disorder and related capability to undergo functional disorder-to-order transitions, as well as because of the structural perturbations induced by functioning [144]. In fact, it is clear that during their functional life, PAs have at least two global possibilities to undergo disorder-to-order transitions, first at binding of metal ions leading to the stabilization of the EF-hands and overall protein fold, and second, at interaction with the partner proteins. Functionality of PAs is further enhanced and controlled by PTMs.
7. Conclusions
Despite several decades of study of PA, its physiological functions are still poorly known. This is surprising since the concentration of PA in some organs and tissues reaches a millimolar level. No target proteins have been revealed for PA so far. It is believed that PA acts as a slow calcium buffer. PA concentration in muscles correlates with the speed of their contraction and relaxation. Numerous experiments on various muscle systems have shown that PA accelerates the relaxation of fast skeletal muscles, not during a single contraction–relaxation cycle, but after tetanus. The effect is not very pronounced and PA gene knock out results in about 30% lower rate constant of Ca2+ decay. Since fast muscles with high PA concentrations are characterized by highly efficient oxygen uptake, it was suggested that one more physiological function of PA in fast muscles could be a protection of these cells from reactive oxygen species. It was found that the oxidation of the PA by reactive oxygen species is conformation-dependent: antioxidant capacity (AOC) value for apo-PA 4–11-fold exceeds that for the Ca2+-loaded protein, while Mg2+-bound PA has AOC similar to that of apo-PA.
The role of PA in neurons is even less understood. In the rat somatosensory cortex, PA is contained only in gamma-aminobutyric acid (GABA) neurons (in hippocampus, cerebellum, and neocortex). PA is thought to regulate calcium-dependent metabolic and electric processes within such neurons. Genetic elimination of PA results in changes in GABAergic synaptic transmission, including an enhancement of synaptic facilitation, a reduction in asynchronous transmitter release, and an increase in the power of gamma oscillations.
Mammalian oncomodulin (OM), the β isoform of PA, is expressed mostly in cochlear outer hair cells and in vestibular hair cells. It has been found that OM knockout mice start to lose their hearing at 1–2 months and are essentially deaf after 3–4 months. For this reason, it was suggested that, in sensory cells, OM maintains auditory function, most likely affecting outer hair cells motility mechanisms. In immune cells, OM seems to be secreted in response to inflammation and its function in this case is facilitation of axon regeneration.
All of the above allows us to conclude that the elucidation of the physiological role of parvalbumins in various tissues and organs is undoubtedly worth continuing.
PA에 대한 수십 년간의 연구에도 불구하고
PA의 생리적 기능은 여전히 잘 알려져 있지 않습니다.
일부 장기와 조직에서 P
A의 농도가 밀리몰 수준에 도달하기 때문에 이는 놀라운 일입니다.
아직까지 PA의 표적 단백질은 밝혀지지 않았습니다.
PA는 느린 칼슘 완충제 역할을 하는 것으로 알려져 있습니다.
근육의 PA 농도는 근육의 수축 및 이완 속도와 상관관계가 있습니다. 다양한 근육 시스템에 대한 수많은 실험에서 PA는 단일 수축-이완 주기가 아니라 파상풍 후 빠른 골격근의 이완을 가속화하는 것으로 나타났습니다. 그 효과는 그다지 뚜렷하지 않으며 PA 유전자가 녹아웃되면 Ca2+ 붕괴 속도 상수가 약 30% 낮아집니다. PA 농도가 높은 빠른 근육은 매우 효율적인 산소 흡수가 특징이기 때문에 빠른 근육에서 PA의 또 다른 생리적 기능은 활성 산소 종으로부터 이러한 세포를 보호하는 것일 수 있다고 제안되었습니다. 활성 산소 종에 의한 PA의 산화는 형태 의존적이라는 것이 밝혀졌습니다. apo-PA의 항산화 능력 (AOC) 값은 Ca2 +-로드 된 단백질의 4-11 배를 초과하는 반면 Mg2 +- 결합 PA는 apo-PA와 유사한 AOC를 갖는 것으로 나타났습니다.
신경세포에서 PA의 역할은 더 잘 알려져 있지 않습니다. 쥐의 체성 감각 피질에서 PA는 해마, 소뇌, 신피질의 감마 아미노부티르산(GABA) 뉴런에만 포함되어 있습니다. PA는 이러한 뉴런 내에서 칼슘에 의존하는 대사 및 전기적 과정을 조절하는 것으로 알려져 있습니다. PA를 유전적으로 제거하면 시냅스 촉진의 향상, 비동기적 전달 물질 방출의 감소, 감마 진동의 힘 증가 등 GABAergic 시냅스 전달에 변화가 생깁니다.
PA의 β 동형체인 포유류 온코모둘린(OM)은 주로 달팽이관 외유모세포와 전정유모세포에서 발현됩니다.
OM 녹아웃 마우스는 1~2개월에 청력을 잃기 시작하여 3~4개월 후에는 본질적으로 청각 장애가 발생한다는 사실이 밝혀졌습니다. 이러한 이유로 감각 세포에서 OM은 청각 기능을 유지하며 외유모세포 운동 메커니즘에 영향을 미칠 가능성이 높다고 제안되었습니다.
면역 세포에서 OM은
염증에 반응하여 분비되는 것으로 보이며,
이 경우 그 기능은 축삭 재생을 촉진하는 것입니다.
위의 모든 것을 통해 다양한 조직과 기관에서 파발민의 생리적 역할에 대한 해명은 의심할 여지없이 계속할 가치가 있다는 결론을 내릴 수 있습니다.
Funding Statement
This research received no external funding.
Author Contributions
Conceptualization, E.A.P.; Validation, E.A.P. and V.N.U.; Literature search and formal analysis, E.A.P. and V.N.U.; Investigation, E.A.P. and V.N.U.; Data curation, E.A.P. and V.N.U.; Writing—original draft preparation, E.A.P.; Writing—review and editing, E.A.P. and V.N.U.; Visualization, E.A.P. and V.N.U. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
1. Permyakov E.A. Parvalbumin. Nova Science Publishers, Inc.; New York, NY, USA: 2006. [Google Scholar]
2. Permyakov E.A., Uversky V.N., Permyakov S.E. Parvalbumin as a pleomorphic protein. Curr. Protein Pept. Sci. 2016;18:780–794. doi: 10.2174/1389203717666161213115746. [PubMed] [CrossRef] [Google Scholar]
3. Permyakov E.A., Kretsinger R.H. Calcium Binding Proteins. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2010. [Google Scholar]
4. Arif S.H. A Ca2+-binding protein with numerous roles and uses: Parvalbumin in molecular biology and physiology. Bioessays. 2009;31:410–421. doi: 10.1002/bies.200800170. [PubMed] [CrossRef] [Google Scholar]
5. Smargiassi M., Daghfous G., Leroy B., Legreneur P., Toubeau G., Bels V., Wattiez R. Chemical basis of prey recognition in thamnophiine snakes: The unexpected new roles of parvalbumins. PLoS ONE. 2012;7:e39560. doi: 10.1371/journal.pone.0039560. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
6. Yin Y., Henzl M.T., Lorber B., Nakazawa T., Thomas T.T., Jiang F., Langer R., Benowitz L.I. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat. Neurosci. 2006;9:843–852. doi: 10.1038/nn1701. [PubMed] [CrossRef] [Google Scholar]
7. Novak Kujundzic R., Steffens W.L., Brewer J.M., Henzl M.T., Ragland W.L. Characterization of avian thymic hormone and chicken parvalbumin 3 target cells. Int. Immunopharmacol. 2013;15:282–288. doi: 10.1016/j.intimp.2012.12.013. [PubMed] [CrossRef] [Google Scholar]
8. Goodman M., Pechere J.F. The evolution of muscular parvalbumins investigated by the maximum parsimony method. J. Mol. Evol. 1977;9:131–158. doi: 10.1007/BF01732745. [PubMed] [CrossRef] [Google Scholar]
9. Nakayama S., Moncrief N.D., Kretsinger R.H. Evolution of EF hand calcium-modulated proteins. II. Domains of several subfamilies have diverse evolutionary histories. J. Mol. Evol. 1992;34:416–448. doi: 10.1007/BF00162998. [PubMed] [CrossRef] [Google Scholar]
10. Moncrief N.D., Kretsinger R.H., Goodman M. Evolution of EF hand calcium-modulated proteins. I. Relationships based on amino acid sequences. J. Mol. Evol. 1990;30:522–562. doi: 10.1007/BF02101108. [PubMed] [CrossRef] [Google Scholar]
11. Nockolds C.E., Kretsinger R.H., Coffee C.J., Bradshaw R.A. Structure of a calcium-binding carp myogen. Proc. Natl. Acad. Sci. USA. 1972;69:581–584. doi: 10.1073/pnas.69.3.581. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
12. Kretsinger R.H., Nockolds C.E. Carp muscle calcium-binding protein. II. Structure determination and general description. J. Biol. Chem. 1973;248:3313–3326. doi: 10.1016/S0021-9258(19)44043-X. [PubMed] [CrossRef] [Google Scholar]
13. Permyakov E.A., Medvedkin V.N., Mitin Y.V., Kretsinger R.H. Noncovalent complex between domain AB and domains CD*EF of parvalbumin. Biochim. Biophys. Acta. 1991;1076:67–70. doi: 10.1016/0167-4838(91)90220-T. [PubMed] [CrossRef] [Google Scholar]
14. Thepaut M., Strub M.P., Cave A., Baneres J.L., Berchtold M.W., Dumas C., Padilla A. Structure of rat parvalbumin with deleted AB domain: Implications for the evolution of EF hand calcium-binding proteins and possible physiological relevance. Proteins. 2001;45:117–128. doi: 10.1002/prot.1131. [PubMed] [CrossRef] [Google Scholar]
15. Henzl M.T., Agah S., Larson J.D. Association of the AB and CDEF domains from rat alpha- and beta-parvalbumin. Biochemistry. 2004;43:10906–10917. doi: 10.1021/bi049254d. [PubMed] [CrossRef] [Google Scholar]
16. Denessiouk K., Permyakov S., Denesyuk A., Permyakov E., Johnson M.S. Two structural motifs within canonical EF-hand calcium-binding domains identify five different classes of calcium buffers and sensors. PLoS ONE. 2014;9:e109287. doi: 10.1371/journal.pone.0109287. [PMC free article] [PubMed] [CrossRef] [Google Scholar]