
Genes (Basel). 2023 Jan; 14(1): 223.
Published online 2023 Jan 14. doi: 10.3390/genes14010223
PMCID: PMC9858982
PMID: 36672964
Parvalbumin: A Major Fish Allergen and a Forensically Relevant Marker
Subham Mukherjee,1,2 Petra Horka,1 Kamila Zdenkova,3 and Eliska Cermakova2,3,*
John H. Postlethwait, Academic Editor
Author information Article notes Copyright and License information PMC Disclaimer
Associated DataData Availability Statement
Abstract
Parvalbumins (PVALBs) are low molecular weight calcium-binding proteins. In addition to their role in many biological processes, PVALBs play an important role in regulating Ca2+ switching in muscles with fast-twitch fibres in addition to their role in many biological processes. The PVALB gene family is divided into two gene types, alpha (α) and beta (β), with the β gene further divided into two gene types, beta1 (β1) and beta2 (β2), carrying traces of whole genome duplication. A large variety of commonly consumed fish species contain PVALB proteins which are known to cause fish allergies. More than 95% of all fish-induced food allergies are caused by PVALB proteins. The authentication of fish species has become increasingly important as the seafood industry continues to grow and the growth brings with it many cases of food fraud. Since the PVALB gene plays an important role in the initiation of allergic reactions, it has been used for decades to develop alternate assays for fish identification. A brief review of the significance of the fish PVALB genes is presented in this article, which covers evolutionary diversity, allergic properties, and potential use as a forensic marker.
파발부민(PVALB)은
저분자량 칼슘 결합 단백질입니다.
PVALB는 많은 생물학적 과정에서의 역할 외에도
빠른 수축 섬유를 가진 근육에서 Ca2+ 전환을 조절하는 데 중요한 역할을 합니다.
PVALB 유전자군은
알파(α)와 베타(β)의 두 가지 유전자 유형으로 나뉘며,
β 유전자는 다시 베타1(β1)과 베타2(β2)의 두 가지 유전자 유형으로 나뉘어
전체 게놈 복제의 흔적을 지니고 있습니다.
일반적으로 섭취하는 다양한 어종에는
생선 알레르기를 유발하는 것으로 알려진
PVALB 단백질이 포함되어 있습니다.
모든 생선으로 인한
식품 알레르기의 95% 이상이
PVALB 단백질에 의해 발생합니다.
수산물 산업이 지속적으로 성장하고 식품 사기가 빈번하게 발생함에 따라 어종 인증은 점점 더 중요해지고 있습니다.
PVALB 유전자는
알레르기 반응의 시작에 중요한 역할을 하기 때문에
수십 년 동안 어종 식별을 위한 대체 분석법을 개발하는 데 사용되어 왔습니다.
이 글에서는
진화적 다양성,
알레르기 특성 및 법의학 마커로서의 잠재적 사용 가능성을 다루는
어류 PVALB 유전자의 중요성에 대해 간략하게 살펴봅니다.
Keywords: parvalbumin, fish identification, allergen, gene diversity, forensic marker
1. Introduction
Parvalbumins (PVALBs) are small-size calcium-binding proteins with molecular weights ranging from 10–12.5 kDa related in structure to calmodulin and troponin C. They are generally expressed in the highest quantity in fast-twitching muscles but are also expressed in other tissues and organs such as the brain and gonads of fishes [1,2]. PVALBs were first discovered in fish and amphibian muscle fibres in 1934 by Deuticke [3] and were later crystallised by Henrotte in 1952 [4] from carp muscle [1]. Despite being called “parvalbumin” by Pechère et al. [5] because of its low molecular weight and high solubility in water, it has no functional similarity to the protein serum albumin. In 1971, Pechère et al. highlighted its binding affinity towards Ca2+ [6]. In the following year, the 3D structure of PVALB, the first protein capable of binding calcium, was published [7].
Parvalbumins play a critical role in many biological processes. An essential function of PVALBs is to regulate the intracellular Ca2+ exchange in fast-twitch muscle fibres. The PVALB proteins are acidic (intracellular isoelectric point, pI: 4.1–5.2) and have a high affinity for Ca2+ and can bind two Ca2+ ions per molecule [8,9]. PVALB’s also aid in the relaxation process of fast-contracting muscles in vertebrates by carrying Ca2+ from troponin C to the sarcoplasmic reticulum via the ATPase pump [10]. Regulating the process is really important because, if Ca2+ switching in muscle fibres is left unchecked, it can cause shifts in Ca2+ homeostasis, ultimately leading to significant health issues such as Alzheimer’s in humans [11]. Moreover, PVALBSs have been observed to contribute to a variety of swimming forms in fish. Swimming form is a specific pattern of swimming behavior, such as a fast start or a C-bend [8,12]. In fish, the PVALB content in muscle varies from 0 to >1.5 mmol per litre [13]. In addition, the muscle relaxation rate varies longitudinally within a fish due to the variation in PVALB expression along its length [14]. Apart from this, PVALBs have also been detected immunohistochemically in non-muscle tissues, including bone, teeth, skin, brain, seminal vesicles, testes, and ovaries [2,15]. The parvalbumin protein belongs to the calcium-binding protein family of food allergens [16], and it has the ability to survive high temperatures such as many other food allergens [17,18], as well as enzymatic digestion and food processing systems [19]. The IgE reactivity of PVALB is, however, reported to decrease when the tissues are heated to 140 °C, as well as when various seafood processing methods are used [20,21].
The PVALB gene also provides an interesting marker for fish identification. The highly conserved four exons and three introns make it an appropriate tool for the authentication of fish species, as well as serving as a tool for forensic applications in case of fish frauds such as species substitution. This genomic marker provides an alternative to mitochondrial-based markers used for identification, which can be highly useful when comparing closely related fish species [22,23,24,25].
Fish muscles usually express 2–5 PVALB isoforms in their white muscle all through development from larval to adult forms [8], whereas a maximum of seven PVALB isoforms were detected in the white muscle of the adult common snook, Centropomus undecimalis [26]. Genetic polymorphism of PVALBs was observed in various fish species, including Cyprinus carpio, Carassius cavatus, Acanthopagrus schlegeli, Tinca tinca, etc. [2,27]. However, due to the wide-ranging distribution, species-specific expression, and unique electrophoretic mobility, together with exceptional stability, make PVALBs an excellent promising molecular marker for species identification [1].
This article reviews the significance of fish PVALB genes. We provide a brief summary about the fish PVALB evolutionary lineage and diversity in teleost fishes. We will also briefly describe the allergenic properties of PV, and its potential as a marker for fish identification, detection of allergens, and forensic application (Figure 1).
파발민(PVALB)은
칼모둘린 및 트로포닌 C와 구조적으로 관련된
분자량 10-12.5 kDa 범위의 작은 크기의 칼슘 결합 단백질로,
일반적으로 빠르게 움직이는 근육에서 가장 많이 발현되지만
물고기의 뇌 및 생식선과 같은 다른 조직과 기관에서도 발현됩니다 [1,2].
PVALB는
1934년 도이티케에 의해 어류와 양서류 근육 섬유에서 처음 발견되었고[3],
이후 1952년 헨로트에 의해 잉어 근육에서 결정화되었습니다[4][1].
분자량이 낮고 물에 대한 용해도가 높기 때문에
페셰르 등[5]에 의해 '파발부민'이라고 불렸지만,
단백질 혈청 알부민과 기능적 유사성은 없습니다.
1971년 Pechère 등은
Ca2+에 대한 결합 친화성을 강조했습니다[6].
이듬해에는
칼슘과 결합할 수 있는 최초의 단백질인 PVALB의 3D 구조가 발표되었습니다[7].
파발체는 많은 생물학적 과정에서 중요한 역할을 합니다.
PVALB의 필수 기능은 빠르게 수축하는 근육 섬유에서 세포 내 Ca2+ 교환을 조절하는 것입니다. PVALB 단백질은 산성(세포 내 등전점, pI: 4.1-5.2)이며 Ca2+에 대한 친화력이 높고 분자당 2개의 Ca2+ 이온과 결합할 수 있습니다[8,9]. 또한 PVALB는 ATPase 펌프를 통해 트로포닌 C에서 소포체로 Ca2+를 운반하여 척추동물에서 빠르게 수축하는 근육의 이완 과정을 돕습니다 [10].
이 과정을 조절하는 것은 매우 중요한데,
근육 섬유의 Ca2+ 전환을 방치하면
Ca2+ 항상성에 변화를 일으켜
궁극적으로 인간의 알츠하이머와 같은 심각한 건강 문제를 일으킬 수 있기 때문입니다 [11].
또한,
PVALBS는
물고기의 다양한 유영 형태에 기여하는 것으로 관찰되었습니다.
수영 형태는 빠른 출발이나 C자 굽힘과 같은 특정 수영 행동 패턴입니다 [8,12].
어류의 경우 근육 내 PVALB 함량은 리터당 0~1.5mmol까지 다양합니다[13].
또한, 근육 이완 속도는 길이에 따른 PVALB 발현의 변화로 인해 어류 내에서 세로로 다양합니다 [14]. 이 외에도 PVALB는 뼈, 치아, 피부, 뇌, 정낭, 고환 및 난소를 포함한 비근육 조직에서도 면역조직화학적으로 검출되었습니다 [2,15].
파발부민 단백질은
식품 알레르겐의 칼슘 결합 단백질 계열에 속하며[16],
효소 소화 및 식품 가공 시스템[19]과 같은
고온에서도 생존할 수 있는 능력을 가지고 있습니다.
그러나
조직을 140°C로 가열하거나
다양한 해산물 가공 방법을 사용할 경우
PVALB의 IgE 반응성이 감소하는 것으로 보고되고 있습니다[20,21].
PVALB 유전자는 또한 어류 식별을 위한 흥미로운 마커를 제공합니다. 고도로 보존된 4개의 엑손과 3개의 인트론은 어종 인증에 적합한 도구일 뿐만 아니라 종 대체와 같은 어류 사기 사건의 경우 법의학 응용 도구로도 사용할 수 있습니다. 이 게놈 마커는 식별에 사용되는 미토콘드리아 기반 마커의 대안을 제공하며, 이는 밀접하게 관련된 어종을 비교할 때 매우 유용할 수 있습니다[22,23,24,25].
어류 근육은
일반적으로 유충에서 성체로 성장하는 동안
백색 근육에서 2~5개의 PVALB 이소폼을 발현하는 반면[8],
성체 스누크인 센트로포무스 운데시말리스(Centropomus undecimalis)의 백색 근육에서는
최대 7개의 PVALB 이소폼이 검출되었습니다[26].
PVALB의 유전적 다형성은 사이프리누스 카르피오, 카라시우스 카바투스, 아칸토파그루스 슐레겔리, 틴카 틴카 등 다양한 어종에서 관찰되었습니다. [2,27]. 그러나 광범위한 분포, 종 특이적 발현, 독특한 전기영동성, 뛰어난 안정성으로 인해 PVALB는 종 식별에 매우 유망한 분자 마커가 될 수 있습니다[1].
이 글에서는 어류 PVALB 유전자의 중요성에 대해 살펴봅니다. 어류 PVALB의 진화 계통과 원시 어류의 다양성에 대해 간략하게 요약합니다. 또한 PV의 알레르기 유발 특성과 어류 식별, 알레르기 유발 물질 검출 및 법의학 적용을 위한 마커로서의 잠재력에 대해서도 간략하게 설명합니다(그림 1).
Overview of the importance and applicability of fish parvalbumin.
2. Parvalbumin Gene Diversity
The PVALB gene family is divided into two gene types, alpha (α) and beta (β), with the β gene further divided into two gene types, beta1 (β1) and beta2 (β2). These two phylogenetically distinct gene types of PV, α and β, have different isoelectric points (α, pI > 5.0; β, pI < 4.5). Since PVALBs belonging to the α-gene type have fewer amino acid residues than the β-gene type, they have higher isoelectric points than the β-gene type. They also differ in their amino acid sequences [28,29]. While α-genes only express 95-111 amino acid residues, β-genes express 106-113 amino acids [30]. They also have different crystal structures, and physiological roles, as well as magnesium and calcium ion affinities. For example, PVALBs of the β-gene type bind more effectively to Ca2+ ions than α-lineage PVALBs (200% better affinity), but in the case of Mg2+ ions, β-gene type PVALBs only have a 16% better affinity [31,32]. In the case of fishes, bony fishes predominantly express β-PVALB in muscle tissue while cartilaginous fishes (e.g., rays and sharks) express α-PVALBs in muscle tissue, resulting in lower allergic incidence in cartilaginous fishes. Through X-ray diffraction spectroscopy, it has been shown that the PVALB protein can be divided into three domains: AB, CD, and EF. While AB domains play a crucial role in protecting the hydrophobic core of the protein as well as hydrophobic parts of the functional EF hands from solvents, CD and EF domains are involved in the calcium-binding system (Figure 2) [33]. The EF domain is the most well-defined and has been used to characterise the canonical EF-hand Ca2+ binding motif [34].
PVALB 유전자군은 알파(α)와 베타(β)의 두 가지 유전자 유형으로 나뉘며, β 유전자는 다시 베타1(β1)과 베타2(β2)의 두 가지 유전자 유형으로 나뉩니다. 계통 발생학적으로 서로 다른 두 가지 유형의 PV, α와 β는 서로 다른 등전점(α, pI > 5.0; β, pI < 4.5)을 가지고 있습니다.
α 유전자 유형에 속하는 PVALB는
β 유전자 유형보다 아미노산 잔기가 적기 때문에
β 유전자 유형보다 더 높은 등전점을 갖습니다.
α 유전자는 95-111개의 아미노산 잔기만 발현하는 반면,
β 유전자는 106-113개의 아미노산을 발현합니다[30].
또한
결정 구조와 생리적 역할,
마그네슘과 칼슘 이온 친화력도 서로 다릅니다.
예를 들어, β- 유전자 유형의 PVALB는 α- 계통 PVALB보다 Ca2+ 이온에 더 효과적으로 결합하지만(200% 더 나은 친화력), Mg2+ 이온의 경우 β- 유전자 유형 PVALB는 16% 더 나은 친화력만 가지고 있습니다[31,32]. 어류의 경우 뼈가 있는 어류는 주로 근육 조직에서 β-PVALB를 발현하는 반면 연골성 어류(예: 가오리, 상어)는 근육 조직에서 α-PVALB를 발현하여 연골성 어류의 알레르기 발생률이 더 낮습니다. X-선 회절 분광법을 통해 PVALB 단백질은 세 가지 영역으로 나눌 수 있음이 밝혀졌습니다: AB, CD, EF. AB 도메인은 단백질의 소수성 코어와 기능성 EF 손의 소수성 부분을 용매로부터 보호하는 데 중요한 역할을 하는 반면, CD와 EF 도메인은 칼슘 결합 시스템에 관여합니다(그림 2) [33]. EF 도메인은 가장 잘 정의되어 있으며 표준 EF-손 Ca2+ 결합 모티프를 특성화하는 데 사용되었습니다 [34].
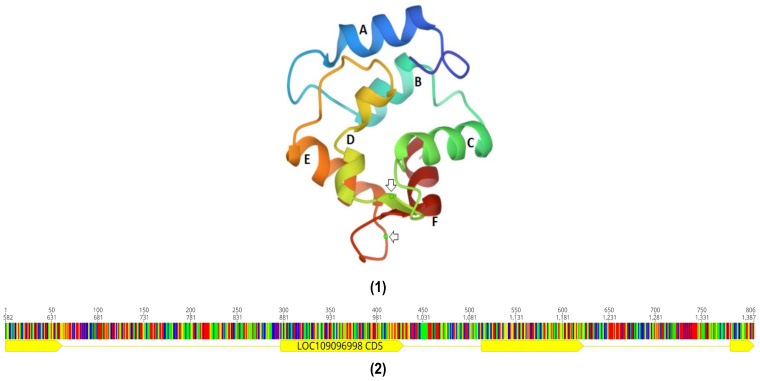
Parvalbumin structure. (1) Carp parvalbumin beta protein ribbon structure. Six helixes (A, B, C, D, E, F) and two Ca2+ ions (Arrow) [35]; (2) Carp parvalbumin gene structure. Four exons (Yellow) and three introns [36].
Regardless of overall structural similarity to β genes, α-PVALB is generally non-allergic in fishes. However, α-PVALBs in frog, chicken, and crocodile meat also act as allergens in humans. While sequencing has revealed that chances of cross-reactivity between fish PVALBs with its mammalian or avian homologs is low and IgE cross-reactivity is unlikely it is still advisable for sensitized consumers to ascertain caution when consuming these products [37]. On the molecular level β isoforms also differ significantly from α isoforms. For example, alignment of the sequencing data of three PVALB gene types of Atlantic salmon (Salmo salar) reveals that a higher similarity is observed between the two β-gene types than between the α-gene and either β-gene types. When PVALB β1 and β2 were compared amongst each other 71.8 % nucleotide similarity was observed. However, the similarity was significantly reduced to 61.1 % when PVALB α and PVALB β isoforms were compared [2].
The parvalbumin gene family, such as that of the HOX gene family, is a conserved gene and carries traces of whole genome duplication [38]. The origin of PVALB-α, PVALB-β1and PVALB-β2 gene types have been attributed to vertebrate ancestors [39]. The diversity of PVALB genes is a possible result of the occurrence of vertebrate-specific whole genome duplication. In a recent study by Mukherjee et al. [2], a high diversity of PVALB genes was observed in teleost fishes. Apart from ancestral vertebrate gene duplication, several teleost lineages such as the Salmoniformes, Cyprinidae, and Sturgeons underwent additional lineage-specific duplication events giving rise to even more clusters of PVALB genes with higher diversity. The study reported a variable number of PVALB gene copies within the teleosts, from seven copies in Esox Lucius (Pike) to 22 copies in S. salar (Atlantic salmon) (Figure 3).
β 유전자와의 전반적인 구조적 유사성에 관계없이
α-PVALB는 일반적으로 생선에서는 알레르기를 일으키지 않습니다.
그러나
개구리, 닭고기, 악어 고기의 α-PVALB는
사람에게도 알레르겐으로 작용합니다.
염기서열 분석 결과
포유류 또는 조류 상동체와
어류 PVALB 간의 교차 반응 가능성은 낮고
IgE 교차 반응 가능성은 낮지만,
민감한 소비자는 이러한 제품을 섭취할 때 주의하는 것이 좋습니다[37].
분자 수준에서 β 이소포름은
α 이소포름과도 크게 다릅니다.
예를 들어, 대서양 연어(살모 살라)의 세 가지 PVALB 유전자 유형의 염기서열 데이터를 정렬한 결과 α 유전자와 두 가지 β 유전자 유형 간의 유사성이 α 유전자 유형과 두 가지 β 유전자 유형 간의 유사성보다 더 높은 것으로 나타났습니다. PVALB β1과 β2를 서로 비교했을 때 71.8%의 뉴클레오티드 유사성이 관찰되었습니다. 그러나 PVALB α와 PVALB β 이소형을 비교했을 때는 유사성이 61.1%로 크게 감소했습니다[2].
HOX 유전자 계열과 같은 파발부민 유전자 계열은 보존된 유전자이며 전체 게놈 복제의 흔적을 가지고 있습니다 [38]. PVALB-α, PVALB-β1 및 PVALB-β2 유전자 유형의 기원은 척추동물 조상으로부터 기인한 것으로 알려져 있습니다[39]. PVALB 유전자의 다양성은 척추동물 특이적인 전체 게놈 복제의 결과일 수 있습니다. 무커지 등[2]의 최근 연구에 따르면, 원시 어류에서 높은 다양성의 PVALB 유전자가 관찰되었습니다. 조상 척추동물 유전자 복제 외에도 연어목, 키프리니과, 철갑상어목과 같은 몇몇 텔레오스트 계통은 추가적인 계통 특이적 복제 사건을 겪으며 더 많은 다양성을 가진 PVALB 유전자 클러스터를 생성했습니다. 이 연구에서는 Esox Lucius(파이크)의 7개에서 S. salar(대서양 연어)의 22개까지 다양한 수의 PVALB 유전자 사본이 보고되었습니다(그림 3).
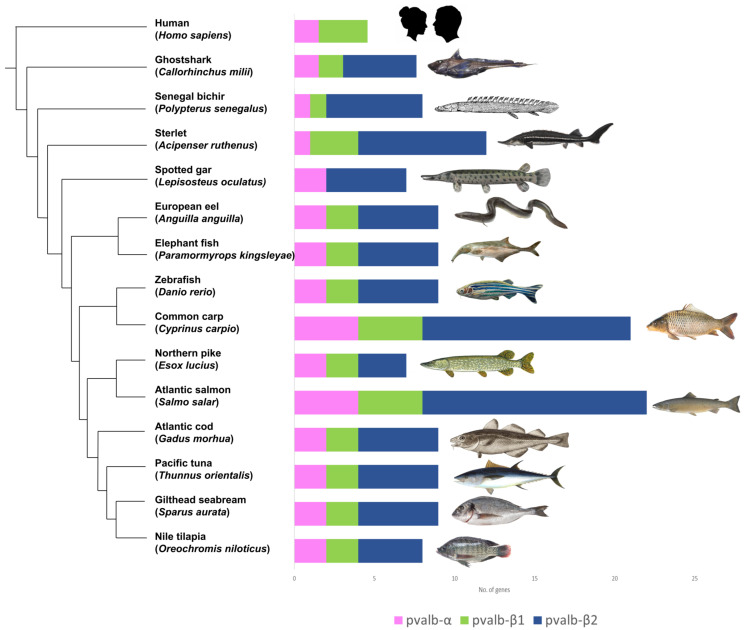
Phylogentic relation of parvalbumin in selected species of the teleost fishes and a non-teleost outgroup. Parvalbumin genes found in the selected fish genomes are shown in groups and coloured by the parvalbumin gene type, i.e., PVALB-α in pink, PVALB-β1 in green, and PVALB-β2 in blue The phylogenetic relation is according to the work of Mukherjee et al. [2].
The diversity of the three PVALB genes was also observed among non-bony fish vertebrates such as Callorhinchus milii (ghost shark), Gallus gallus (chicken), Homo sapiens (human), Rattus norvegicus (mouse), and Xenopus laevis (frog). Amongst the three gene types, the PVALB β2 gene was the most diverse in teleost fishes, with salmon possessing up to 14 copies of the ancestral PVALB β2. Homo sapiens (humans) possess one PVALB α, one PVALEF, two oncomodulin {OCM (OCM, OCM2)}, and three calmodulin (CALM) genes. Oncomodulin genes OCM and OCM2 are both similar to the fish PVALB β1 gene. Belonging to the EF-hand protein family, oncomodulin genes express proteins to increase the calcium-ion binding affinity. These proteins are also found in early embryonic cells in the placenta and can also be found in tumors [40].
While this gene (PVALB β2) is most diverse in teleost fishes, it is absent in mammals such as humans and mice [2]. Due to the presence of all three isoforms in a single species, as observed in teleost’s fishes, a layered complexity is added for diagnosing, detecting, and effectively managing allergic PVALB [41]. So, albeit having α, β1, and β2, the gene types are also present in multiple copies. Phylogenetic analysis along with structural and biochemical investigations have assigned α and β PVALBs as separate clusters [2]. While the α subtype is present in humans and other vertebrates such as the house mouse (Mus musculus), subtype β2 is absent in them. The allergenicity of these two gene types may be due to the distantly separated clusters of PVALBs.
텔레오스트 어류의 일부 종과 텔레오스트가 아닌 아웃그룹의 파발부민의 계통학적 관계.
선택된 어류 게놈에서 발견된 파발부민 유전자를 그룹으로 표시하고 파발부민 유전자 유형에 따라 색상을 지정했습니다(예: 분홍색은 PVALB-α, 녹색은 PVALB-β1, 파란색은 PVALB-β2) 계통학적 관계는 Mukherjee 등[2]의 연구에 따릅니다.
세 가지 PVALB 유전자의 다양성은 뼈가 없는 어류 척추동물인 칼로린쿠스 밀리(Callorhinchus milii, 유령 상어), 갈루스 갈루스(Gallus gallus, 닭), 호모 사피엔스(Homo sapiens, 인간), 라투스 노르베기쿠스(Rattus norvegicus, 쥐), 제노퍼스 라비스(Xenopus laevis, 개구리) 등에서도 관찰되었습니다. 세 가지 유전자 유형 중 PVALB β2 유전자는 원시 어류에서 가장 다양했으며, 연어는 최대 14개의 조상 PVALB β2 유전자를 보유하고 있었습니다. 호모 사피엔스(인간)는 1개의 PVALB α, 1개의 PVALEF, 2개의 온코모둘린{OCM(OCM, OCM2)}, 3개의 칼모둘린(CALM) 유전자를 보유하고 있습니다. 온코모둘린 유전자 OCM과 OCM2는 모두 어류의 PVALB β1 유전자와 유사합니다. EF-손 단백질 계열에 속하는 온코모둘린 유전자는 칼슘 이온 결합 친화력을 증가시키는 단백질을 발현합니다. 이 단백질은 태반의 초기 배아 세포에서도 발견되며 종양에서도 발견될 수 있습니다 [40].
이 유전자(PVALB β2)는 원시 어류에서 가장 다양하지만, 인간과 생쥐와 같은 포유류에는 없습니다 [2]. 텔레오스트 어류에서 관찰된 것처럼 한 종에 세 가지 이소형이 모두 존재하기 때문에 알레르기성 PVALB를 진단, 감지 및 효과적으로 관리하기 위한 계층적 복잡성이 추가됩니다 [41]. 따라서 α, β1, β2를 가지고 있지만 유전자 유형도 여러 사본으로 존재합니다. 구조적 및 생화학적 조사와 함께 계통학적 분석을 통해 α 및 β PVALB를 별도의 클러스터로 지정했습니다[2]. α 아형은 인간과 집쥐(Mus musculus)와 같은 다른 척추동물에 존재하는 반면, β2 아형은 존재하지 않습니다. 이 두 유전자 유형의
3. Parvalbumin—A Major Fish Allergen
Food allergy is an immunologically based adverse reaction to food or food additives. Allergies caused by foods are deemed a significant hazard to public health, particularly for those sensitive to an allergic reaction. Peanuts, soy, milk, shellfish, fish, and tree nuts are some common foods that induce allergic reactions [42]. These food sources contain high levels of allergens, which remain stable when processed and digested in the body [43]. While some allergens degrade during the process of digestion, their fragments are still identified by IgE antibodies that instigate an allergic reaction [44].
Nowadays, fish allergy is one of the most frequently occurring food allergies among children and adults [45]. Fish is an integral part of the human diet and nutrition since it is rich in essential lipid-soluble vitamins, polyunsaturated fatty acids (such as docosahexaenoic acid and eicosapentaenoic acid), and essential amino acids. Even though fish intake in landlocked countries continues at a reasonably stable state, the overall demand for fish and fish products continues to increase worldwide [45,46].
PVALBs are responsible for more than 95% of fish-induced food allergies [28]. Studies provide evidence that the PVALB protein is a fish allergen for a wide range of commonly consumed species, including salmon, carp, mackerel, tuna, and pilchard [47,48]. Due to the high cross-reactivity of β-PVALB from different species, more than 90% of people sensitive to fish usually have allergic reactions to several species of fish. Researchers found a 50% possibility of reacting to PVALB from more than one fish species [49,50,51]. The allergen cross-reactivity can be evaluated by comparing amino acid sequences of isoallergen. While certain fishes such as S. salar (Atlantic salmon) and Rastrelliger kanagurta (Indian mackerel) possess only one PVALB isoallergen, others such as Gadus morhua (Atlantic cod), Lates calcarifer (Baramundi), and Clupea harengus (Atlantic herring) contain more than one (Table 1). Fish species that are closely related also exhibit marked clinical cross-reactivity. A significant number of food-allergic individuals may suffer severe health problems due to unintentionally consuming products containing undeclared seafood [52]. Cross-reactive PVALB epitopes are located in highly conserved protein regions, especially at the ion-binding sites [53]. Oral allergy syndrome and rhinitis are general clinical manifestations, as well as diarrhea, abdominal pain, angioedema, urticaria, asthma, and, in severe cases, life-threatening anaphylactic reactions.
파르발부민- 주요 생선 알레르기 유발 물질
식품 알레르기는
식품이나 식품 첨가물에 대한 면역학적인 이상 반응입니다.
식품으로 인한 알레르기는
특히 알레르기 반응에 민감한 사람들에게
공중 보건에 심각한 위험을 초래하는 것으로 간주됩니다.
땅콩, 콩, 우유, 조개 및 갑각류, 생선, 견과류는
알레르기 반응을 유발하는 대표적인 식품입니다[42].
이러한 식품에는
높은 수준의 알레르겐이 포함되어 있으며,
이는 체내에서 가공 및 소화될 때 안정적으로 유지됩니다[43].
일부 알레르겐은
소화 과정에서 분해되지만,
그 조각은 여전히 알레르기 반응을 유발하는 IgE 항체에 의해 식별됩니다 [44].
오늘날
생선 알레르기는
어린이와 성인 사이에서 가장 빈번하게 발생하는
식품 알레르기 중 하나입니다 [45].
생선은
필수 지용성 비타민,
고도 불포화 지방산(도코사헥사에노산, 에이코사펜타엔산 등),
필수 아미노산이 풍부하기 때문에
인간의 식단과 영양에서 없어서는 안 될 중요한 부분입니다.
내륙 국가의 생선 섭취량은 상당히 안정적인 상태를 유지하고 있지만, 전 세계적으로 생선 및 수산물의 전반적인 수요는 계속 증가하고 있습니다[45,46].
PVALB는
생선으로 인한 식품 알레르기의 95% 이상을 유발합니다 [28].
연구에 따르면
연어, 잉어, 고등어, 참치, 정어리 등
일반적으로 소비되는 다양한 어종에 대해
PVALB 단백질이 생선 알레르겐이라는 증거가 있습니다 [47,48].
다른 종의 β-PVALB는 교차 반응성이 높기 때문에
생선에 민감한 사람의 90% 이상이
일반적으로 여러 종의 생선에 알레르기 반응을 보입니다.
연구자들은
두 가지 이상의 어종에서 나온
PVALB에 반응할 가능성이
알레르겐 교차 반응성은
동종 알레르겐의 아미노산 서열을 비교하여 평가할 수 있습니다.
S. 살라(대서양 연어)와
라스트렐리거 카나구르타(인도 고등어)와 같은 특정 어류는
PVALB 동종 알레르겐을 하나만 가지고 있지만,
가두스 모화(대서양 대구), 라테스 칼카리퍼(바라문디), 클루페 하렌구스(대서양 청어) 같은 다른 어류는
둘 이상을 포함하고 있습니다(표 1).
밀접한 관련이 있는 어종은
임상적으로도 뚜렷한 교차 반응성을 보입니다.
상당수의 식품 알레르기가 있는 사람은
신고되지 않은 해산물이 포함된 제품을 의도치 않게 섭취하여
심각한 건강 문제를 겪을 수 있습니다[52].
교차 반응성 PVALB 에피토프는
고도로 보존된 단백질 영역,
특히 이온 결합 부위에 위치합니다 [53].
구강 알레르기 증후군과 비염은
일반적인 임상 증상이며
설사, 복통, 혈관 부종, 두드러기, 천식,
심한 경우 생명을 위협하는 아나필락시스 반응을 일으킬 수 있습니다.
Table 1
Fish and non-fish parvalbumin allergens registered with the WHO (www.allergen.org (accessed on 15 November 2022)).
Name of AllergenOrganismBiochemical NameGenbank Nucleotide Accession no.ReferenceScientific NameCommon Name
| Fish species | |||||
| Clu h 1 | Clupea harengus | Atlantic herring | β-parvalbumin | FM178220 | [61] |
| FM178221 | |||||
| FM178222 | |||||
| Cten i 1 | Ctenopharyngodon idella | Grass carp | β-parvalbumin | MK140606 | [62] |
| Cyp c 1 | Cyprinus carpio | Common carp | β-parvalbumin | AJ292211 | [63] |
| AJ292212 | |||||
| Gad c 1 | Gadus callarias | Baltic cod | β-parvalbumin | [64] | |
| Gad m 1 | Gadus morhua | Atlantic cod | β-parvalbumin | AY035584 | [65] |
| AM497927 | |||||
| AY035585 | |||||
| AM497928 | |||||
| lat c 1 | Lates calcarifer | Baramundi | β-parvalbumin | AY688372 | [66] |
| KF021278 | |||||
| AY626068 | |||||
| KF021279 | |||||
| AY688373 | |||||
| Lep w 1 | Lepidorhombus whiffiagonis | Megrim, whiff, turbot fish | β-parvalbumin | AM904681 | [19] |
| Onc m 1 | Oncorhynchus mykiss | Rainbow trout | β-parvalbumin | not specified | [67] |
| Pan h 1 | Pangasianodon hypophthalmus | Striped catfish | β-parvalbumin | XM_026916202 | [68] |
| XM_026947968 | |||||
| Ras k 1 | Rastrelliger kanagurta | Indian mackerel | parvalbumin | KX527884 | [69] |
| Sal s 1 | Salmo salar | Atlantic salmon | β-parvalbumin 1 | X97824 | [70] |
| Sar sa 1 | Sardinops sagax | Pacific pilchard | β-parvalbumin | FM177701 | [47] |
| Sco s 1 | Scomber scombrus | Atlantic mackerel | parvalbumin | FM994926 | [48] |
| Seb m 1 | Sebastes marinus | Ocean perch, redfish | β-parvalbumin | FM178218 | [71] |
| FM178219 | |||||
| Sole s 1 | Solea solea | Sole | parvalbumin | [72] | |
| Thu a 1 | Thunnus albacares | Yellow fin | β-parvalbumin | FM178217 | [73] |
| Xip g 1 | Xiphias gladius | Swordfish | β-parvalbumin | FM202668 | [19] |
| Non-fish Species | |||||
| Cro p 1 | Crocodylus porosus | Australian saltwater crocodile | β-parvalbumin | XM_019542160 | [74] |
| Cro p 2 | α-parvalbumin | XM_019544844 | |||
| Gal d 8 | Gallus domesticus | Chicken | α-parvalbumin | FM994924 | [75] |
| [76] | |||||
| Ran e 1 | Rana esculenta (Pelophylax esculentus) | Edible frog | α-parvalbumin | AJ315959 | [77] |
| Ran e 2 | Rana esculenta (Pelophylax esculentus) | Edible frog | β-parvalbumin | AJ414730 | |
For allergy sufferers, the only effective way to prevent an adverse reaction in the event of exposure to the allergenic food is to avoid seafood altogether or, in the case of accidental exposure, to use therapeutic treatment (e.g., antihistamines, corticosteroids, epinephrine) [54]. However, it has been shown that even patients with extreme fish sensitivities can consume certain kinds of fish, such as tuna, without an untoward event [55,56]. In addition, molecule-specific epitope regions are known, such as PVALBs from salmonid fish, explaining a limited cross-reactivity with these species. Nonetheless, there are substantial differences in PVALB content between fish species, and these variations correlate with differences in the allergenicity of fish PVALBs [28,57]. For example, Kuehn et al. [28] demonstrated that the PVALB level in fish muscles is up to 100 times higher for carp than mackerel or tuna. They found an average of <0.05 mg/g PVALB in tuna; 30.7 mg/g in mackerel; 12.5 mg/g in salmon, trout, and cod; and >2.5 mg/g in carp, herring, and redfish. The amount of PVALB depends not only on the fish species but also on the method of preparation. PVALB allergenicity decreases due to fish processing through cooking, baking, and smoking; due to this variability, people with a clinically relevant sensitization to PVALB may still eat processed fish with a lower concentration of PVALB without a reaction. In the case of fish allergen PVALB, isoforms and the extent of thermal processing influence antibody reactivity. Saptarshi et al. [18] validated this by comparing PVALBs in a wide-range of fishes (raw and heated fish extracts) from the Asia-Pacific region through immunoblotting experiments. They found variations in the thermal stability of PVALB within the tested fish genera, and their results demonstrate that heat processing the antigen significantly affects the reactivity of antibodies to PVALBs. Human antibodies reacted less strongly to multimeric bony fish PVALBs after heating, whereas antibodies lost reactivity completely in cartilaginous fish.
Apart from clinical advances, it has become imperative to improve consumer protection through an accurate food labelling system preventing potentially life-threatening risks for sensitized/allergic individuals [58]. According to recent European Union (EU) regulations, food manufacturers are required to declare the presence of 14 food groups classified as potentially allergenic, namely fish, crustaceans, molluscs, celery, mustard, sesame seeds, gluten, tree nuts, peanuts, milk, eggs, soybeans, lupins and sulphites, and highlighting them from the list of other ingredients [59].
As of 2022, there are more than 290 entries in the Allergome database (www.allergome.org) for fish PV and its isoforms and allergens [60]. Out of these 290, 27 isoforms from 17 fish species are registered and documented with the World health organization (WHO) and the International Union of Immunological Societies (IUIS) (Table 1).
알레르기 환자의 경우,
알레르기 유발 식품에 노출되었을 때 부작용을 예방하는 유일한 효과적인 방법은
해산물을 완전히 피하거나 실수로 노출되었을 경우
치료적 치료(예: 항히스타민제, 코르티코스테로이드, 에피네프린)를 사용하는 것입니다[54].
그러나
생선 과민증이 극심한 환자도
참치와 같은 특정 종류의 생선을 섭취해도 별다른 이상 반응 없이 섭취할 수 있는 것으로 나타났습니다[55,56].
또한 연어류의 PVALB와 같은
분자 특이적 에피토프 영역이 알려져 있어
이러한 어종과의 교차 반응성이 제한적이라고 설명합니다.
그럼에도 불구하고
어종 간 PVALB 함량에는 상당한 차이가 있으며,
이러한 차이는 어류 PVALB의 알레르기 유발성 차이와 관련이 있습니다[28,57].
예를 들어, Kuehn 등[28]은
잉어의 경우
고등어나 참치에 비해
생선 근육의 PVALB 수치가 최대 100배까지 높다는 사실을 입증했습니다.
참치에서는 평균 0.05 mg/g 미만,
고등어에서는 30.7 mg/g,
연어, 송어, 대구에서는 12.5 mg/g,
잉어, 청어, 홍어에서는 2.5 mg/g 이상의 PVALB를 발견했습니다.
PVALB의 양은
어종뿐만 아니라
조리 방법에 따라 달라집니다.
조리, 굽기, 훈제 등을 통한 생선 가공으로 인해
PVALB 알레르기 유발성은 감소하며,
이러한 가변성으로 인해 임상적으로 PVALB에 민감하게 반응하는 사람은
PVALB 농도가 낮은 가공 생선을 먹어도 반응 없이 섭취할 수 있습니다.
생선 알레르겐 PVALB의 경우, 이소형과 열처리 정도가 항체 반응성에 영향을 미칩니다. Saptarshi 등[18]은 면역 블 롯팅 실험을 통해 아시아 태평양 지역의 다양한 생선(날 생선 및 가열 생선 추출물)의 PVALB를 비교하여 이를 검증했습니다. 연구팀은 실험 대상 어종 내에서 PVALB의 열 안정성에 차이가 있음을 발견했으며, 그 결과 항원을 열처리하는 것이 PVALB에 대한 항체의 반응성에 큰 영향을 미친다는 사실을 입증했습니다. 인간 항체는 가열 후 다합체 뼈 어류 PVALB에 덜 강하게 반응한 반면, 연골 어류에서는 항체가 완전히 반응성을 잃었습니다.
임상적 발전과는 별개로,
민감성/알레르기 질환자에게 잠재적으로 생명을 위협할 수 있는 위험을 방지하는 정확한 식품 표시 시스템을 통해 소비자 보호를 개선하는 것이 필수적이 되었습니다[58].
최근 유럽연합(EU) 규정에 따르면
식품 제조업체는
생선, 갑각류, 연체동물, 셀러리, 겨자, 참깨, 글루텐, 견과류, 땅콩, 우유, 계란, 대두, 루핀, 아황산염 등
잠재적 알레르기 유발 식품으로 분류된 14가지 식품군을 다른 성분 목록에서 강조하여 표시해야 합니다[59].
2022년 현재, 어류 PV와 그 동형 및 알레르겐에 대한 Allergome 데이터베이스(www.allergome.org)에는 290개 이상의 항목이 있습니다[60]. 이 290개 중 17개 어종의 27개 이소폼이 세계보건기구(WHO)와 국제면역학회연맹(IUIS)에 등록되어 문서화되어 있습니다(표 1).
4. Forensic Application of Parvalbumin
The seafood industry is ever-increasing and as the industry expands, the issue of authentication of fish species becomes more imperative. With the increase in fish consumption and an uncertain supply and demand chain, cases of the surreptitious substitution of one species with another (fish fraud) are on the rise. While fish fraud not only amounts to economic deception, it can also have a detrimental effect on consumers’ health due to species-specific antigenicities discussed above and environmental management programs for endangered species. Regulatory authorities such as US FDA, FAO, and EU have established laws for labelling fish products to prevent product substitutes, however, these regulations can be difficult to enforce when morphological fish identification is not possible. Hence, apart from the enforcement of labelling regulations, research into various analytical methods for fish identification is carried out to overcome challenging situations such as precooked and frozen seafood.
Fishes are particularly unidentifiable through their external features in the case of landlocked countries since most fish is imported in the form of compact frozen blocks of meat or fillets [78]. Taxonomical identification is an ideal method for identifying fish species to prevent adulteration of fish, however, it is compromised when distinguishing features are removed (for example, head, fins, scales, and skin in fish) or if the specimen has been cooked. It may also be difficult to ascertain the geographic origin and also to visually identify fish due to the phenotypic resemblances of some fish species [79]. In many instances, globally, fraudsters take advantage of this fact and intentionally mislabel fish products by substituting a species of high worth with a low-priced alternative. Experts anticipate an increase in cases of food fraud due to COVID-19 regulations since there have been reduced private sector food inspections and audits and limitations in supply and demand [80,81]. Therefore, it is essential that standardized, accurate, and simple fish identification methods should be developed that have global use.
A number of assays and methodologies have been used to tackle the problem of fish identification in the past few decades. Since the PVALB gene plays an important role in inducing allergenic reactions, it has been used for decades to design assays to be used as an alternate approach for fish authentication. The assays can be broadly divided into protein-based assays that include electrophoretic [22,82], chromatographic [83] or immunological [84] methods, and DNA-based assays (PCR with species-specific or universal primers, DNA microarray) [85,86,87,88], and biosensor based assays [89,90].
수산물 산업은 계속 성장하고 있으며, 산업이 확장됨에 따라 어종에 대한 인증 문제가 더욱 중요해지고 있습니다. 생선 소비가 증가하고 수급망이 불확실해지면서 한 어종을 다른 어종으로 은밀하게 대체하는 사례(생선 사기)가 증가하고 있습니다. 생선 사기는 경제적 사기에 해당할 뿐만 아니라 위에서 언급한 종별 항원성 및 멸종 위기 종에 대한 환경 관리 프로그램으로 인해 소비자의 건강에도 해로운 영향을 미칠 수 있습니다.
미국 FDA, FAO, EU 등의 규제 당국에서는 대체품 사용을 방지하기 위해 수산물 표시 관련 법률을 제정하고 있지만, 형태학적 어류 식별이 불가능한 경우 이러한 규정을 시행하기 어려울 수 있습니다. 따라서 표시 규정 시행과는 별개로, 전처리 및 냉동 수산물과 같은 어려운 상황을 극복하기 위해 어류 식별을 위한 다양한 분석 방법에 대한 연구가 진행되고 있습니다.
특히 내륙국의 경우 대부분의 생선이 소형 냉동 덩어리 또는 필레 형태로 수입되기 때문에 외형적인 특징만으로는 식별이 불가능합니다[78]. 분류학적 식별은 어류의 혼입을 방지하기 위해 어종을 식별하는 이상적인 방법이지만, 생선의 머리, 지느러미, 비늘, 껍질 등의 특징이 제거되거나 표본이 조리된 경우 식별이 어려워집니다. 또한 일부 어종의 표현형적 유사성으로 인해 지리적 원산지를 확인하거나 어류를 육안으로 식별하기 어려울 수도 있습니다[79].
전 세계적으로 사기꾼들은 이러한 사실을 악용하여 고가의 어종을 저가의 대체품으로 대체하여 의도적으로 수산물의 원산지를 허위로 표시하는 경우가 많습니다. 전문가들은 코로나19로 인해 민간 부문의 식품 검사 및 감사가 축소되고 공급과 수요가 제한됨에 따라 식품 사기 사례가 증가할 것으로 예상하고 있습니다[80,81]. 따라서 전 세계적으로 사용할 수 있는 표준화되고 정확하며 간단한 어류 식별 방법을 개발하는 것이 필수적입니다.
지난 수십 년 동안 어류 식별 문제를 해결하기 위해 다양한 분석법과 방법론이 사용되어 왔습니다. PVALB 유전자는 알레르기 반응을 유도하는 데 중요한 역할을 하기 때문에 수십 년 동안 어류 인증을 위한 대체 접근법으로 사용할 분석법을 설계하는 데 사용되어 왔습니다. 분석법은 크게 전기영동[22,82], 크로마토그래피[83] 또는 면역학적[84] 방법을 포함하는 단백질 기반 분석법과 DNA 기반 분석법(종 특이적 또는 범용 프라이머를 사용한 PCR, DNA 마이크로어레이)[85,86,87,88], 바이오센서 기반 분석법[89,90]으로 나눌 수 있습니다.
4.1. Protein-Based Assays
Parvalbumin proteins can be regarded as a suitable biomarker for fish species authentication and have been detected and quantified using a variety of protein-based assays [91]. PVALB proteins are expressed in high concentrations in fish muscle and also have high interspecies variability. The interspecies variability of PVALB sequences is fundamental for the discrimination of different fish species as assessed by earlier proteomic analyses performed on species belonging to the Merlucciidae family (hake’s) [92]. PVALB solubility in aqueous buffers makes the extraction protocol both easy and extremely quick. The structural stability of PVALBs even under harsh conditions such as heat is paramount to utilising these biomarkers also for the authentication of fish species sold as thermally processed products [93,94].
Earlier, isoelectric focusing (IEF) was one of the most widely used methods for fish identification [95]. Urea IEF and IEF in immobilized pH gradients (IPG) have great practical significance in the analysis of both fresh and boiled samples. However, in urea IEF, standard marker proteins cannot be used due to modification of their conformation, which impacts their isoelectric points. [95,96]. In place of standard marker proteins, known fish PVALBs can serve as marker proteins in IEF gels for unknown PVALBs due to their thermostable properties and may also be employed in database generation for the differentiation and identification of diverse fish or other specimens [8]. Dobrovolov et al. [97] demonstrated the ability to differentiate various species of sturgeons and their origin using the IEF of sarcoplasmic protein (general muscle protein such as lactate dehydrogenase, malate dehydrogenase, or malic enzyme). Using a similar principle, PVALB was detected in three sturgeon species, Acipenser baeri, A. gueldenstaedtii, A. ruthenus [98]. Authentication of closely related scombrid, catfish, and tilapia species by isoelectric focusing of PVALB also provided a rapid screening method for identification [22]. IEF was also used to study mislabeling in cases of various Alaskan flatfishes in German meat markets [99].
In addition to IEF, tandem mass spectrometry has been used to define the structure of protein isoforms that are essential to understanding cross-reactivity among various allergenic proteins [100]. More recently, exploiting the improved performance of new instruments such as Fourier-transform ion-cyclotron resonance (FTICR) mass spectrometers and linear ion trap (LIT) mass spectrometers, innovative strategies for the extensive characterization of PVs have been proposed. These studies led to the de novo sequencing of 25 isoforms from all commercial species of the Merlucciidae family and the rapid and direct detection of the presence of fish allergens in all of the investigated food products [94,101]. Detection of allergens with high levels of sensitivity was also achieved by employing an optimized protein chip [102]. These assays were useful in detecting fish allergens thus increasing consumer safety.
MALDI-MS can be used in the quality control processes including the main issues of fish authentication and fraud detection. Apart from MALDI-MS, the application of MALDI-TOF in the mass spectra of sarcoplasmic proteins allows the authentication of fish species. Targeted proteomics has been applied to assess fish authenticity and detect allergens. Proteomics offers tools potentially suitable as routine tests in food authentication [103]. PVALBs exhibit a high ionization efficiency in MALDI-TOF MS analysis so that, regardless of the complexity of the analyzed sarcoplasmic extracts, the obtained mass spectra predominantly show signals originating from these proteins [100,104]. Proteomic studies integrating two-dimensional electrophoresis (2-DE) with MALDI-TOF MS peptide mass mapping for protein identification allowed the characterization of the 2-DE PVALB-specific pattern and the definition of a set of specific tryptic peptides suitable for the identification of nine hake species [105,106].
Studies have shown that immunoassays, including indirect ELISA and Western blotting, are the primary tools for detecting fish allergen changes following processing [107]. Despite being primary tools, immunoassays might be inaccurate due to cross-reactivity with nontarget allergenic proteins, and they might not be specific enough to detect allergens [108]. MALDI-MS also have certain disadvantages, such as in some cases, the inability of the system to differentiate between two related species may be due to the inherent similarity of the organisms themselves. Another reason that similar species may be incorrectly identified is a lack of sufficient spectra in the database. If this occurs, it is possible to obtain an incorrect species-level identification or no identification at all [109]. Hence, to overcome the limitations possessed by protein-based methods, DNA-based approaches for fish identification are intrinsically independent of biomolecular interactions and are thus slowly gaining more interest than protein-based methods [110].
4.2. Using DNA-Based Assays for Fish Identification
The genetic identification of species is based on DNA polymorphisms or genetic variations caused by naturally occurring mutations in the DNA [111]. The PVALB gene is a conserved gene with highly conserved exons in the protein-coding part of the PVALB gene, separated by three introns that are unique among various fish species [88]. PVALB can be used as an interesting universal marker for fish identification and allergen detection, as Sun et al. [25] and Rehbein [88] demonstrated. Both universal and species-specific assays have been designed in recent times for better detection of PVALB genes (Table 2).
Table 2
Universal and species-specific primers that have been used for detection of PVALB genes.
OrganismPrimer NamePrimer Sequence (5′-3′)Amplification Length (BP)Amplification RegionReferenceScientific NameCommon Name
| Universal | IFF232 | GACAAGAGCGGCTTCATTGAGG | 268 | β-parvalbumin | [122] | |
| IFF233 | TCAACTCCAATCTTGCCATCACCAT | Exon 3 to Exon 4 | ||||
| Universal | IFF 233a | TCAATACCGATCTTGCCATCACCGT | NA | [123] | ||
| IFF 233b | TCAACTCCGATCATGCCATCACCAT | |||||
| Universal | SUN-F | CAGGACAAGAGTGGCTTCAT | 57 | β2-parvalbumin | [25] | |
| SUN-R | GAAGTTCTGCAGGAACAGCTT | Exon2 to Exon 3 | ||||
| probe | AGGAGGAYGAGCT | |||||
| C. harengus | Atlantic herring Pacific herring | CluHaPaF | CCGCTGATGATGTGAAGAAG | 189 | β2-parvalbumin | [124] |
| Clupea pallasii | CluHaPaR | GCAGGAACAGCCTGAGAGAG | Exon 2 to Exon 3 | |||
| Cyprinus carpio | Carp | MA-f | ACAAGCTTATGGCTTTCGCCGGAATTCTGA | β-parvalbumin | [125] | |
| MA-r | ATCGGATCCTATGCCTTGATCATGGC | |||||
| G. morhua | Atlantic cod | rGad m 1.01 | ATGGCATTCGCTGGAATTCTCG | 599 | ||
| rGad m 1.02 | ATGGCTTTCGCCGGAATTCTG | 797 | ||||
| Lophius piscatorius Lophius budegassa | White anglerfish | DAS-F | ACAACTTTCCCCGAGAAGC | 196 | β2-parvalbumin | [126] |
| Black-bellied anglerfish | DAS-R | ACAACATCACAGTTTAAGTTTTGC | Exon 2 to Exon 3 | |||
| Oncorhynchus mykiss | Rainbow trout | 601F9 forward | AGACAGAGACACAGGTTGGCTTACTATTCT | 75 | β-parvalbumin | [24] |
| 601G0 reverse | TTTACGACATAGGGAGCAGCTTACTATTCT | Intron 2 | ||||
| Paralichthys olivaceus | Japanese flounder | sunF | GATGACACCATATGTCTCTGGCATCTAAGCTGTCTG | 327 | β-parvalbumin | [127] |
| sunR | GTGTCCTCGAGTTACTGTTTCACCATCGCCGC | |||||
| S. salar | Atlantic salmon | 601F5 forward | AGACAGAGACACAGGTTGGCTTACTATTCT | 126 | β-parvalbumin | [24] |
| 601F6 reverse | TTTACGACATAGGGAGCAGCTTACTATTCT | Intron 2 | ||||
| S. salar | Atlantic salmon | Sense PV | AGYGGCTTYATHGARGARGAYGARYT | 430 | β2-parvalbumin | [70] |
| Antisense PV1 | YTGYTTNACNAANACNGCRAAYTC | Exon 2 to Exon 4 | ||||
| Antisense PV2 | GAATTCRTCRACHCCDATYTTHCC | |||||
| S. salar | Atlantic salmon | IFF 156 | ATGGCCTGTGCCCATCTGTGC | 300 | β1-parvalbumin | [122] |
| IFF 157 | GGACTTCGAGGCAAAGCCAAT | Exon 1 to Exon 2 | ||||
| S. salar | Atlantic salmon | Psal1 | CTGTGCCCATCTGTGCAAGG | 650 | β1-parvalbumin | [128] |
| Oncorhynchus mykiss | Rainbow trout | Psal2 | CCAATCATGCCATCACCATCG | Exon1 to Exon 3 | ||
| Salmo trutta | Brown trout | Psal3 | TACCGATGCAGAGACAAAGG | 931 | β1-parvalbumin | |
| Salvelinus alpinus | Arctic char | Psal4 | GTCTTGGGCAATATTGTTCC | 3′ end of Exon 3 to Exon 4 | ||
| Scomber japonicus | Mackerel | SJ9 | CCCTACAAAGCAAAAACATC | 1500 | β-parvalbumin | [129] |
| SJ487 | GCATAGGAGGAAAGGICTCT | |||||
| SJ106 | GTAGITTCGACCACAAAAAGTT | 190 | β-parvalbumin | |||
| SJG441r | ACTGCTGTATAGGTGATAGG | Exon 2 to Intron 2 | ||||
| SJG107f | AGCTATTCTGTATCGCTTCG | 284 | β-parvalbumin | |||
| SGG297r | GGTGTGAGTCTTACTTCAGC | Intron 1 to Intron 2 | ||||
| S. scombrus Trachurus trachurus | Atlantic mackerel Atlantic horse mackerel | Pval1Fw | CTGAAGCTGTTCCTGCAGAACTT | 87 | β-parvalbumin | [130] |
| Pval1Rev | GCTGTCACCGGCCTTGAG | |||||
| Pval1Probe | [6FAM]TCCGACGCCGAGACCAAGGC[TAM] | Intron 2 to Exon 3 | ||||
| Spondyliosoma cantharus | Black seabream | 1189B6 | TGAGCTGAAGTAAGACACTCAGGAA | 78 | β-parvalbumin | [23] |
| 1189B7 | TCTAAAATGTTGTCTTGGTGCCTTAG | |||||
| 1273H9(probe) | TGCACACTTGAGCAAGCAATGGCC | Intron 2 | ||||
| Spondyliosoma cantharus | Black seabream | 601F7 forward | AGACAGAGACACAGGTTGGCTTACTATTCT | 79 | β-parvalbumin | [24] |
| 601F8 reverse | TTTACGACATAGGGAGCAGCTTACTATTCT | Intron 2 | ||||
| Thunnus albacares | Yellowfin tuna | ALB4F | AGGATTGGATTTTCTGTCTTAGCTT | 227 | β-parvalbumin | [22] |
| ALB4R | TCAGTTTGTGTCAATTGGTCTGTAG | Intron 2 | ||||
| PARVT1F | GGGGTTGGAGATGAATGGCA | 785 | β-parvalbumin | |||
| PARVT1R | GAGTCACCGGCCATGAGAAA | Intron 1 to Exon 3 | ||||
| PARVT2F | ACAGCTGCCGACTCTTTCAA | 670 | Parvalbumin β | |||
| PARVT2R | CGGCCATGAGAAATGCCTTG | Intron 1/Exon 2 to Exon 3 |
NA = Not available in the reference.
DNA-based methodologies follow an indirect approach to detecting allergens because they identify the DNA sequences from the allergenic food components rather than detecting the allergenic protein itself [112]. DNA is overall more stable than proteins, particularly when subjected to thermal treatments. Even though DNA may fragment at high temperatures, it is still detectable [25]. DNA-based methodologies thus have a significant advantage over other methods and as a result, DNA-based methods are especially useful to analyze highly processed foodstuffs, emphasizing their important role in the management of allergens in the food industry [113,114].
The most frequent markers used for fish identification are found in the mitochondrial genome, such as cytochrome oxidase 1 (COI) and Cytochrome b (Cyt b). Due to the fact that mitochondrial genes are a part of a large number of copies in fish tissues, and their mutation rate is much higher than that of nuclear genes, mitochondrial gene loci are usually relied upon for species identification in fish, primarily as a result of their characteristics such as being part of a haploid genome with a high copy number [88,115]. Due to the fact that mitochondrial genes are small and mitochondria are numerous and relatively easy to isolate, mitochondrial genes are widely used for species identification. Additionally, the number of mitochondrial DNA (mtDNA) gene sequences available in public databases has rapidly increased, making it relatively easy to compare a test sequence with previously identified samples.
However, in the last few years, markers residing in the nuclear genome have also started to play an essential role in constructing such species-identification assays. Although mitochondrial DNA has quite a few advantages when compared to nuclear DNA, some disadvantages must be considered [116]. Although mtDNA markers can separate species, they can sometimes be uninformative while separating closely related species, such as in the case of Thunnus species [117] and in cases of mixed products. The mixed product can be manufactured by combing premium quality tuna with tuna of lower quality and price. The sequencing of mtDNA also has the significant disadvantage of potentially introducing nuclear mitochondrial pseudogenes (numts), which are mitochondrial-derived non-functional nuclear sequences [118,119]. In contrast, amplifying nuclear sequences (markers) can overcome these problems while providing fairly high levels of uniqueness even in closely related fish species [23,88,120].
The identification of certain species has also been proposed using novel nuclear regions, such as the flatfish genome [121]. DNA can be amplified even in highly processed foods since nuclear DNA (nDNA) barcodes tend to be shorter than mtDNA barcodes. Next-generation sequencing (NGS) can be conducted on DNAs extracted from these foods as they are easy to read. As a result, species can be identified even in samples containing several species [121].
Previous reports showed that the PVALB protein of bony fishes could be encoded by different paralogs genes that contain the same number of exons and introns but whose introns differ in size and nucleotide sequence, such as the Parv β1 polymorphic site in salmonoids, which was demonstrated by Muñoz-Colmenero et al. [128]. Each orthologous exon contains an identical number of nucleotides in all the paralogs, for example, the exon sequence of PVALB β1 of carp (Cyprinus carpio) and rainbow trout (Oncorhynchus mykiss) is 330 bp long [131,132,133]. Rencova et al. [124] demonstrated a fast, simple, specific, and sensitive PCR assay for the detection of PVALB in two closely related herring species (C. harengus and C. pallasii). Additionally, PVALB species-specific primers were used to authenticate closely related species of scombrid, catfish, and tilapia [22]. These results validate the use of DNA-based assays for cheap, routine screening methods for PVALB allergens in fish and food products.
Conserved PVALB exon sequences can be used to design universal PCR primers that amplify a species-specific intron, as well as regions of the exons flanking the intron, from even very distantly related fish species, such that fish species identification could be achieved by using probes that target a species-specific intron region [134]. EPIC (Exon primed intron crossing) PCR makes use of this property demonstrating the suitability of nuclear intron sequences as molecular markers for PCR-based species determination and subsequent real-time PCR-based quantification of the extent of each species in a complex mixed sample [25,88]. The use of the PVALB gene for fish identification and their quantification in commercial products is well documented in the literature. Results obtained confirmed the possibility of using the PVALB gene for forensic application in the fish trade and food industry.
4.3. Methods of Parvalbumin Allergen Quantification
Apart from the traditional assays based on protein and DNA detection, biosensor detection of PVALB for fish authentication is a developing field. Biosensors are integrated receptor-transducer devices that convert biological recognition events into measurable chemical, and physical signals proportional to the target concentration. Depending on the target allergen, the receptor might be an antibody raised against it, a single-strand DNA molecule that hybridizes with the allergen-specific DNA fragment, or an aptamer that recognizesthe allergen directly. To detect PVALB a fluorescence sensor was developed by Jiang et al. [89] exhibiting the possibility of quantification of fish PVALB. They also demonstrated its utility for food allergen and detection. Similarly, kinetic analysis by a surface plasmon resonance biosensor was performed to understand the parvalbumin antigen-antibody interaction, providing a fast and powerful tool for allergen detection and quantification [135]. Recently a gold nanoparticle aptasensor was developed for PVALB detection [136]. While gold-based nanoparticles offer an excellent platform for developing rapid, low-cost, portable biosensors for food safety detection, they also have certain drawbacks. For example, colour changes of gold particles can be difficult to interpret in cases of low concentration. Further stability issues of the sensor may change over time giving inaccurate results [137]. While biosensors provide a good alternative in the field of food safety and allergen detection, it is necessary to address the drawbacks before moving forward.
5. Future Outlook
The PVALB gene, usually present in fish’s muscle tissue, is a major fish allergen affecting humans. The presence of three gene types (PVALB α, PVALB β1, and PVALB β2) makes detecting and managing these allergens difficult. A high level of cross-reactivity between species is observed without proper knowledge of the cause of the cross-reactivity. Though significant innovations and efforts have been made in recent times to study and control food allergies, further work is still required. While the scientific community has managed to identify new isoforms successfully, some work is still needed to develop standardised methodologies and assessment tools to detect and quantify PVALBs easily, quickly, and efficiently. Developing reliable, rapid, on-site allergen-detection methods showing high accuracy and sensitivity are future research needs for seafood products that will benefit the food industry and make food products healthier for consumers. However, avoiding allergenic seafood intake is still the only standard way for clinical protection of seafood-allergic patients. Consequently, it is imperative to conduct in-depth research into the therapeutic hypoallergenic treatment of PVALB in order to decrease the risk of seafood allergy and provide a safe environment for global consumers. Studying trends in the incidence of fish allergies is also critical to understanding the burden of allergic disease. Unfortunately, no robust studies have been conducted to assess food/fish allergy prevalence trends over time. This lapse could be the result of the data gap that exists between recorded statistics and real allergy diagnostics based on DBPCFC (Double Blind Placebo Controlled Food Challenge).
With the increase in cases of fish substitutions and fish fraud, reliable methods are needed to detect and identify fish products. The PVALB gene has high variability which makes it a reliable marker for fish identification. PVALB assays could be used as a tool to control species mislabeling of samples containing closely related fish species. At the same time, efforts have been made to develop authentication tools for commercial purposes by cataloguing SNPs. There is a need to find out and catalogue more SNPs of species of commercial interest. Since most studies are focused on the northern hemisphere, data available from the southern hemisphere is still sparse. Filling this data gap is essential in cataloguing SNPs enabling the development of more reliable and efficient tools that can be applied around the globe.
Funding Statement
This research was financially supported by the Ministry of Agriculture of the Czech Republic [grant No. QK1910231 and the institutional support MZE-RO0318].
Author Contributions
Conceptualization, S.M. and E.C.; formal analysis, S.M.; investigation, S.M., E.C., P.H., K.Z.; resources, S.M., E.C., P.H., K.Z.; data curation, S.M.; writing—original draft preparation, S.M., E.C., P.H., K.Z.; writing—review and editing, S.M. and E.C.; visualization, S.M. and E.C.; supervision, E.C.; project administration, E.C., P.H., K.Z.; funding acquisition, E.C. and P.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
1. Arif S.H., Jabeen M., Hasnain A. Biochemical characterisation and thermostable capacity of parvalbumins: The major fish-food allergens. J. Food. Biochem. 2007;31:121–137. doi: 10.1111/j.1745-4514.2007.00104.x. [CrossRef] [Google Scholar]
2. Mukherjee S., Bartoš O., Zdeňková K., Hanák P., Horká P., Musilova Z. Evolution of the Parvalbumin Genes in Teleost Fishes after the Whole-Genome Duplication. Fishes. 2021;6:70. doi: 10.3390/fishes6040070. [CrossRef] [Google Scholar]
3. Deuticke H.J. Űber die Sedimentationskonstante von Muskelproteinen. Physiol. Chem. 1934;224:216–228. doi: 10.1515/bchm2.1934.224.5-6.216. [CrossRef] [Google Scholar]
4. Henrotte J.G. A crystalline constituent from myogen of carp muscles. Nature. 1952;169:968–969. doi: 10.1038/169968b0. [PubMed] [CrossRef] [Google Scholar]
5. Pechère J.F. Muscular parvalbumins as homologous proteins. Comp. Biochem. Physiol. 1968;24:289–295. doi: 10.1016/0010-406X(68)90978-X. [PubMed] [CrossRef] [Google Scholar]
6. Pechère J.F., Capony J., Ryden L. The primary structure of the major parvalbumin from hake muscle. Eur. J. Biochem. 1971;23:421–428. doi: 10.1111/j.1432-1033.1971.tb01636.x. [PubMed] [CrossRef] [Google Scholar]
7. Nockolds C.E., Kretsinger R.H., Coffee C.J., Bradshaw R.A. Structure of a calcium binding carp myogen. Proc. Natl. Acad. Sci. USA. 1972;69:581–584. doi: 10.1073/pnas.69.3.581. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
8. Arif S.H. A Ca2+-binding protein with numerous roles and uses: Parvalbumin in molecular biology and physiology. BioEssays. 2009;31:410–421. doi: 10.1002/bies.200800170. [PubMed] [CrossRef] [Google Scholar]
9. Heizmann C.W. Ca2+-Binding proteins of the EF-hand superfamily: Diagnostic and prognostic biomarkers and novel therapeutic targets. Methods Mol. Biol. 2019;1929:157–186. [PubMed] [Google Scholar]
10. Nogueira L., Gilmore N.K., Hogan M.C. Role of parvalbumin in fatigue-induced changes in force and cytosolic calcium transients in intact single mouse myofibers. J. Appl. Physiol. 2022;132:1041–1053. doi: 10.1152/japplphysiol.00861.2021. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
11. Ge M., Chen S., Huang Y., Chen W., He L., Zhang Y. Role of calcium homeostasis in Alzheimer’s Disease. Neuropsychiatr. Dis. Treat. 2022;18:487. doi: 10.2147/NDT.S350939. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
12. Berchtold M.W., Brinkmeier H., Műntener M. Calcium ion in skeletal muscle: Its crucial role for muscle function, plasticity and disease. Physiol. Rev. 2000;80:1215–1265. doi: 10.1152/physrev.2000.80.3.1215. [PubMed] [CrossRef] [Google Scholar]
13. Gillis J.M. Relaxation of vertebrate skeletal muscle. A synthesis of the biochemical and physiological approaches. Biochem. Biophys. Acta. 1985;811:97–145. doi: 10.1016/0304-4173(85)90016-3. [PubMed] [CrossRef] [Google Scholar]
14. Coughlin D.J. Aerobic muscle functions during steady swimming in fishes. Fish Fish. 2002;3:63–78. doi: 10.1046/j.1467-2979.2002.00069.x. [CrossRef] [Google Scholar]
15. Celio M.R., Heizmann C.W. Calcium-binding protein parvalbumin as a neuronal marker. Nature. 1981;293:300–302. doi: 10.1038/293300a0. [PubMed] [CrossRef] [Google Scholar]
16. Radauer C., Bublin M., Wagner S., Mari A., Breiteneder H. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J. Allergy Clin. Immunol. 2006;121:847–852. doi: 10.1016/j.jaci.2008.01.025. [PubMed] [CrossRef] [Google Scholar]
17. Lopata A.L., Jeebhay M.F. Airborne seafood allergens as a cause of occupational allergy and asthma. Curr. Allergy Asthma Rep. 2013;13:288–297. doi: 10.1007/s11882-013-0347-y. [PubMed] [CrossRef] [Google Scholar]