
교차반응 에피토프
1) 사스 바이러스 감염 Primary allergen IgE epitope --> specific IgE 생성..
코로나 바이러스 감염 Seconcary allergen --> allergenic cross reactivity 반응
2) 땅콩 allergen IgE epitope...
견과류 ....
Review article
Cross-reactive epitopes and their role in food allergy
Author links open overlay panelSandip D. Kamath MSc, PhD a b, Merima Bublin MSc, PhD a, Katsumasa Kitamura MD, PhD c, Teruaki Matsui MD, PhD c, Komei Ito MD, PhD c d, Andreas L. Lopata MSc, PhD b e f g
Show more
Add to Mendeley
Share
Cite
https://doi.org/10.1016/j.jaci.2022.12.827Get rights and content
Under a Creative Commons license
open access
Allergenic cross-reactivity among food allergens complicates the diagnosis and management of food allergy. This can result in many patients being sensitized (having allergen-specific IgE) to foods without exhibiting clinical reactivity. Some food groups such as shellfish, fish, tree nuts, and peanuts have very high rates of cross-reactivity. In contrast, relatively low rates are noted for grains and milk, whereas many other food families have variable rates of cross-reactivity or are not well studied. Although classical cross-reactive carbohydrate determinants are clinically not relevant, α-Gal in red meat through tick bites can lead to severe reactions. Multiple sensitizations to tree nuts complicate the diagnosis and management of patients allergic to peanut and tree nut. This review discusses cross-reactive allergens and cross-reactive carbohydrate determinants in the major food groups, and where available, describes their B-cell and T-cell epitopes. The clinical relevance of these cross-reactive B-cell and T-cell epitopes is highlighted and their possible impact on allergen-specific immunotherapy for food allergy is discussed.
식품 알레르겐 간의 알레르기 교차 반응성 Allergenic cross-reactivity 은
식품 알레르기의 진단과 관리를 복잡하게 만듭니다.

이로 인해 많은 환자가
임상적 반응성을 보이지 않으면서도
식품에 감작(알레르기 항원 특이 IgE 보유)을 일으킬 수 있습니다.
조개류, 생선, 견과류, 땅콩과 같은
일부 식품군은
교차 반응성이 매우 높습니다.
반면
곡류와 우유는 상대적으로 낮은 비율을 보이는 반면,
다른 많은 식품군은 교차 반응률이 다양하거나 잘 연구되지 않았습니다.
고전적인 교차 반응 탄수화물 결정 인자는
임상적으로 관련이 없지만
진드기에 물린 붉은 육류의 α-Gal은 심각한 반응을 일으킬 수 있습니다.

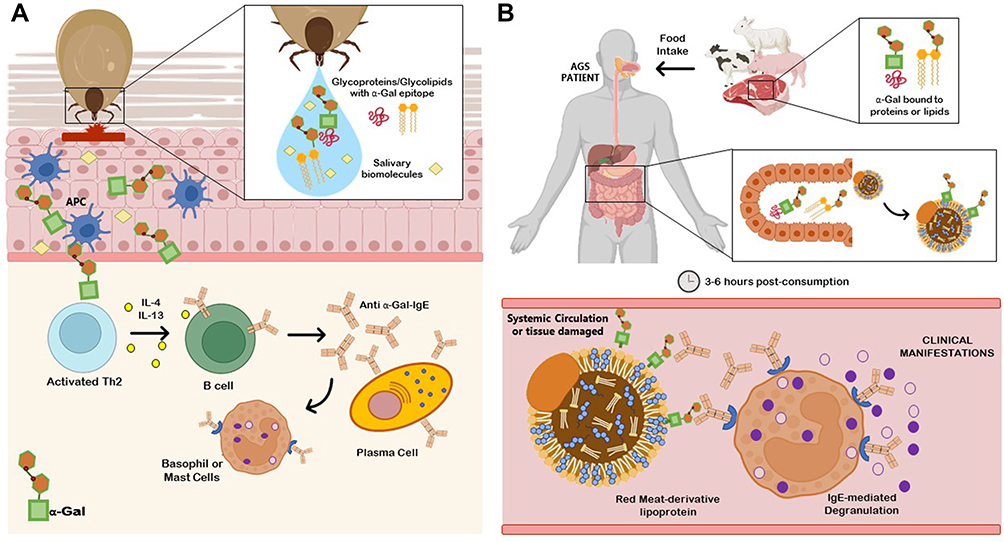
진드기가 소를 물면 소의 APC(수지상세포)가 활성화, 알파-갈 생성
알파갈이 조직내로 들어가 Th2 활성화
IL4, 13분비
B세포 활성화
Anti-a-Gal-IgE 분비
호염기구, 비만세포에서 히스타민 분비
그 음식을 인간이 먹으면 3~6시간 후
만성염증으로 증상이 나타나는 것을 '알파갈 증후군'이라고 함.
견과류에 대한 다중 과민 반응은
땅콩과 견과류 알레르기가 있는 환자의
진단과 관리를 복잡하게 만듭니다.
이 리뷰에서는
주요 식품군의 교차 반응성 알레르겐과
교차 반응성 탄수화물 결정 인자에 대해 논의하고,
가능한 경우 이들의 B세포 및 T세포 에피토프에 대해 설명합니다.
이러한
교차 반응성 B세포 및 T세포 에피토프의 임상적 관련성을 강조하고
식품 알레르기에 대한
알레르겐 특이 면역 요법에 미칠 수 있는 영향에 대해 논의합니다.
Key words
Allergens
allergenic cross-reactivity
B-cell
clinical cross-reactivity
cross-reactive epitope
cross-reactive IgE
food allergy
IgE antibody
T-cell
tropomyosin
Abbreviations used
α-Gal
Galactose-α-1,3-galactose
CCD
Cross-reactive carbohydrate determinant
CMA
Cow’s milk allergy
CM
Cow’s milk
CN
Casein
HDM
House dust mite
HMW
High molecular weight
WDEIA
Wheat-dependent exercise-induced anaphylaxis
Allergenic cross-reactivity can be clinically manifest or irrelevant. In vitro diagnosis of food allergy and its management is often hampered by cross-reacting food proteins.1,2 IgE binding to cross-reactive clinically irrelevant allergens results in false-positive results on in vitro diagnostic tests. However, unidentified sources of cross-reactive allergens of clinical relevance may lead to unintentional exposure and allergic reactions, posing a health risk for the affected individuals. The concept of cross-reactivity between related or unrelated allergen sources is extensively addressed in the literature. However, this information is often not accompanied with data on identifying specific allergens or epitopes. The most common view in the current literature is that cross-reactive allergenic proteins present with a high primary amino acid sequence identity of above 70%.3 However, relevant IgE-binding epitopes are often below 20 amino acids in length and allow for a much better assessment of allergenic cross-reactivity.4 In addition, shared cross-reactive T-cell epitopes could explain some of the clinical desensitization to food allergens observed after successful pollen immunotherapy5; however, the cross-reactive T-cell epitopes have been studied for very few allergens. Well-characterized cross-reactive B-cell and T-cell epitopes between food allergens will not only assist in developing more specific and accurate molecular diagnosis but also contribute to the development of lead candidates for targeted immunotherapy. In this review, we summarize the basic concepts of allergenic cross-reactivity, discuss about protein and carbohydrate epitopes, and provide an overview of the cross-reactive allergens belonging to the major food allergen groups.
알레르기 교차 반응성은
임상적으로 나타나거나 관련이 없을 수 있습니다.
식품 알레르기의 체외 진단 및 관리는
종종 교차 반응하는 식품 단백질로 인해 방해를 받습니다.1,2
임상적으로 관련이 없는
교차 반응성 알레르겐에 결합하는 IgE는
체외 진단 테스트에서 위양성 결과를 초래합니다.
그러나
임상적으로 관련성이 있는 교차 반응성 알레르겐의 출처가 확인되지 않은 경우
의도하지 않은 노출과 알레르기 반응으로 이어질 수 있어
영향을 받는 사람에게 건강상의 위험을 초래할 수 있습니다.
관련성이 있거나
관련이 없는 알레르겐원 간의 교차 반응성 개념은
문헌에서 광범위하게 다루어지고 있습니다.
그러나 이러한 정보는
특정 알레르겐이나
에피토프를 식별하는 데이터와 함께 제공되지 않는 경우가 많습니다.
현재 문헌에서 가장 일반적인 견해는
교차 반응성 알레르기 유발 단백질은
70% 이상의 높은 1차 아미노산 서열 동일성을 가지고 존재한다는 것입니다.3
그러나
관련 IgE 결합 에피토프는
종종 길이가 20 아미노산 미만이며
알레르기 교차 반응성을 훨씬 더 잘 평가할 수 있습니다.4
또한
공유 교차 반응 T 세포 에피토프는
성공적인 꽃가루 면역 요법 후 관찰되는
식품 알레르기 항원에 대한 임상적 둔감화의 일부를 설명할 수 있지만5
교차 반응 T 세포 에피토프는
극소수의 알레르기 항원에 대해 연구되어 왔습니다.

잘 특성화된 식품 알레르겐 간의
교차 반응성 B세포 및 T세포 에피토프는
보다 구체적이고 정확한 분자 진단을 개발하는 데 도움이 될 뿐만 아니라
표적 면역 치료를 위한 주요 후보를 개발하는 데도 기여할 것입니다.
이 리뷰에서는
알레르기 교차 반응성의 기본 개념을 요약하고
단백질 및 탄수화물 에피토프에 대해 논의하며
주요 식품 알레르겐 그룹에 속하는
교차 반응성 알레르겐에 대한 개요를 제공합니다.
Concept of allergenic cross-reactivity and the role of IgE (B-cell) and T-cell epitopes
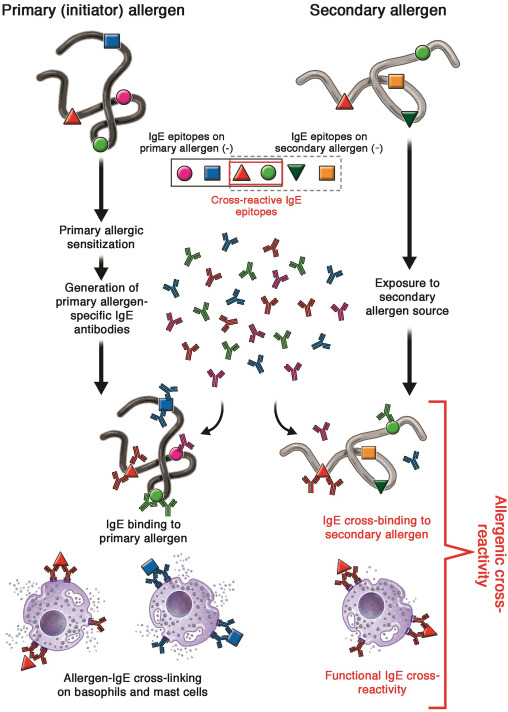
Cross-reactivity in allergy is a broad term used to define the ability of (secondary) allergen(s) to recognize IgE antibodies and/or invoke a cellular (T-cell, mast cell, or basophil) response in the body upon exposure, which has been already sensitized to a primary (initiator) allergen that shares 1 or more epitope with the secondary allergen. These shared regions are defined as cross-reactive epitopes. IgE cross-reactivity is often recognized when allergic symptoms arise to an allergen source without prior exposure. However, IgE cross-reactivity might be clinically manifest or irrelevant.
알레르기의 교차 반응성은
2차 알레르겐과 1개 이상의 에피토프를 공유하는 1차(개시) 알레르겐에
이미 감작된 (2차) 알레르겐이 노출 시
IgE 항체를 인식하거나
신체에서 세포(T세포, 비만 세포 또는 호염기구) 반응을 유발하는 능력을 정의하는 데
사용되는 광범위한 용어입니다.
IgE cross-reactivity can be defined as the relationship between 1 antibody and 2 or more allergens.6 Sensitization occurs to a primary allergen via a TH2 response, leading to the generation of allergen-specific IgE antibodies.7 These IgE antibodies may be directed to several conformational and/or to linear epitopes on the allergen.8 When the individual is later exposed to a food source containing homologous proteins/allergens via ingestion, inhalation, or contact, the preformed primary allergen-specific IgE antibodies are able to recognize these secondary allergens via cross-reactive epitopes, and may lead to cross-linking on basophils and mast cells, resulting in mediator release and subsequent clinical symptoms (Fig 1). The secondary allergen may be a complete or incomplete allergen in that it may or may not be capable of inducing primary allergic sensitization by itself (cosensitization).6,9 In some cases, the route of exposure may differ between the primary and secondary allergen. For example, primary sensitization to shrimp tropomyosin may occur via ingestion, but secondary exposure and cross-reactive IgE binding to dust mite tropomyosin occurs via inhalation.10 In addition, the IgE antibody’s binding affinity to the secondary allergen may be weaker as compared with that to the primary allergen, depending on the number of cross-reactive epitopes and binding affinity. All these factors, in addition to the physicochemical stability and amino acid sequence homology or structural homology of the secondary allergen, play a role in the clinical relevance of IgE cross-reactivity.
IgE 교차 반응성은
하나의 항체와 2개 이상의 알레르겐 사이의 관계로 정의할 수 있습니다.6
감작성은 TH2 반응을 통해
1차 알레르겐에 발생하여 알레르겐 특이 IgE 항체를 생성합니다.7
이러한 IgE 항체는
알레르겐의 여러 형태 및/또는선형 에피토프로 향할 수 있습니다.8
several conformational and/or to linear epitopes
나중에
섭취, 흡입 또는 접촉을 통해
상동 단백질/알레르겐이 포함된 식품 공급원에 노출되면
미리 형성된 1차 알레르겐 특이 IgE 항체는
교차 반응성 에피토프를 통해 이러한 2차 알레르겐을 인식할 수 있으며
호염기구와 비만 세포에서 교차 결합을 유발하여
매개체 방출과 그에 따른 임상 증상을 유발할 수 있습니다(그림 1).
2차 알레르겐은
그 자체로 1차 알레르기 감작을 유발할 수 있거나
그렇지 않을 수 있다는 점에서
완전하거나 불완전한 알레르겐일 수 있습니다(코감작 Cosensitization ).6,9
어떤 경우에는
1차 알레르겐과 2차 알레르겐의 노출 경로가 다를 수 있습니다.
예를 들어,
새우 트로포마이오신에 대한 1차 감작성은
섭취를 통해 발생할 수 있지만,
집먼지 진드기 트로포마이오신에 대한
2차 노출 및 교차 반응성 IgE 결합은
흡입을 통해 발생합니다.10
또한
교차 반응성 에피토프의 수와 결합 친화도에 따라
2차 알레르겐에 대한 IgE 항체의 결합 친화도가
1차 알레르겐에 비해 약할 수 있습니다.
이차 알레르겐의 물리화학적 안정성 및
아미노산 서열 상동성 또는 구조적 상동성 외에도
이러한 모든 요인이 IgE 교차 반응성의 임상적 관련성에 영향을 미칩니다.
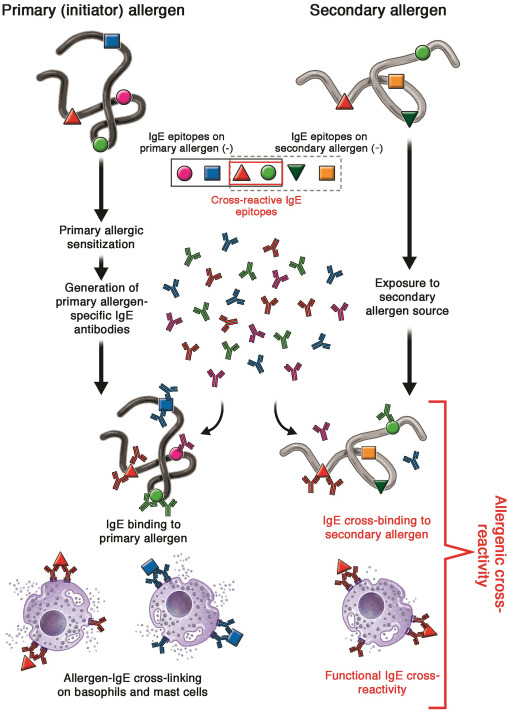
Fig 1. Simplified schematic representation of the mechanism of IgE-mediated allergenic cross-reactivity. The process of primary allergic sensitization ultimately leads to the generation of IgE antibodies targeted against antigenic epitopes on the initiator allergen. These primary allergen-specific IgE antibodies can recognize secondary allergen(s), containing 1 or more homologous epitopes, and lead to IgE cross-linking and mediator release from basophils and mast cells.
IgE 매개 알레르기 교차 반응의 메커니즘을 단순화한 모식도.
일차 알레르기 감작 과정은 궁극적으로 개시 알레르겐의 항원 에피토프를 표적으로 하는 IgE 항체의 생성으로 이어집니다. 이러한 1차 알레르겐 특이 IgE 항체는 1개 이상의 상동 에피토프를 포함하는 2차 알레르겐을 인식하고 호염기구와 비만 세포에서 IgE 교차 결합 및 매개체 방출을 유도할 수 있습니다.
T-cell cross-reactivity can be defined as the reaction of T cells to more than 1 peptide-MHC ligand.11 T-cell cross-reactivity is possible for several reasons including MHC binding promiscuity and degeneracy in peptide-TCR recognition as a mechanism that has evolved to recognize a wide range of external antigenic peptides.12 However, in terms of allergenic T-cell cross-reactivity, the most likely cause and mechanism is sequence homology of the T-cell cross-reactive peptide residues among closely related allergen sources.13 A cross-reactive T-cell epitope is able to stimulate memory T cells and induce subsequent IgE production (Fig 2). A well-known example is the Bet v 1 immunodominant T-cell epitope Bet v 1142-156, and its cross-reactivity to homologous peptides from PR-10–like food allergens from apple, peach, pear cherry, hazelnut, celery, and carrot.14,15 The presence of this dominant peptide was also detected after ex vivo antigen processing and MHC class II presentation.16 Cross-reactive T-cell epitope peptides have also been shown to play a role in CD4+ memory T-cell survival, in absence of the primary priming allergen.17 In the context of predicting T-cell cross-reactive epitopes among closely related allergens, the peptide sequence homology is known to play an important role; higher the homology, stronger the cross-reactivity.13 However, the structural stability of closely related allergens may also play a role, which may not be associated with sequence homology, in the generation of homologous T-cell peptides, and subsequent T-cell cross-reactivity.18
T세포 교차 반응성은
1개 이상의 펩타이드-MHC 리간드에 대한
T세포의 반응으로 정의할 수 있습니다.11
T세포 교차 반응성은
광범위한 외부 항원 펩타이드를 인식하도록
진화한 메커니즘으로서
펩타이드-TCR 인식의 MHC 결합 무차별성 및
퇴행성 등 여러 이유로 가능합니다.12
그러나
알레르기성 T세포 교차 반응성의 측면에서
가장 가능성이 높은 원인과 메커니즘은
밀접하게 관련된 알레르겐 공급원 간의
T세포 교차 반응성 펩타이드 잔기의 서열 상동성입니다.13
교차 반응성 T세포 에피토프는
기억 T세포를 자극하고
후속 IgE 생성을 유도할 수 있습니다(그림 2).
잘 알려진 예로는
사과, 복숭아, 배 체리, 헤이즐넛, 셀러리, 당근의
PR-10 유사 식품 알레르겐의 상동 펩티드에 대한 교차 반응성인
Bet v 1 면역 우세 T세포 에피토프 Bet v 1142-156이 있습니다.14,15
이 우성 펩타이드의 존재는
생체 외 항원 처리 및 MHC 클래스 II 제시 후에도 검출되었습니다.16
교차 반응성 T 세포 에피토프 펩타이드는 또한
1차 프라이밍 알레르겐이 없을 때
CD4+ 기억 T 세포 생존에 역할을 하는 것으로 나타났습니다.17
밀접하게 관련된 알레르겐 중
T세포 교차 반응성 에피토프를 예측하는 맥락에서
펩타이드 서열 상동성은 중요한 역할을 하는 것으로 알려져 있으며,
상동성이 높을수록 교차 반응성이 강합니다.13
그러나
밀접하게 관련된 알레르겐의 구조적 안정성은
서열 상동성과 관련이 없을 수 있는 역할도 할 수 있으며,
동종 T세포 펩타이드의 생성 및
후속 T세포 교차 반응성에서 중요한 역할을 할 수 있습니다.18
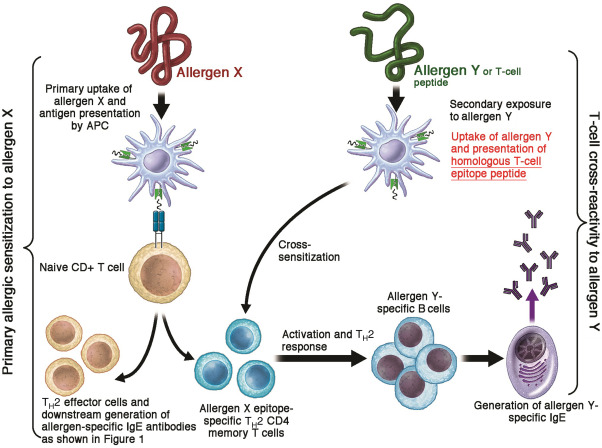
Fig 2. A simplified representation of T-cell epitope-mediated allergenic cross-reactivity. Primary allergen (allergen X) exposure and subsequent antigen presentation by dendritic cells to naive T cells in the presence of IL-4 lead to the differentiation and expansion of allergen-specific TH2 cells. Later exposure to secondary allergen (allergen Y) containing homologous T-cell epitopes leads to cross-sensitization by activating primary allergen-specific memory T cells, and subsequent generation of secondary allergen-specific IgE antibodies.
T세포 에피토프 매개 알레르기 교차 반응성의 단순화된 표현.
1차 알레르겐(알레르겐 X)에 노출되고
이후 수지상 세포가 IL-4가 있는 상태에서
순진한 T 세포에 항원을 제시하면
알레르겐 특이적인 TH2 세포가 분화 및 확장됩니다.
나중에
상동 T세포 에피토프를 포함하는 2차 알레르겐(알레르겐 Y)에 노출되면
1차 알레르겐 특이 기억 T세포가 활성화되고
2차 알레르겐 특이 IgE 항체가 생성되어
교차 감작 cross-sensitization 이 일어납니다.
Cross-reactive epitopes in the major food allergen groups
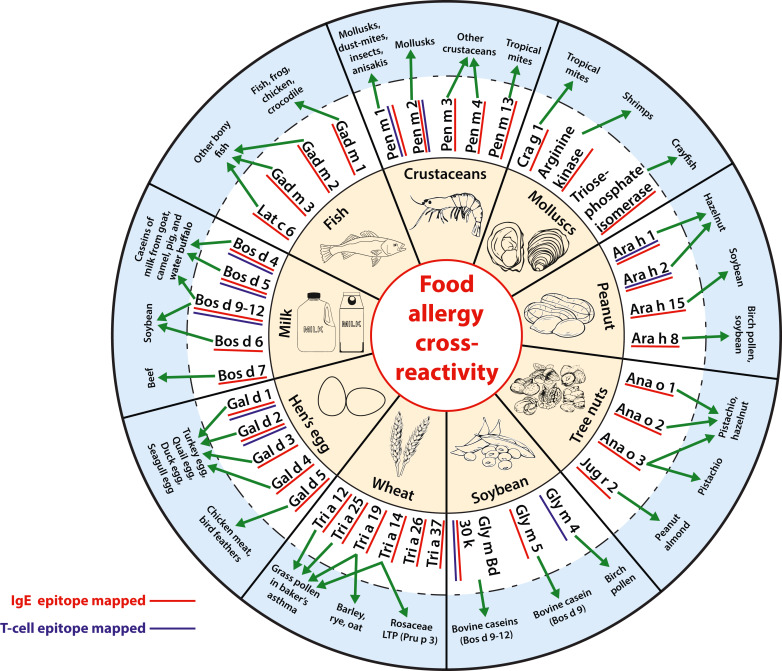
The clinically relevant cross-reactive allergens belonging to the major food groups are summarized below, and a brief overview on the current knowledge on cross-reactive IgE- or T-cell epitopes provided (Fig 3).
주요 식품군에 속하는
임상적으로 관련된 교차 반응성 알레르겐은
아래에 요약되어 있으며,
교차 반응성 IgE 또는
T세포 에피토프에 대한 현재 지식에 대한 간략한 개요가 제공됩니다(그림 3).
생선 -- 개구리, 닭, 악어
새우 --
땅콩 - 헤이즐넛, 콩
밀(글루텐) - grass pollen(목초 꽃가루), 보리(barley), 호밀(rye), 귀리(oat)
달걀 - 다른 알, 닭고기
우유 - 소고기, 콩, 염소, 돼지고기
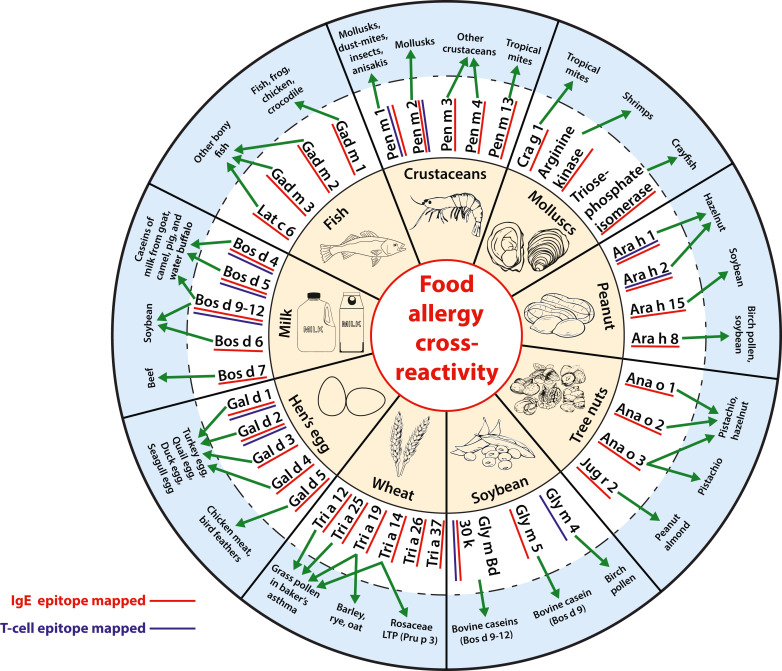
FIG 3. An overview of the clinically relevant cross-reactive allergens belonging to the major food groups, and the secondary allergen sources against which cross-reactivity is documented.
Legumes 콩류
Peanut and soybean are the most significant allergen sources of the Fabaceae family and consequently the best characterized concerning cross-reactivity of their allergens.
땅콩과 대두는
파바과에서 가장 중요한 알레르겐 공급원이며, 따라서
알레르겐의 교차 반응성과 관련하여
가장 잘 특징지어지는 식품입니다.
Peanut allergy
Sensitization to peanut can be associated with sensitization to other members of the Fabaceae family such as soy and lupine, with tree nuts, or with pollen. Among peanut-allergic children, up to 67% were sensitized to other legumes and up to 28% had confirmed allergy to at least 1 other legume.19,20 Similarly, allergy to tree nuts is common among children with peanut allergy, with up to 86% having sensitization to tree nuts and up to 40% clinically confirmed tree nut allergy.21
All known peanut allergens were determined to comprise 85% of the total protein content of peanut, whereas seed storage proteins of the 2S albumin (Ara h 2, 6, and 7), the vicilin (Ara h 1), and the legumin (Ara h 3) protein families together accounted for 75%.22 In addition, the allergens of these 3 families have been identified as major allergens in other legumes and tree nuts. Consequently, frequent cosensitization of peanut-allergic individuals to other legumes and tree nuts has been interpreted by cross-reactive epitopes present in homologous allergens from the 3 protein families. Although, for the majority, the sequential IgE epitopes have been identified, their cross-reactivity was only rarely investigated. Apart from a conserved pattern of 8 cysteine residues, the sequence of Ara h 2 shows very low sequence identities (<36%) to 2S albumins from other legumes and tree nuts. However, their structural similarity has been proposed as the immunologic basis for the observed coallergies.23
Ara h 1 shows 39% to 53% sequence identities with vicilins from tree nuts and botanically related legumes. Using homology modeling, 5 surface-exposed epitopes of Ara h 1 have been predicted on the basis of the conformational similarity to be cross-reactive with Gly m 5, Jug r 1, Ana o 1, and Cor a 11.24 However, in inhibition assays with IgE-binding peptides from Ara h 1, 2, and 3 and corresponding peptides from walnut allergens (Jug r 1, 2, and 4), no relevant cross-reacting IgE antibodies could be detected in sera from peanut- and walnut-allergic patients.25 Similarly to IgE cross-reactive epitopes, T-cell cross-reactive epitopes between peanut and other seeds are poorly defined. In 4 of 5 patients with peanut and hazelnut coallergy, cross-reactive T-cell response was driven by cross-reactivity to Ara h 1 and 2, but the specificity of cross-reactive T-cell epitopes was not defined.26 Peanut oleosins (Ara h 10, 11, 14, and 15) might be a cause of IgE cross-reactivity to oil-contained seeds. The linear IgE epitope DKARDVKDRAKDYAG, localized in the C-terminal domain of Ara h 15 with high sequence identity to other seed-derived oleosins, was recognized by IgE from soybean- and rapeseed-allergic patient.27 Peanut allergens belonging to the Bet v 1 (Ara h 8), the profilin (Ara h 5), the defensin (Ara h 12 and 13), and the cyclophilin (Ara h 18) protein families are mostly involved in pollen-associated food allergy. Furthermore, the nonspecific lipid transfer proteins (Ara h 9, 16, and 17) are involved in the so-called nonspecific lipid transfer protein syndrome.28 One IgE-binding surface area on Bet v 1 and Ara h 8, identified by using phage-displayed epitope mimics, was shown to be involved in IgE cross-reactivity to Gly m 4.29
Although cross-reactivity has been commonly recognized between members of the same protein family, several lines of evidence demonstrated IgE cross-reactivity between members of different protein families of seed storage proteins. It was demonstrated that IgE cross-reactive to Ara h 1, 2, and 3 comprised the major fraction of IgE specific to these allergens in sera from peanut-allergic patients.30 The cross-reactive IgE antibodies manifested identical gene rearrangements in unrelated individuals as well as high affinity and cross-reactivity to the peanut allergens.31 The 3 Ara h 2 epitopes implicated in this cross-reactivity (1: WLQGDRRCQSQLER, 2: SYGRDPYSPSQDPYS, and 3: PDRRDPYSPSPYDRR)30 have also been identified as immunodominant epitopes in different studies.32, 33, 34 The molecules named covalent heterobivalent inhibitors containing only 1 immunodominant epitope of Ara h 2 (DPYSPOHSDRRGAGSS) and 1 of Ara h 6 (QDRQ) yielded an almost complete inhibition of basophil degranulation to peanut extract in in vitro cellular assays with patients’ sera.35 IgE- and T-cell cross-reactivities between Ara 1, 2, and 3 were also observed in mice.36
땅콩에 대한 감작은
콩이나 루핀과 같은
다른 콩과 식물, 견과류 또는 꽃가루에 대한 감작과 관련이 있을 수 있습니다.
땅콩 알레르기가 있는 어린이 중
최대 67%가 다른 콩과 식물에 민감하게 반응하고
최대 28%가 최소 1개 이상의 다른 콩과 식물에 알레르기가 확인되었습니다.19,20
마찬가지로,
땅콩 알레르기가 있는 어린이 중
최대 86%가 견과류에 민감하게 반응하고
최대 40%가 임상적으로 견과류 알레르기가 확인되는 등 견과류에 대한 알레르기도 흔합니다.21
알려진 모든 땅콩 알레르겐은
땅콩 전체 단백질 함량의 85%를 차지하는 것으로 확인되었으며,
2S 알부민(Ara h 2, 6, 7), 비실린(Ara h 1), 콩류(Ara h 3) 단백질 군의
종자 저장 단백질이 75%를 차지합니다.22
또한 이 3개 군의 알레르겐은 다른 콩류 및 견과류의 주요 알레르기 유발 물질로 확인되었습니다. 따라서 땅콩 알레르기가 있는 사람이 다른 콩과 식물과 견과류에 자주 과민 반응을 보이는 것은 세 단백질 군의 상동 알레르겐에 존재하는 교차 반응성 에피토프에 의한 것으로 해석되었습니다. 대부분의 경우 순차적 IgE 에피토프가 확인되었지만, 교차 반응성은 거의 조사되지 않았습니다. 8개의 시스테인 잔기의 보존된 패턴을 제외하고, 아라 h 2의 서열은 다른 콩과 식물 및 견과류의 2S 알부민과 매우 낮은 서열 동일성(<36%)을 보입니다. 그러나 이들의 구조적 유사성은 관찰된 공동 알레르기의 면역학적 근거로 제안되었습니다.23
아라 h 1은 견과류 및 식물 관련 콩과 식물의 비실린과 39%~53%의 염기서열 동일성을 보여줍니다. 상동성 모델링을 사용하여 Ara h 1의 표면 노출 에피토프 5개는 형태적 유사성을 기반으로 Gly m 5, Jug r 1, Ana o 1 및 Cor a 11과 교차 반응할 것으로 예측되었습니다. 24 그러나 Ara h 1, 2, 3의 IgE 결합 펩티드와 호두 알레르겐의 해당 펩티드(Jug r 1, 2, 4)를 사용한 억제 분석에서는 땅콩 및 호두 알레르기 환자의 혈청에서 관련 교차 반응 IgE 항체를 검출할 수 없었습니다.25 IgE 교차 반응 에피토프와 유사하게 땅콩과 다른 종자 사이의 T세포 교차 반응 에피토프는 제대로 정의되지 않았습니다. 땅콩과 헤이즐넛 동시 알레르기 환자 5명 중 4명에서 교차 반응성 T세포 반응은 아라 h 1과 2에 대한 교차 반응성에 의해 주도되었지만 교차 반응성 T세포 에피토프의 특이성은 정의되지 않았습니다.26 땅콩 올레오신(아라 h 10, 11, 14, 15)은 기름 함유 종자에 대한 IgE 교차 반응의 원인이 될 수 있습니다. 다른 종자 유래 올레오신과 높은 염기서열 동일성을 가진 Ara h 15의 C-말단 도메인에 국한된 선형 IgE 에피토프 DKARDVKDRAKDYAG는 대두 및 유채 알레르기 환자의 IgE에서 인식되었습니다.27 땅콩 알레르겐은 Bet v 1(Ara h 8), 프로필린(Ara h 5), 디펜신(Ara h 12 및 13), 사이클로필린(Ara h 18) 단백질 계열에 속하며, 대부분 꽃가루와 관련된 식품 알레르기에 관여합니다. 또한 비특이적 지질 전달 단백질(Ara h 9, 16, 17)은 소위 비특이적 지질 전달 단백질 증후군에 관여합니다.28 파지 표시 에피토프 모방을 사용하여 확인된 Bet v 1과 Ara h 8의 IgE 결합 표면 영역 하나는 Gly m 4.29에 대한 IgE 교차 반응성에 관여하는 것으로 나타났습니다.
같은 단백질 계열의 구성원 간에는 교차 반응성이 일반적으로 인정되었지만, 종자 저장 단백질의 다른 단백질 계열 구성원 간에는 IgE 교차 반응성이 입증된 증거가 몇 가지 있습니다. 땅콩 알레르기 환자의 혈청에서 Ara h 1, 2, 3에 교차 반응하는 IgE가 이들 알레르겐에 특이적인 IgE의 주요 부분을 차지하는 것으로 입증되었습니다.30 교차 반응하는 IgE 항체는 땅콩 알레르겐에 대한 높은 친화력과 교차 반응성뿐만 아니라 관련 없는 개인에서 동일한 유전자 재배열을 나타냈습니다.31 이 교차 반응성에 관여하는 3개의 아라 h 2 에피토프(1: WLQGDRRCQSQLER, 2: SYGRDPYSPSQDPYS, 3: PDRRDPYSPSPYDRR)30는 다른 연구에서도 면역 우세 에피토프로 확인되었습니다.32, 33, 34 Ara h 2의 면역 우세 에피토프 1개(DPYSPOHSDRRGAGSS)와 Ara h 6의 1개(QDRQ)만을 포함하는 공유 이성질체 억제제라는 분자는 환자의 혈청을 사용한 시험관 내 세포 분석에서 땅콩 추출물에 대한 호염기구 탈과립화를 거의 완벽하게 억제했습니다.35 Ara 1, 2 및 3 사이의 IgE 및 T 세포 교차 반응도 생쥐에서 관찰되었습니다.36
Soybean allergy
Primary soybean allergy is associated with sensitization to soybean vicilin Gly m 5 (7S globilin, β-conglycinin), legumin Gly m 6 (11S globulin, glycinin), 2S albumin Gly m 8, and the oil body–associated Gly m Bd 30K and Gly m Bd 28K. The seed storage proteins, Gly m 8, Gly m 5, and Gly m 6, are major contributors (80%) to the protein content of this seed and are recognized as potential diagnostic markers for severe allergic reactions to soybean.37,38 Linear epitopes of Gly m 6.0201 (GSNILSGFAPEF) and Gly m 6.0501 (GSVLSGFSKHFL) overlapped with a previously identified epitope hot spot (HS#2) of legumins from peanut and tree nuts.39 However, further studies are necessary to confirm the cross-reactivity including inhibition assays and histamine release assays to confirm the ability of these epitopes to inhibit IgE and activate effector cells.
In birch-endemic regions, soybean allergy is based on cross-reactivity between birch pollen allergen Bet v 1 and its related allergen Gly m 4 in soybean.40 Investigation of the conformational IgE epitope profile of soybean allergen Gly m 4 using Gly m 4–type model proteins harboring individual and multiple putative epitopes found 4 putative IgE-binding areas suitable to discriminate allergic and tolerant subjects.41 However, the study did not investigate how those epitopes are involved in cross-reactivity with Bet v 1. By a combination of different bioinformatics tools, predicted Gly m 4 T-cell epitopes AKADALFKAIEAYLL and ADALFKAIEAYLLAH42 share high sequence identity (80%) to the immunodominant Bet v 1 T-cell epitope TLLRAVESYLLAHSD” (aa 142-156), and thus could be responsible for cross-reactivity at the T-cell level.14
In addition, Gly m 5 and Gly m Bd 30K cross-reactive epitopes have been described to be involved in reactions to a soybean protein formula in patients allergic to cow’s milk (CM) (summarized by Bublin and Breiteneder3). Three peptides on Gly m 5 and 4 peptides on Bos d 9 (α-casein [CN]) with a common core motif were identified using a Bos d 9 (α-CN)-specific mAb.43 Recently, application of cross-reactive soybean allergen Gly m Bd 30K peptide NKIQDKVTIDGY comprising an immunodominant cross-reactive T-cell and IgG epitope was shown to prevent IgE-mediated milk sensitization in mice through the induction of blocking IgG.44
Tree nut allergy
The tree nut allergy prevalence varies from less than 1% to approximately 3%.21 Walnut, hazelnut, cashew, pistachio, almond, Brazil nut, and macadamia are the typically reported tree nut allergen sources. Most of the patients with allergy to tree nut are sensitized to multiple nuts, but strong clinically relevant cosensitization was found only between highly botanically related cashew and pistachio as well as between walnut and pecan (summarized by Cox et al1). Their concurrent allergies and extensive in vitro IgE cross-reactivity have been interpreted by the high sequence identities (≥70%) of their homologous allergens from the vicilin, legumin, or 2S albumin protein families. 2S albumins Ana o 1 from cashew and Pis v 3 from pistachio share 81% sequence identity. Using molecular modeling, a surface patch comprising 2 of the previously identified Ana o 3 linear IgE epitopes was predicted to be part of a conformational epitope responsible for cross-reactivity to Pis v 1.23 At the T-cell level, it has been demonstrated that cashew allergens Ana o 1 (vicilin) and Ana o 2 (legumin) share cross-reactive T-cell epitopes to pistachio and/or hazelnut but not to walnut.45 In Central-Northern Europe, hazelnut, walnut, and almond are often involved in the so-called pollen-food allergy syndrome due to the cross-reactivity of Bet v 1–specific IgE to hazelnut Cor a 1.04, walnut Jug r 5, and almond Pru du 1.46,47 A possible cross-reactive epitope might be located in the highly conserved glycine-rich region.47
Similar to peanuts, cross-reactive epitopes between unrelated allergens from cashew and hazelnut have been identified. In a recent study using individual cashew and hazelnut allergens, it was shown that the intraspecies cross-reactivity between unrelated allergens belonging to the 2S albumins, vicilins, and legumins families was higher than the intraprotein family cross-reactivity between the 2 nut species.48 Moreover, IgE with high affinity to Ana o 3 cross-reacted not only with the unrelated cashew nut allergens Ana o 1 and 2 but also with the hazelnut allergen Cor a 9. These cross-reactive IgE might be responsible for cross-reactivity between unrelated tree nuts. Peptide QRQCQQRCE from the N-terminal polypeptide (alpha-hairpinin) of walnut vicilin Jug r 2 was identified to share similar physicochemical properties to the immunodominant epitope of 2S albumin Ara h 2 (DRRCQSQLE). A rabbit antibody raised against the peptide was cross-reactive not only to Ara h 2 but also to almond legumin Pru du 6 and walnut vicilin Jug r 2.49 Nevertheless, the clinical relevance of IgE cross-reactivity of these allergens is unknown and requires more research.
Shellfish allergy
Shellfish (crustacean and mollusk) allergy affects approximately 3% of the general population.50,51 Because of the increasing number of allergic cases to shellfish, diagnostics and food-labeling practices define crustacean and mollusk allergy separately.
Crustacean allergy
Edible crustaceans are decapods, containing several members of species such as shrimps, crabs, lobsters, and crayfish.52 Many diverse species with homologous allergens are consumed in different regions of the world, posing a challenge to designing diagnostic tools and immunotherapeutic solutions. In individuals with crustacean allergy, clinical or immunologic cross-reactivity has been observed to mollusks, inhaled insects, edible insects, mites, and anisakis. Several cross-reactive crustacean allergens have been identified and characterized including tropomyosin, arginine kinase, myosin light chain, sarcoplasmic calcium-binding protein, fatty acid–binding protein, triose phosphate isomerase, filamin, troponin C, and hemocyanin. Several novel allergens were recently discovered in shrimps using a transcriptomic approach.53
Tropomyosin or Pen m 1 is the major crustacean allergen and a cross-reactive invertebrate pan allergen.54 Extensive epitope mapping analysis identified 8 IgE-binding epitopes in Pen a 155 and Lit v 1.56 Most of the IgE-binding epitopes are identical among crustaceans, and exhibit more than 70% amino acid sequence identity to tropomyosin in other sources as shown by multiple sequence alignment analysis.54,57 Several IgE epitopes that have been experimentally elucidated in other crustacean species, such as Siberian prawn58 and Mud crab,59 show a high homology with IgE epitopes of Pen a 1 as well as to dust mite, Der p 10, and cockroach, Bla g 7. Recent studies have shown that only 30% to 40% of crustacean-allergic individuals may be primarily sensitized to tropomyosin.10,60,61 This implies that there are other crustacean allergens that might play a role in allergic sensitization, and responsible for cross-reactivity between shellfish and other invertebrates.
Arginine kinase (Pen m 2) is a cross-reactive allergen against other invertebrates, particularly to molluscs62 and mites.63 Recently, IgE epitopes have been elucidated in Mud crab using phage display library, and cross-reactive epitopes have been elucidated using informatics analysis.57,64,65 Similarly, 3 conformational IgE epitopes from myosin light chain were elucidated from crayfish and cross-reactivity demonstrated to other crustacean species.66 Recently, a new shrimp allergen, fatty acid–binding protein, or Lit v 13, was identified and characterized, with a cross-reactive IgE epitope located in regions amino acid 40-85 and 107-136, with reactivity to Blo t 13 of tropical mites.67
In crustacean species, T-cell epitopes have been identified to a lesser extent. Shrimp tropomyosin T-cell epitopes were identified in Pen a 1 using cytokine release and CD4 T-cell proliferation assays in shellfish-allergic subjects.68 The immunodominant T-cell epitopes were also identified in Met e 1 and Pen m 1,18,69 as well as for Pen m 2.70 T-cell epitope-specific cross-reactivity among crustacean allergens in allergic patients has not been experimentally demonstrated. However, higher IL-4+/IFN-γ+ ratios in dividing CD4+ and CD56+ lymphocytes were shown in crustacean-allergic patients as compared with nonatopics on exposure to shrimp extract.71 Generation of T-cell peptides and cross-reactivity of shrimp tropomyosin to dust mite, cockroach, and anisakis tropomyosin was shown to be dependent on structural stability, more than on amino acid sequence identity.18 The role of allergen stability on T-cell peptide generation has implications on how cross-sensitization and cross-allergenicity may occur between ingested shellfish allergens and other inhalant/ingested invertebrate allergens.
Mollusk allergy
Edible mollusks are mainly classified into bivalves (oyster, clams, mussels), gastropods (abalone, snails), and cephalopods (octopus, squid). Worldwide, more than 300 different mollusk species are consumed. Oyster, abalone, squid, and octopus among other edible species are frequently implicated mollusks in food allergy, with most of the cases exhibiting gastrointestinal symptoms. Similar to crustaceans, tropomyosin is the major mollusk allergen.72 Arginine kinase from oyster has been shown to have similar IgE epitopes as Pen m 2.62 Recently, triose phosphate isomerase was characterized as a novel octopus allergen with 8 linear and 1 conformational IgE-binding epitopes and cross-reactivity demonstrated to crayfish.73,74 Recent developments in proteomics and transcriptomics analysis have helped with the rapid identification of novel allergens and their cross-reactive epitopes in Pacific oyster, which is one of the most widely consumed mollusk.72,75,76
Allergy to mollusks is widely considered to be as a result of primary allergic sensitization to shrimps or other crustaceans, and clinical symptoms on ingestion of mollusks as a result of cross-reactive anticrustacean IgE antibodies. Oyster tropomyosin elicits IgE cross-reactivity to shrimp tropomyosin even though they share very low amino acid sequence identity.54 However, our recent study demonstrated that a mollusk tropomyosin (Hal l 1) from abalone is capable of generating IgE antibodies in a mouse model, independent of previous exposure to crustacean tropomyosin. These abalone tropomyosin-specific IgE antibodies could bind to shrimp tropomyosin, Pen m 1.77 This study opens up the possibility that individuals may be susceptible to primary sensitization to mollusk allergens that may contain cross-reactive IgE epitopes, which could lead to cross-allergenicity to crustaceans or other invertebrates. Currently, no experimental data are available on T-cell epitopes, cross-reactive or otherwise, among mollusk allergens and presents an avenue for future research.
Fish allergy
Fish are divided into the super classes of bony fish (Osteichthyes), comprising most edible fish worldwide, and cartilaginous fish (Chondrichthyes), which include rays, skates, and sharks.54,78 The prevalence of fish allergy is approximately 1% of the world population, with higher frequency among children (up to 5%) and regions with high fish consumption and among fish-processing workers (up to 36%).54,79 Sensitization occurs usually via ingestion, but also through skin contact and inhalation, resulting in different symptoms, including anaphylaxis, asthma, and dermatitis.
Fish allergens have been identified in most parts of the fish, including fish muscle, skin, bones, roe, and blood. Most well-studied allergens are heat stable; however, increasingly less-stable allergens are identified, due to reduced food processing and more regional studies. Registered allergens include parvalbumin, aldolase A, β-enolase, tropomyosin, creatine kinase, collagen, triosephosphate isomerase, pyruvate kinase, l-lactate dehydrogenase, glucose 6-phosphate isomerase, glyceraldehyede-3-phosphate, and vitellogenin.
The best-studied allergen is the calcium-binding protein parvalbumin, ranging in molecular weight from 10 kDa to 13 kDa and sensitization ranges from 70% to 95%, depending on the study population. Most fish express different molecular isoforms of parvalbumin (up to 5), most likely being responsible for differential clinical reactivity, resulting in monosensitivity (eg, salmon and cod) but mostly multiple sensitivity.80 Parvalbumins cluster into 2 distinct phylogenetic lineages of parvalbumin, with β-parvalbumins being predominantly expressed in bony fish, causing most of the reported IgE-mediated allergic reactions. Cartilaginous fish contain predominantly α-parvalbumins, with much lower IgE reactivity.81,82 β-Parvalbumins also contain a greater proportion of acidic amino acid residues and have an isoelectric point (pI) below 4.8. Other established allergens include enolases and aldolases from cod, salmon, tuna, carp, and catfish, with IgE reactivities ranging from 13% to 56%. In addition, collagen and tropomyosins have been identified in several fish species.83 Although in vitro cross-reactivity has been demonstrated for most allergens in different fish species, the clinical relevance is yet to be confirmed.
IgE cross-reactivity seems to be limited between α- and β-parvalbumins. In contrast, frequent cross-reactivity is seen between beta-homologues, due to the very high structural homology, particularly in the 2 calcium-binding regions.84 More than 16 different parvalbumins are registered with the International Union of Immunological Societies (IUIS) database, and large amino acid sequence diversity between species such as cod, carp, and salmon has been demonstrated.85 Notably, parvalbumin isoforms from the same species often share less than 68% amino acid sequence identity as shown for barramundi and rainbow trout, adding complexity to correct diagnosis.86 Parvalbumins of the α-lineage share only 43% to 60% amino acid sequence identity with β-parvalbumins, explaining the lower allergenicity of cartilaginous fish.82
IgE-binding epitopes of β-parvalbumin are identified in about 4 different patches87; however, none share identical epitopes. Although the most conserved amino acid region is in the first calcium-binding site, the most common IgE-binding sites are between position 25 and 45.87 A second frequent IgE epitope is in the second calcium-binding site, explaining reduced antibody reactivity after depletion of calcium ions from parvalbumin. One epitope in the first 20 amino acids of the N-terminal region has only been demonstrated for salmon, and probably explains the monosensitivity seen to this fish species.88 The number of linear epitopes and IgE reactivity to the C-terminal epitope seem to correlate with severity of allergic reactions.89 Clinically relevant cross-reactivity to nonfish parvalbumins has also been demonstrated, for frog (Ran e 2), chicken (Gal d 8), and recently for crocodile (Cro p 1, Cro p 2).90,91
Because parvalbumin is the most frequent IgE-binding fish allergen, a strategy for immunotherapy using hypoallergenic parvalbumins has been developed. Mutations in the 2 calcium-binding sites resulted in significantly reduced IgE binding. The immune response observed in sensitized mice and rabbits, supported by peptide-specific antibody responses, clearly demonstrated IgG-driven protection against allergic reactions.92 These finding are supported by a recent study using 7 overlapping peptides to identify IgE- and IgG4-binding epitopes on Asian seabass parvalbumin.87 Patients demonstrated patient-specific antibody-binding profiles; however, peptide recognition differed between antibody isotypes.
Hen’s egg allergy
Egg allergy is one of the most frequent food allergies in children worldwide and can affect up to 10%.93,94 Sensitization and exposure occur usually via ingestion, but skin contact and inhalation of aerosolized particles has also been reported. Although up to 75% of allergic children seem to outgrow egg allergy during later childhood, severe symptoms are common in the early years, including vomiting, abdominal pain, and urticaria. Importantly, the remaining allergic children experience allergy into adulthood.95 The early identification of children with persistent egg allergy is critical to management and component-resolved diagnosis seems to identify several egg white allergens as good predictors.
Egg white contains 4 major allergens, ovomucoid (Gal d 1), ovalbumin (Gal d 2), ovotransferrin (Gal d 3), and egg lysozyme (Gal d 4), whereas alpha-livetin (Gal d 5) is present in egg yolk. Other less well-characterized allergens include phosvitin, apovitellenins-I and -VI as well as ovomucin. Clinical cross-reactivity occurs between various bird egg proteins (eg, hen, turkey, duck, seagull, and quail96), probably due to 1 or several of these allergens. Reduced allergenicity is shown for baked eggs (180°C, >30 minutes), indicating the presence of conformational IgE epitopes. IgE binding to specific allergens can assist in determining the degree of clinical reactivity, persistence of sensitization, and cross-reactivity to other bird egg proteins. Elevated IgE level to the heat-stable Gal d 1 might indicate sustained egg allergy to all forms of egg. The less-stable allergens Gal d 2, Gal d 3, and Gal d 4 indicate a higher risk of clinical reactions to raw and slightly heated egg. Less studied are the allergens in egg yolk, which seem to predominantly affect adults. Clinical cross-reactivity to Gal d 5 can cause the bird-egg syndrome, and sensitization to airborne avian allergens.97 In addition, it was observed in children that Gal d 5 specific IgE was strongly correlated with persistent egg allergy.95
Several studies analyzed the linear epitopes of Gal d 2 and identified up to 5 epitopes, of which some had β-turns and β-sheets exposed on the protein surface structure.98 Epitope studies of Gal d 1 identified 3 intradomain disulfide bonds in each of the 3 domains, leading to its high stability. Up to 9 IgE epitopes were identified in these 3 domains. Subsequently, hypoallergenic variants of Gal d 1 have been produced in several studies, through disruption of stabilizing disulphide bonds in domain III, resulting in much reduced IgE binding.99 Gal d 4 has 4 disulfide bonds, and 3 IgE epitopes have been identified so far. T-cell epitope studies have only been conducted for Gal d 1100 and Gal d 2101 in a BALB/c mouse model.
Cow’s milk allergy
Cow’s milk allergy (CMA) is one of the most common food allergies worldwide (0.5%-7.5% in westernized countries).91 CN and whey constitute approximately 80% and 20% of CM protein, respectively. CNs (αs1-, αs2-, β-, and κ-CN, Bos d 8), beta-lactoglobulin (Bos d 5), and α-lactalbumin (Bos d 4) are considered major allergens, whereas BSA (BSA, Bos d 6), lactoferrin, and immunoglobulin (Bos d 7) are considered minor allergens.
CN is a phosphoprotein that interacts with calcium phosphate and presents in the micelle structure in CM. The labile structure impedes heat denaturation and aggregation, and specific IgE antibodies preferentially recognize sequential epitopes. However, CN is easily degraded by digestive enzymes such as pepsin and trypsin, so major IgE-binding epitopes exist at amino acid sequence sites that are not cleaved by these enzymes.91,102 IgE epitope mapping has been investigated using an overlapping peptide array technique.103 Although the suggested IgE and IgG4 epitopes were varied among the reports, the identified epitopes were related to the diagnosis of CMA,104 acquisition of natural tolerance,105 and the outcome of oral immunotherapy.106 Tetramer-guided T-cell epitope mapping identified 23 T-cell epitopes, and suggested a possibility of epitope spreading in subjects with persistent CMA.107
When considering the cross-reactivity between mammalian milk, the composition of each protein fraction and sequential homologies are important. The total protein content in milk from the order Artiodactyla (cow, sheep, goat, camel, and pig) is higher than that from the order Perissodactyla (horse and donkey). The ratio of CN to whey protein is very similar among the family Bovidae (cow, sheep, goat), whereas the milk from the family Equidae (horse, donkey) has a lower ratio, which is even lower in human milk. The highest sequential homologies are observed between CM and other Bovidae (84%-91% in CNs, 71%-97% in whey proteins). Lower homologies are associated with the milk from Camelidae (camel), Suidae (pig), Equidae, and humans. Consistent with these compositional and sequential properties, immunologic cross-antigenicity has been demonstrated by inhibition assays between CM and Bovidae (60%-89%), but much less between CM and Equidae. Clinically, high concordance of skin prick test positivity (63%-100%) and reactivity of oral food challenge test (92%) is confirmed between CM and Bovidae, whereas these are lower between CM and Camelidae, Suidae, and Equidae (1%-31%). Although it is difficult to prove true clinical cross-reactivity, the remission of most goat and sheep milk allergies after CM oral immunotherapy provides indirect evidence of the clinical cross-reactivity between them.108,109
Patients with CMA sometimes react to raw or weakly heated bovine meat, due to the presence of common allergenic proteins, including serum albumins (Bos d 6) and immunoglobulins (Bos d 7).1 In contrast, 73% to 93%1,110,111 of beef-allergic patients, especially sensitized to Bos d 6, were shown to react to CM. Although there is no evidence of direct clinical cross-reactivity, approximately 10% of patients with CMA are reported to be allergic to soybean.112 Moreover, several studies have shown immunologic cross-antigenicity between CM and the Gly m 5,113 Gly m Bd 28k,3 and Gly m Bd 30k.44,114
Although amino acid sequence homology between αs1-CNs and β-CN is quite low (4%), specific IgE levels between them were strongly correlated, and simultaneously decreased during CM oral immunotherapy.115,116 Moreover, complete IgE inhibition was observed by β-CN against αs1-CN in ELISA. The partial amino acid sequences of αs1-CN (E61-E70) and β-CN (I12-E21) showed a low propensity distance value (5.30) under in silico analysis,117 which suggests high sequential homology. These conserved sequences have been known as CN phosphopeptide, which has the core motif of “SSSEE,” consisting of phosphorylated serine residues. Patients with severe CMA exhibit allergic reactions to CN phosphopeptide–containing products such as oral care products, chewing gums, topical creams, and toothpaste.118 These findings suggest that partial cross-reactive epitopes, but not the entire sequential homology, play a vital role in the cross-antigenicity between CN fractions.
Wheat allergy
Wheat is among the 5 most common food allergens in children; the prevalence varies from 0.4% to 4% depending on age and region.91,119 Wheat proteins are classified as water/salt-soluble fraction (albumin/globulin) and water/salt-insoluble fraction (gluten). Gluten is a large disulfide-bonded polymer composed of gliadin and glutenin. Gliadins are characterized as glutamine-rich, alcohol-soluble grain storage prolamins and are classified as α/β-, γ-, and ω-gliadins. Glutenins are alcohol-insoluble, acid/base-soluble proteins classified into high molecular weight (HMW) and low molecular weight.
Twenty-eight wheat allergens are listed in the WHO-IUIS nomenclature database.91 Proteins constituting gluten are the major allergens in immediate-type wheat allergy in children and wheat-dependent exercise-induced anaphylaxis (WDEIA), with ω-5 gliadin (Tri a 19) and HMW-glutenin (Tri a 26) reported as major components.4,91,116 Many proteins in water/salt-soluble fraction, including α-amylase inhibitor (Tri a 15, 28-30), nonspecific lipid transfer protein (Tri a 14), wheat profilin (Tri a 12), thioredoxin (Tri a 25), thiol reductase homolog (Tri a 27), serpin (Tri a 33), serine protease inhibitor (Tri a 39), and peroxidase were found to be significant allergens in patients with baker’s asthma and immediate-type wheat allergy.4,91,120, 121, 122 α-Purothionin (Tri a 37) is associated with severe wheat allergy,123 and IgG and IgE reactivity to α-purothionin has been reported to be useful in distinguishing between wheat allergy and sensitization.124
Epitope studies have identified a consensus sequence consisting of QQX1PX2QQ (X1 = L, F, S, I; X2 = Q, E, G) in ω-5-gliadin as the IgE-binding epitope in Japanese and European patients with WDEIA.4,125 IgE from patients with WDEIA also reacts strongly with HMW-glutenin, and QQPGQ, QQPGQGQQ, and QQSGQGQ have been identified as IgE-binding epitopes.126 These sequences are present repeatedly in the primary structure of ω-5 gliadin and HMW-glutenin, respectively. However, a consensus sequence QPQQPFPQ in γ-gliadin and ω-2 gliadin was identified in patients with WDEIA after transdermal sensitization to hydrolyzed wheat protein. It was identical to the epitope sequence identified in European patients orally sensitized with hydrolyzed wheat protein.127,128 In these cases, deamidation of glutamine (Q) residue, converted to glutamate residue (E), strengthens the IgE-binding affinity, and the substitution of 3 glutamines, QPEEPFPE, increases its recognition. Thus, the deamidation of gluten generates neo-epitopes responsible for hydrolyzed wheat protein allergy.129 T-cell epitopes have been studied in celiac disease, and it is known that the deamidation of specific motifs in gluten generates effective T-cell epitopes. Although an association between the HLA-DPB1∗02:01:02 allele and WDEIA has been reported,130 T-cell epitopes in wheat allergy are not yet elucidated.
Wheat has a wide range of in vitro cross-reactivity among other grains of the grass Poaceae family. Prolamine is considered responsible for the cross-reactivity between gliadin in wheat, hordein in barley, secalin in rye, and avenin in oat.91,121,131 Among wheat-allergic patients, cross-reactive grain allergies confirmed by oral food challenge are observed in 8% to 56% for barley, 12% for rye, and 7% to 20% for oat.119,132, 133, 134, 135 Early studies of patients with WDEIA showed that γ-3 hordein in barley and γ-70 and γ-35 secalin in rye cross-react with ω-5 gliadin.131 Inhibition ELISA confirmed that ω-5 gliadin inhibited the binding of IgE to solid-phase γ-3 hordein and γ-secalins. These proteins are major storage proteins of the endosperm, containing unusually rich proline and glutamate residues in their primary structure, and are highly homologous to each other.136,137
For immediate-type allergy in children, inhibition ELISA studies using serum from patients with comorbid wheat and barley allergy showed that wheat completely inhibited the binding of IgE to barley solid phase, even at lower concentrations than barley.135,138 Immunoblotting138 and ELISA135 inhibition showed that multiple barley fractions were inhibited with wheat, indicating that multiple fractions, not only gliadin, might be involved in cross-reactivity between wheat and barley. In addition, in patients with oral food challenge–confirmed wheat and barley allergy, successful wheat oral immunotherapy simultaneously ameliorated the barley allergy.138 This indicated that in the clinical cross-reactivity between wheat and barley, mostly wheat is the primary allergen and barley is the cross-sensitized allergen. Other types of wheat allergy involving cross-reactivity have been reported, suggesting that IgE produced by sensitization to grass pollen–derived proteins cross-reacts with peroxidase-I and beta-glucosidase in wheat foods, resulting in immediate wheat allergy or WDEIA.139 There are no reports on wheat allergy immunotherapy with IgE epitopes. Although reductions in all gliadin and glutenin component-specific IgE have been reported in oral immunotherapy for patients with immediate wheat allergy, no specific component was found to correlate with the efficacy of oral immunotherapy.140
Cross-reactive carbohydrate determinants and their role in food allergy
Glycosylation is one of the common post-translational modification of proteins in most organisms. Carbohydrate moieties can vary in structure and complexity, and may play a role in protein functionality, structural stability, solubility, and protein transport. Carbohydrate determinants found on glycoproteins from plants, nonprimate mammals, and invertebrates do not occur in humans. Therefore, these glycans are highly immunogenic and capable of inducing a strong antibody response. These carbohydrate moieties are termed as cross-reactive carbohydrate determinants (CCDs). The presence of α 1,3-linked core fucose (plant and insect) and β1,2-linked xylose (plant and helminth) motifs on N-glycans is high among plant and invertebrate allergens and exhibit antibody cross-reactivity.141 Anti-CCD IgE antibodies are found primarily in individuals with multiple sensitizations to plant glycoprotein allergens. In a study by Holzweber et al,142 it was shown that 22% of allergic patient sera contained anti-CCD IgE antibodies. Similarly, 10% to 50% of patients with zucchini, celery, carrot, or tomato allergy had anti-CCD IgE.143 The presence of anti-CCD IgE antibodies does not correlate with clinically relevant allergic symptoms in most, if not all, cases. This may be due to the presence of anti-CCD IgG antibodies that act as blocking antibodies.144 Inhibition of these anti-CCD IgEs can increase the diagnostic efficiency.145 In an interesting study, CCDs have also been shown to play a role in cross-reactive antibodies between peanut allergen Ara h 1 and Schistosoma mansoni egg antigens and cross-binding abolished on removal of glycan groups.146
In contrast to the low clinical effects of classical CCDs, the IgE-mediated clinical response to galactose-α-1,3-galactose (α-Gal) is well established. Anti–α-Gal IgE antibodies are produced in individuals on exposure to glycoproteins in tick saliva after a tick bite, and can bind to α-Gal found in red meat that is bound to either proteins or lipids, and lead to cross-linking on the surface of basophils and mast cells. Interestingly, α-Gal–induced red meat allergy elicits a delayed-onset allergic response usually after 3 to 6 hours of ingestion.147,148 In contrast, immediate allergic reaction and anaphylaxis was observed to intravenous administration of cetuximab, a therapeutic drug known to contain α-Gal.149 Recently, it was shown that α-Gal from glycolipids, and not glycoproteins, is able to cross the intestinal monolayer and trigger an allergic reaction. This may explain the delay in allergic reaction to red meat allergy due to the slow digestion and absorption process of lipids.150 It is interesting to note that in case of both classical CCDs and α-Gal–based CCDs, the primary sensitization can occur through percutaneous exposure via an arthropod (insect sting or tick bite). However, current literature do not show any evidence of CCDs playing a role in arthropod-related food allergy (crustacean, mollusk, or edible insects) and may be a potential topic of future research in understanding the role of glycoproteins in allergic sensitization and cross-reactivity.
Allergy to galacto-oligosaccharides, an ingredient that is present in milk formulations and dairy products, may be associated with primary sensitization to dust mites.151 Glycoproteins present in tropical mites may induce cross-reactive IgE antibodies, which may in turn bind to dietary galacto-oligosaccharides. Such cross-reactivity may also explain allergy to galacto-oligosaccharide–supplemented beverages shown among a group of oyster shuckers having sea squirt allergy.152 Because of the growing knowledge and clinical significance of the role of carbohydrate epitopes in allergic diseases, CCDs are included as potential allergenic epitopes in the WHO/IUIS Allergen Nomenclature.153
Role of cross-reactive epitopes in allergen-specific immunotherapy for food allergy
The presence of cross-reactive epitopes in allergen preparations used for allergen immunotherapy plays an important role in the safety and efficacy of the treatment and in its objective of achieving desensitization and clinical tolerance.
One example of immunotherapy and concurrent sensitization/desensitization is that of house dust mite (HDM) immunotherapy and sensitization or allergy to shellfish. IgE sensitization and clinical symptoms were seen in a patient after receiving HDM immunotherapy, and IgE binding to snail in another patient.154 In a separate study, 1 patient showed a decrease in specific IgE to both Der p 10 and Pen a 1 after receiving HDM immunotherapy as well as loss of reactivity toward seafood.155 A study by Asero156 showed in a 3-year follow-up study that none of the 70 patients receiving HDM immunotherapy developed sensitization or clinical symptom to shrimps and reported regular consumption of crustaceans and mollusks. Most studies focus on tropomyosin as the major cross-reacting allergen. However, several other HDM and shrimp allergens belong to the same allergen families, including arginine kinase, myosin light chain, sarcoplasmic calcium-binding protein, hemocyanin, fatty acid–binding protein, and filamin C, which play a role in HDM/shrimp cross-reactivity.10,157
This phenomenon has also been observed in pollen-related food allergy syndrome. A recent study showed that patients on a 5-grass pollen sublingual tablet immunotherapy declared good tolerance to offending plant-based foods.5 Patients on subcutaneous immunotherapy with birch pollen extract showed tolerance to soy milk, although some patients elicited systemic reactions in the rapid escalation phase of the treatment.158 In a clinical study, allergen-specific immunotherapy with fresh apples showed an increased tolerance to consumption and decreased skin reactivity, as well as decreased conjunctivitis reactivity to birch extract.159
Based on the extensive cross-reactivity between tree nuts as well as on the unexpected cross-reactivity between unrelated allergens described above, there is great potential for single nut therapy or single allergen to have therapeutic effects for multiple nut allergies. Current research into walnut allergy reaffirms what animal models demonstrated, that cross-desensitization between tree nuts might occur.160,161 Elizur et al162 showed that walnut oral immunotherapy can be an effective way to induce desensitization to walnut as well as cross-desensitization to pecan and hazelnut in patients who were allergic to the 3 tree nuts.162 Furthermore, a recent study in an anaphylactic mouse model showed that vaccination against either Ara h 1 or Ara h 2 was sufficient to induce protection against the whole peanut extract consisting of multiple allergens.163
The presence or modification of cross-reactive epitopes can be controlled in protein- or peptide-based immunotherapy for food allergy. Well-characterized cross-reactive T-cell epitopes may help in desensitization to multiple food sources as elucidated for cashew allergens.45 Factoring in the presence or absence of cross-reactive T- or B-cell epitopes is important while developing immunotherapeutic lead candidates for major food-group allergies such as fish or shellfish. Because of the vast number of consumed species, presence of multiple sensitizing allergens, and the structural and immunologic differences among the allergens they contain, it is a challenge to design a single peptide- or protein-based candidate. Current advances in immunoinformatic tools may help in deciding specific shared cross-reactive peptide sequences covering a range of different allergen sources. However, it should be noted that structural differences among allergens from the same protein family might result in differential cross-reactivity.18,164
Conclusions and future directions
It is clear that allergenic cross-reactivity plays a central role in the initiation and progress of allergic reactions to food. Identification and characterization of B-cell and T-cell cross-reactive epitopes in clinically relevant food allergens is important for improving our understanding of mechanisms of clinical cross-sensitization and cross-reactivity, as well as to design improved diagnostic assays to be able to predict cross-reactivity to the initiator allergen source, with high sensitivity and specificity. More importantly, it is important to take into account the presence of cross-reactive epitopes in the development of protein- or peptide-based molecules for allergen-specific immunotherapy. Cross-reactive food allergen epitopes could potentially be harnessed to induce desensitization and tolerance to a wider range of related food sources.
알레르기 교차 반응성이
식품에 대한 알레르기 반응의
시작과 진행에 중심적인 역할을 한다는 것은 분명합니다.
임상적으로 관련된 식품 알레르겐에서
B세포 및 T세포 교차 반응성 에피토프를 식별하고 특성화하는 것은
임상 교차 감작 및 교차 반응성의 메커니즘에 대한 이해를 높이고
높은 민감도와 특이도로 개시 알레르겐원에 대한 교차 반응성을 예측할 수 있도록
개선된 진단 분석법을 설계하는 데 중요합니다.
더 중요한 것은
알레르겐 특이 면역 치료를 위한 단백질 또는
펩타이드 기반 분자를 개발할 때 교차 반응성 에피토프의 존재를 고려하는 것이 중요하다는 점입니다.
교차 반응성 식품 알레르겐 에피토프는
잠재적으로 더 광범위한 관련 식품 공급원에 대한
탈감작과 내성을 유도하는 데 활용될 수 있으며,
아라 1, 2, 3 사이의 세포 교차 반응성은 생쥐에서도 관찰되었습니다.36
References