

The good and the bad of T cell cross-reactivity: challenges and opportunities for novel therapeutics in autoimmunity and cancer
T cells are main actors of the immune system with an essential role in protection against pathogens and cancer. The molecular key event involved in this absolutely central task is the interaction of membrane-bound specific T cell receptors with peptide-MHC complexes which initiates T cell priming, activation and recall, and thus controls a range of downstream functions. While textbooks teach us that the repertoire of mature T cells is highly diverse, it is clear that this diversity cannot possibly cover all potential foreign peptides that might be encountered during life. TCR cross-reactivity, i.e. the ability of a single TCR to recognise different peptides, offers the best solution to this biological challenge. Reports have shown that indeed, TCR cross-reactivity is surprisingly high. Hence, the T cell dilemma is the following: be as specific as possible to target foreign danger and spare self, while being able to react to a large spectrum of body-threatening situations. This has major consequences for both autoimmune diseases and cancer, and significant implications for the development of T cell-based therapies. In this review, we will present essential experimental evidence of T cell cross-reactivity, implications for two opposite immune conditions, i.e. autoimmunity vs cancer, and how this can be differently exploited for immunotherapy approaches. Finally, we will discuss the tools available for predicting cross-reactivity and how improvements in this field might boost translational approaches.
T세포는
병원균과 암으로부터 우리 몸을 보호하는 데
필수적인 역할을 하는 면역 체계의 주축입니다.
이 절대적으로 핵심적인 임무에 관여하는 분자적 핵심 사건은
막 결합 특이적 T세포 수용체와
펩타이드-MHC 복합체의 상호작용으로,
이는 T세포 프라이밍, 활성화 및 리콜을 시작하여 다양한 다운스트림 기능을 제어합니다.
교과서에서는
성숙한 T 세포의 레퍼토리가 매우 다양하다고 가르치지만,
이러한 다양성이 일생 동안 마주칠 수 있는
모든 잠재적 외부 펩타이드를 포괄할 수는 없습니다.
TCR 교차 반응성,
즉 단일 TCR이 다른 펩타이드를 인식하는 능력은
이러한 생물학적 과제에 대한 최상의 솔루션을 제공합니다.
실제로
TCR 교차 반응성은
놀라울 정도로 높다는 보고가 있습니다.
따라서
T세포의 딜레마는 다음과 같습니다.
외부의 위험을 표적으로 삼아
최대한 구체적으로 자신을 보호하면서도
신체를 위협하는 다양한 상황에 반응할 수 있어야 한다는 것입니다.
이는
자가 면역 질환과 암 모두에 중대한 영향을 미치며,
T 세포 기반 치료법 개발에도 중요한 의미를 갖습니다.
이 리뷰에서는
T세포 교차 반응성에 대한 필수적인 실험적 증거,
자가면역과 암이라는 두 가지 상반된 면역 상태에 대한 시사점,
그리고 이를 면역 치료 접근법에 어떻게 다르게 활용할 수 있는지 제시할 것입니다.
마지막으로
교차 반응성을 예측하는 데 사용할 수 있는 도구와
이 분야의 개선이 어떻게 중개적 접근법을 향상시킬 수 있는지에 대해 논의할 것입니다.
1 Introduction: basics on TCR cross-reactivity
T cells are essential players of the adaptive immunity that are not only responsible for long-term immune memory, but also orchestrate innate and adaptive immune responses. Immature T cells undergo a strict selection in the thymus which leads to the release of mature, largely self-tolerant, T cells. Each of these cells bears several 10.000 copies of a unique kind of T cell receptor (TCR) that results from the assembly of two recombined TCR chains (in most cases α and β) (1, 2). The TCRαβ interacts with antigens presented as peptides by cell membrane-bound molecules of the major histocompatibility complex (pMHC) on the antigen presenting cell (APC) or on the target cell, for example, after pathogen infection (Figure 1A). Importantly, the binding of peptides to the various MHC allelic products is subject to specific rules (anchor or preferred residues) (4, 5). The diversity of the TCRαβ T cell repertoire is high, but not unlimited. Based on the V, D and J fragments´ recombination at the two chain loci, the theoretical number of single TCRs is estimated to reach at least 1015. In fact, the sum of all different TCRs present in the human blood has been estimated to be much less, in the range of 2.5 x 107 for naïve T cells and approximately 100-fold lower for memory T cells (6–8). The number of potential pathogen- (and tumour-) derived epitopes presented as pMHC throughout life might well exceed this number of T cell clones. It became therefore progressively clear that the clonal selection theory, which proposed that one lymphocyte/receptor is available for each single antigen, needed to be revised, and that cross-reactivity, i.e. the ability of single TCRs to recognise multiple peptide sequences, is a frequent event (9–11).
1 소개: TCR 교차 반응성에 대한 기본 사항
T세포는
장기 면역 기억을 담당할 뿐만 아니라
선천성 및 적응성 면역 반응을 조율하는 적응 면역의 필수적인 역할을 합니다.
미성숙 T 세포는
흉선에서 엄격한 선택을 거쳐
대부분 자기 내성이 있는 성숙한 T 세포로 방출됩니다.
이 세포들 각각은 두 개의 재조합된
TCR 사슬(대부분의 경우 α 및 β)의 조립으로 인해 생성되는
독특한 종류의 T세포 수용체(TCR)를
TCRαβ는 예를 들어
병원체 감염 후 항원 제시 세포(APC) 또는
표적 세포에서 주요 조직 적합성 복합체(pMHC)의 세포막 결합 분자에 의해
펩타이드로 제시된 항원과 상호 작용합니다(그림 1A).
중요한 것은
펩타이드와 다양한 MHC 대립 유전자 생성물에 대한 결합은
특정 규칙(앵커 또는 선호 잔기)의 적용을 받는다는 점입니다(4, 5).
TCRαβ T 세포 레퍼토리의 다양성은 높지만
무제한은 아닙니다.
두 사슬 좌위에서 V, D, J 단편의 재조합에 근거하여
이론적으로 단일 TCR의 수는
최소 1015개에 이르는 것으로 추정됩니다.
실제로
사람의 혈액에 존재하는
모든 다른 TCR의 합은
순진한 T세포의 경우 2.5 x107 범위에서 훨씬 적고
기억 T세포의 경우 약 100배 낮은 것으로 추정되었습니다(6-8).
일생 동안 pMHC로 나타나는 잠재적인 병원체(및 종양) 유래 에피토프의 수는
이 T세포 클론의 수를 훨씬 초과할 수 있습니다.
따라서
단일 항원마다 하나의 림프구/수용체를 사용할 수 있다고 제안한
클론 선택 이론은 수정이 필요하며
교차 반응성, 즉
단일 TCR이 여러 펩타이드 서열을 인식하는 능력이
빈번하게 발생한다는 것이 점차 분명해졌습니다(9-11).
Figure 1
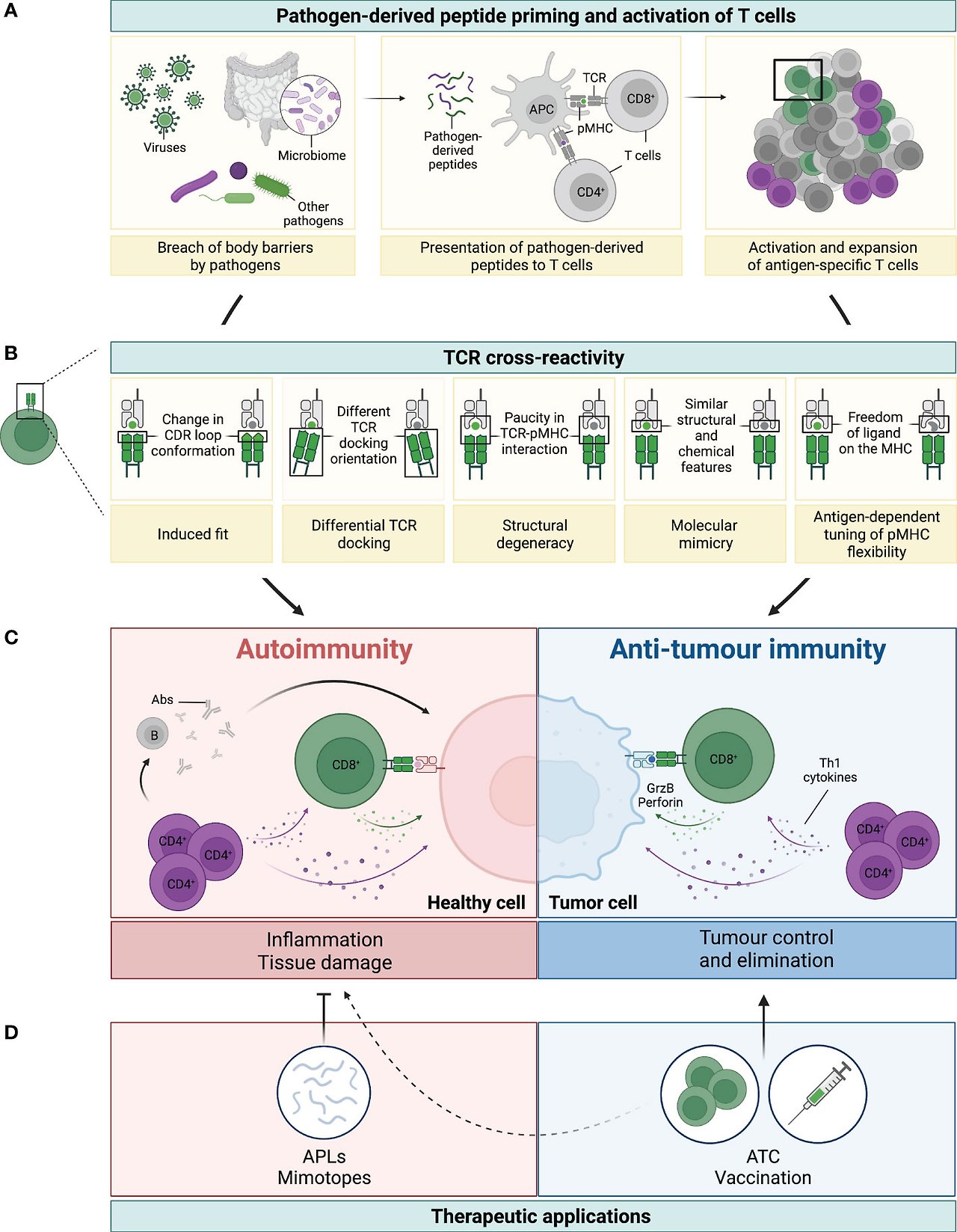
Figure 1 Legend: TCR cross-reactivity: a double-edged sword. (A) Microorganisms, such as viruses, microbiota or other pathogens, can penetrate body barriers and get into contact with our immune system (left panel). Processing and presentation of pathogen (foreign)-derived peptides by antigen-presenting cells (APCs) on MHC-class I (represented by the green dot) and MHC-class II (represented by the purple dot) primes and drives the activation of CD8+ and CD4+ T cells, respectively (middle panel). A polyclonal population of activated T cells then proliferates and expands to fight the invading microorganism (right panel). (B) Several mechanisms have been reported to be involved in TCR cross-reactivity. This figure has been adapted from reference (3). A representative CD8+ T cell and its TCR is shown in green interacting with the pathogen-derived peptide (in green) presented by an MHC-class I molecule (grey). Due to cross-reactivity, the same TCR can interact with another peptide (shown in grey). (C) If this peptide is presented by healthy cells (depicted in pink) or by cancer cells (depicted in blue) this can ultimately result in either autoimmunity or anti-tumour immunity, respectively. On the one hand, recognition of healthy tissues by cross-reactive TCRs (mainly from CD4+, but also from CD8+ T cells), leads to inflammation and tissue damage with deleterious consequences. Secretion of Th1 cytokines by CD4+ T cells (colored in purple) can directly affect healthy cells, but also support the activation of auto-reactive B (grey) and CD8+ T (green) cells, which then secrete Abs and cytotoxic molecules (i.e. granzyme B and perforin, illustrated with the green arrow and dots), respectively (left panel in C). On the other hand, recognition of tumour antigens by cross-reactive T cells can prompt cell killing and tumour elimination, highlighting the contrasting impact of TCR cross-reactivity in this setting (right panel in C). (D) TCR cross-reactivity can be exploited for therapeutic applications. Usage of mimotopes or APLs to drive a Th1 to Th2 or regulatory switch in autoimmunity is an attractive strategy to reduce tissue inflammation and its damage. In cancer, usage of cross-reactive TCRs in adoptive T cell therapy (ATC), or of pathogen-derived peptides for vaccination are promising nascent strategies. Potential side effects against healthy tissues of this novel anti-cancer therapies (represented by the dotted line) need to be carefully considered to prevent damage and severe toxicities.
전설:
TCR 교차 반응성: 양날의 검.
(A) 바이러스, 미생물 또는 기타 병원체와 같은 미생물은 신체 장벽을 통과하여 우리 면역 체계와 접촉할 수 있습니다(왼쪽 패널).MHC 클래스 I(녹색 점으로 표시)과 MHC 클래스 II(보라색 점으로 표시)의 항원 제시 세포(APC)에 의한 병원체(외부) 유래 펩타이드의 처리 및 제시가 각각 CD8+ 및 CD4+ T 세포의 활성화를 촉진하고 유도합니다(가운데 패널).활성화된 T 세포의 다클론 집단은 침입한 미생물과 싸우기 위해 증식하고 확장합니다(오른쪽 패널).
(B) TCR 교차 반응성에는 여러 가지 메커니즘이 관여하는 것으로 보고되었습니다.이 그림은 참고자료 (3)에서 각색한 것입니다.대표적인 CD8+ T 세포와 그 TCR이 병원체 유래 펩타이드(녹색)와 상호작용하는 MHC 클래스 I 분자(회색)와 상호작용하는 것을 녹색으로 표시했습니다.교차 반응성으로 인해 동일한 TCR이 다른 펩타이드(회색으로 표시)와 상호 작용할 수 있습니다.
(C) 이 펩타이드가 건강한 세포(분홍색으로 표시) 또는 암세포(파란색으로 표시)에 의해 제시되면 궁극적으로 각각 자가 면역 또는 항종양 면역을 초래할 수 있습니다. 한편으로, 교차 반응성 TCR(주로 CD4+ 및 CD8+ T세포)이 건강한 조직을 인식하면 염증과 조직 손상을 유발하여 해로운 결과를 초래할 수 있습니다. CD4+ T 세포(보라색)에 의한 Th1 사이토카인의 분비는 건강한 세포에 직접 영향을 줄 수 있지만 자가 반응성 B(회색) 및 CD8+ T(녹색) 세포의 활성화를 지원하여 각각 Abs 및 세포 독성 분자(예: 녹색 화살표와 점으로 표시된 그랜자임 B 및 퍼포린)를 분비합니다( C의 왼쪽 패널). 반면에 교차 반응하는 T 세포가 종양 항원을 인식하면 세포 사멸과 종양 제거를 촉진할 수 있으며, 이 설정에서 TCR 교차 반응성의 대조적인 영향이 강조됩니다( C의 오른쪽 패널).
(D) TCR 교차 반응성은 치료 응용 분야에 활용할 수 있습니다. 자가 면역에서 Th1에서 Th2로 또는 조절 스위치를 유도하기 위해 미모토프 또는 APL을 사용하는 것은 조직 염증과 그 손상을 줄이기 위한 매력적인 전략입니다. 암에서는 입양 T 세포 치료(ATC)에 교차 반응성 TCR을 사용하거나 백신 접종을 위해 병원체 유래 펩타이드를 사용하는 것이 유망한 초기 전략입니다. 이 새로운 항암 요법의 건강한 조직에 대한 잠재적 부작용(점선으로 표시)은 손상과 심각한 독성을 예방하기 위해 신중하게 고려해야 합니다.
Cross-reactivity is commonly observed when testing nearly identical peptides which differ only in 1 or 2 amino acids (aa) (for a total length of 8-10 aa for a CD8+ T cell epitope presented by MHC-class I). This is physiologically highly relevant for fighting rapidly mutating viruses like HIV, SARS-Cov-2 or dengue viruses (9, 12–14). An interesting example is that of HIV elite controllers who are often HLA-B*5701+, an allelic product which, according to in silico models, is recognised by T cells with high cross-reactive potential (15). Heterologous immunity, whereby T cells cross-react with different viruses, is also frequently reported and has been reviewed elsewhere (16). In addition, many examples of T cells reacting to very different aa sequences are known (17, 18). Kersh and colleagues claimed that a peptide is recognised as long as it contains a motif for binding to the MHC and one key residue for the TCR (18). This concept was refined by the observation that no single residue was strictly required for recognition, if the available residues allow for a sufficient affinity between the MHC and TCR molecules (19). Thus, peptides not sharing a single residue may productively interact with the same TCR. A similar flexibility was also observed for the length of the MHC-class II peptide, with some CD4+ T cells requiring as little as four aa for recognition as long as these optimally fit to the MHC and TCR (20). In contrast, in a more recent study based on a unique experimental approach, Birnbaum et al. tested a set of murine and human CD4+ T cell clones and observed that the diversity of the peptide sequences recognised by single TCRs could be smaller than previously thought (21). Still, pluriallelic restriction, as well as alloreactivity have also been experimentally observed (22–24), increasing the number of cross-reactivity scenarios. The structural features that rule cross-recognition have been described in detail (3, 14, 17, 23, 25). They include several mechanisms of conformational adaptation of the TCR and pMHC units (e.g. changes in the TCR docking, displacement of the CDR loop), as summarised in Figure 1B. Hence, TCR-pMHC interactions are not rigidly conserved, but rather allow for considerable flexibility within the confines of some general orientation and binding rules. It is also important to note that in vivo, T cell cross-reactivity is very likely fine-tuned by the set of co-receptors (inhibitory or activating) and adhesion molecules that the T cell expresses at a given time (26).
A very convincing hint that our T cell immunity is shaped by cross-reactivity was provided in the elegant study of Su et al. (27): a search for HLA-DRB1*0401 restricted CD4+ T cells specific for HIV-, CMV- and HSV-derived epitopes in the blood of virus-unexposed healthy donors revealed that although the frequency of such cells was very low (< 10 cells per million), a large fraction (variable between individuals but in average > 50%) were found in the CD45RO+ subset. Looking at HIV-specific cells more precisely, the authors confirmed that these CD45RO+ cells represent a memory cell pool by assessing IFN-γ production, sequencing the TCR, and analysing further memory markers by gene expression. In addition, such cells were not found in umbilical cord blood. Finally, cross-reactivity of HIV-specific T cell clones with a range of bacterial- or algae-derived peptides suggested that such cells had been primed by unrelated antigens. Also relevant for vaccination, the authors further showed that Influenza-specific clones derived after Flu vaccination were able to recognise related peptidic sequences derived from other microbes. Similar observations were done by the group of F. Sallusto that demonstrated that HIV-specific CD4+ T cells could be detected in both the naive and memory T cell subsets (defined with the two markers CD45RA and CCR7) of HIV-unexposed healthy donors (28). The large majority (>80%) of the HIV-epitopes activating memory T cells matched strongly with human microbiome aa sequences. A further notable observation in this report was that both the specificity and the frequency of these HIV-specific T cells were different across donors. This highlights the inter-individual variability of T cell responses, likely to be shaped by both MHC-polymorphism and the environment.
Despite clear evidences about the cross-reactive nature of the TCR, it remains unclear how many single peptides can a unique TCR recognise in “real life”. According to early estimates, it should be approx. 106 (29). Meanwhile, there is evidence from several studies that individual T cell clones can indeed sense over a million different peptides in the context of a single MHC molecule (30–32). Studying the cross-reactive repertoire of an autoimmune HLA-A*0201 CD8+ T cell clone which recognises a 10 aa-long preproinsulin-derived peptide, Sewell and co-workers showed that many of the “cross-reactive” peptides were better agonists than the original one, despite some sequences differing in up to 7 out of the 10 aa positions (31). Based on the assumption that only 1% of all peptides will end up being presented on MHC, they also estimated the “true” frequency of cross-reactivity to be approximately 1 in 104 peptides (11, 31). This is in the same range as the frequency of 1 in 3x104 found by Ishizuka et al. when using a peptide library derived from pathogen sequences (33).
교차 반응성은
일반적으로 1개 또는 2개의 아미노산(aa)만 다른 거의 동일한 펩타이드를 검사할 때 관찰됩니다(MHC 클래스 I에 의해 제시된 CD8+ T 세포 에피토프의 경우 총 길이가 8-10 aa).
이는 생리적으로 HIV, SARS-Cov-2 또는 뎅기 바이러스와 같이 빠르게 변이하는 바이러스와 싸우는 데 매우 관련이 있습니다(9, 12-14). 흥미로운 예로 인실리코 모델에 따르면, 교차 반응 가능성이 높은 T 세포에 의해 인식되는 대립 유전자 산물인 HLA-B*5701+인 HIV 엘리트 컨트롤러의 경우가 있습니다(15).T 세포가 다른 바이러스와 교차 반응하는 이종 면역도 자주 보고되고 있으며 다른 곳에서 검토되었습니다(16).또한, 매우 다른 AA 서열에 반응하는 T 세포의 많은 예가 알려져 있습니다(17, 18).Kersh와 동료들은 펩타이드가 MHC에 결합하는 모티프와 TCR의 핵심 잔기를 하나만 포함하면 인식된다고 주장했습니다(18).이 개념은 사용 가능한 잔기가 MHC와 TCR 분자 사이에 충분한 친화력을 허용하는 경우 단일 잔기가 인식에 반드시 필요하지 않다는 관찰에 의해 구체화되었습니다(19).따라서 단일 잔기를 공유하지 않는 펩타이드는 동일한 TCR과 생산적으로 상호 작용할 수 있습니다.MHC 클래스 II 펩타이드의 길이에 대해서도 유사한 유연성이 관찰되었는데, 일부 CD4+ T 세포는 MHC와 TCR에 최적으로 맞는 한 인식에 최소 4개의 aa만 필요로 합니다(20).이와는 대조적으로, 독특한 실험적 접근법을 기반으로 한 최근 연구에서 Birnbaum 등은 일련의 쥐와 인간 CD4+ T 세포 클론을 테스트한 결과 단일 TCR이 인식하는 펩타이드 서열의 다양성이 이전 생각보다 작을 수 있다는 것을 관찰했습니다(21). 그럼에도 불구하고 교차 반응성 시나리오의 수를 증가시키는 복수 제한과 전반응성도 실험적으로 관찰되어(22-24) 교차 반응성 시나리오의 수가 증가했습니다. 교차 인식을 지배하는 구조적 특징이 자세히 설명되어 있습니다(3, 14, 17, 23, 25). 여기에는 그림 1B에 요약된 것처럼 TCR과 pMHC 단위의 형태 적응 메커니즘(예: TCR 도킹의 변화, CDR 루프의 변위)이 포함됩니다. 따라서 TCR-pMHC 상호 작용은 엄격하게 보존되는 것이 아니라 몇 가지 일반적인 방향 및 결합 규칙의 범위 내에서 상당한 유연성을 허용합니다. 또한 생체 내에서 T 세포 교차 반응성은 T 세포가 주어진 시간에 발현하는 공동 수용체(억제 또는 활성화) 및 부착 분자의 집합에 의해 미세 조정될 가능성이 매우 높다는 점에 유의하는 것이 중요합니다(26).
바이러스에 노출되지 않은 건강한 기증자의 혈액에서 HIV, CMV 및 HSV 유래 에피토프에 특이적인 HLA-DRB1*0401 제한 CD4+ T 세포를 검색한 결과, 이러한 세포의 빈도는 매우 낮았지만(백만 개당 10세포 미만) CD45RO+ 하위 집합에서 많은 부분(개인마다 다르지만 평균 > 50%)이 발견되었습니다(27). HIV 특이 세포를 더 정확하게 살펴보면 저자들은 IFN-γ 생산을 평가하고, TCR을 시퀀싱하고, 유전자 발현에 따른 추가 메모리 마커를 분석하여 이러한 CD45RO + 세포가 메모리 세포 풀을 나타내는 것을 확인했습니다. 또한 제대혈에서는 이러한 세포가 발견되지 않았습니다. 마지막으로, 다양한 박테리아 또는 조류 유래 펩티드와 HIV 특이적 T 세포 클론의 교차 반응성은 이러한 세포가 관련 없는 항원에 의해 프라이밍되었음을 시사했습니다. 또한 백신 접종과 관련하여 저자들은 독감 백신 접종 후 파생된 인플루엔자 특이 클론이 다른 미생물에서 파생된 관련 펩티드 서열을 인식할 수 있다는 사실을 추가로 보여주었습니다. 살루스토 박사 연구팀도 유사한 관찰을 통해 HIV에 노출되지 않은 건강한 기증자의 순진한 T 세포와 기억 T 세포 서브세트(CD45RA와 CCR7의 두 마커로 정의)에서 HIV 특이 CD4+ T 세포가 모두 검출될 수 있음을 입증했습니다(28). 기억 T 세포를 활성화하는 HIV 에피토프의 대다수(>80%)가 인간 마이크로바이옴 aa 서열과 강하게 일치했습니다. 이 보고서에서 주목할 만한 또 다른 관찰은 이러한 HIV 특이적 T 세포의 특이성과 빈도가 기증자마다 다르다는 것입니다. 이는 MHC 다형성과 환경에 의해 형성될 수 있는 T 세포 반응의 개인 간 가변성을 강조합니다.
TCR의 교차 반응성에 대한 명확한 증거가 있음에도 불구하고, '실제'에서 얼마나 많은 단일 펩타이드를 고유한 TCR이 인식할 수 있는지는 아직 불분명합니다. 초기 추정에 따르면 약 106개(29)가 될 것으로 예상됩니다. 한편, 여러 연구에서 개별 T세포 클론이 단일 MHC 분자의 맥락에서 실제로 백만 개 이상의 서로 다른 펩타이드를 감지할 수 있다는 증거가 있습니다(30-32). 10aa 길이의 프리프로인슐린 유래 펩타이드를 인식하는 자가 면역 HLA-A*0201 CD8+ T 세포 클론의 교차 반응 레퍼토리를 연구한 Sewell과 동료들은 10aa 위치 중 최대 7개의 서열이 다른 일부 펩티드에도 불구하고 많은 "교차 반응" 펩타이드가 원래의 것보다 더 나은 작용제임을 보여주었습니다(31). 연구진은 전체 펩타이드의 1%만이 MHC에 제시된다는 가정을 바탕으로 교차 반응성의 "실제" 빈도를 104개 펩타이드 중 약 1개로 추정했습니다(11, 31). 이는 병원체 서열에서 추출한 펩타이드 라이브러리를 사용할 때 이시즈카 등이 발견한 3x104분의 1의 빈도와 동일한 범위입니다(33).
2 T cell cross-reactivity and autoimmunity
The most obvious and detrimental consequence of T cell cross-reactivity to vast numbers of individual peptides is the risk of developing autoimmunity (Figure 1C, left panel). Although self-reactive T cells are deleted in the thymus, weakly cross-reactive T cells may survive and become activated in the periphery through the recognition of epitopes from infectious agents (microorganism antigens, MoAs), a phenomenon known as “molecular mimicry”. Memory T cells can be stimulated by peptide concentrations more than 50-fold lower than those required to stimulate naïve T cells (34, 35). It is, therefore, likely that a memory T cell could be stimulated by a cross-reactive self-peptide with an affinity for the TCR that is far lower than that of the original pathogen-derived peptide. This goes in line with the quite frequent observation that infections can precipitate autoimmune diseases (36), and is of particular interest for novel therapies (37, 38). In autoimmunity, preferentially TCR cross-reactivity of CD4+ T cells has been analysed as a consequence of their central role in the development of autoimmune disorders. This is in contrast to cancer where analysis of cytotoxic anti-tumour response, i.e. CD8+ T cells is more important.
Here, we mainly present three examples for the involvement of TCR cross-reactivity in the induction of autoimmune diseases: one resulting from a bacterial infection, i.e. rheumatic fever; another induced by a food component, i.e. celiac disease; and a third example representative for the many autoimmune disorders for which no clear connection to an environmental agent has been found, as for instance multiple sclerosis.
수많은 개별 펩타이드에 대한 T 세포 교차 반응성의 가장 명백하고 해로운 결과는 자가 면역이 발생할 위험이 있다는 것입니다(그림 1C, 왼쪽 패널).
자가 반응 T 세포는 흉선에서 삭제되지만, 약한 교차 반응 T 세포는 "분자 모방"으로 알려진 현상인 감염원(미생물 항원, MoA)의 에피토프를 인식하여 말초에서 생존하고 활성화될 수 있습니다. 기억 T 세포는 순진한 T 세포를 자극하는 데 필요한 농도보다 50배 이상 낮은 펩타이드 농도로도 자극할 수 있습니다(34, 35). 따라서 기억 T 세포는 원래 병원체 유래 펩타이드보다 훨씬 낮은 TCR에 대한 친화력을 가진 교차 반응성 자가 펩타이드에 의해 자극될 가능성이 높습니다.이는 감염이 자가면역 질환을 촉발할 수 있다는 매우 빈번한 관찰과 일치하며(36), 특히 새로운 치료법(37, 38)에 대한 관심의 대상이 되고 있습니다.자가면역에서는 CD4+ T 세포의 TCR 교차 반응성이 자가면역 질환의 발병에 있어 중심적인 역할을 하는 것으로 분석되었습니다.이는 세포 독성 항종양 반응, 즉 CD8+ T 세포의 분석이 더 중요한 암과는 대조적입니다.
여기에서는 주로 자가면역질환의 유발에 TCR 교차 반응성이 관여하는 세 가지 예, 즉 류마티스 열과 같은 박테리아 감염, 셀리악병과 같은 식품 성분에 의한 유발, 다발성 경화증과 같이 환경 요인과의 명확한 연관성이 발견되지 않은 많은 자가면역질환을 대표하는 세 가지 예를 제시합니다.
2.1 Rheumatic fever (RF)
Acute rheumatic fever is a typical example of systemic autoimmunity which occurs subsequently to an infection, namely with group A β-haemolytic streptococci (39, 40). It can affect synovial joints, cardiac valves and the brain, resulting in clinical features as arthritis, carditis, chorea, erythema marginatum and subcutaneous nodules. Molecular mimicry between group A streptococci and heart tissue was first described by Kaplan in 1960 (41). In the early 1980s, the role of both humoral and cellular autoimmune responses was reported in several studies (42). The cross-reactive antibody (Ab) response against S. pyogenes has been well described (43, 44). Meanwhile, it is clear that also T cell-mediated immune reactions play an important role in RF (40, 44, 45). Three types of protein antigens present on the S. pyogenes surface are M, T, and R proteins. M protein is the most virulent one and shares structural similarities with various host proteins, including cardiac myosin, laminin, vimentin, and tropomyosin (43, 46, 47). During this cellular response, streptococcal antigens are presented via MHC-class II molecules and activate autoreactive T cells (40). Indeed, T cells from patients with RF recognise different alpha (α)-helical coiled-coil proteins such as streptococcal M protein, myosin, laminin, and tropomyosin, and identical epitopes on the N-terminal portions of both streptococcal M protein and cardiac myosin were identified (45). In addition, in the valvular tissue and myocardium of patients with RF, T cells with three patterns of cross-reactivity were found: 1) cardiac myosin and valve-derived proteins, 2) cardiac myosin and streptococcal M peptides, and 3) cardiac myosin, streptococcal M peptides and valve-derived proteins (48). Potential sites of mimicry were revealed in the S2- and light meromyosin (LMM)-region of human cardiac myosin peptides and distinct peptides in the B repeat region of streptococcal M protein (peptides B2 and B3A) (45). Other mechanisms which are involved in the pathogenesis of RF are epitope spreading and TCR degeneracy. Ellis et al. investigated the degeneracy of the cross-reactive T cell responses towards different α-helical proteins such as human cardiac myosin, laminin, tropomyosin, and streptococcal M protein, and observed a mosaic of different T cell clones reacting with at least six distinct α-helical proteins demonstrating different degrees of cross-reactivity (45). Moreover, T cells are activated in RF when auto-Abs interact with the endothelium cells, leading to upregulation of vascular cell adhesion molecule 1 (VCAM-1) and facilitating increased T cell infiltration into the heart valve (49). These activated auto-reactive T cells produce inflammatory cytokines and lead to valve damage but also promote activation of B cells which produce cross-reactive Abs. Due to the destruction of valvular tissue, epitope spreading may occur, thus enhancing the humoral and cellular autoimmune reaction.
Another crucial streptococcal antigen is N-acetyl ß D-glucosamine (GlcNac), a carbohydrate moiety of the bacteria cell wall (43). In a neurologic manifestation of RF, the Sydenham chorea, T cells as well as Abs that recognise this bacterial antigen have been shown to cross-react with the brain cell antigens lysogangliosides and tubulin (39, 50, 51). The humoral responses correlate with clinical symptoms and mediate neuronal cell signalling (52).
급성 류마티스 열은 A형 β- 용혈성 연쇄상구균(39, 40) 감염에 이어 발생하는 전신 자가 면역의 전형적인 예입니다.
활액 관절, 심장 판막 및 뇌에 영향을 미쳐 관절염, 심장염, 무도병, 홍반 변연 및 피하 결절과 같은 임상적 특징을 초래할 수 있습니다. A군 연쇄상구균과 심장 조직 사이의 분자 모방은 1960년 Kaplan에 의해 처음 설명되었습니다(41). 1980년대 초, 체액성 및 세포성 자가 면역 반응의 역할이 여러 연구에서 보고되었습니다(42).
S. 파이오제네스에 대한 교차 반응성 항체(Ab) 반응은 잘 설명되어 있습니다(43, 44).한편, T 세포 매개 면역 반응도 RF에서 중요한 역할을 하는 것이 분명합니다(40, 44, 45).S. 파이오제네스 표면에 존재하는 세 가지 유형의 단백질 항원은 M, T, R 단백질입니다. M 단백질은 가장 독성이 강하며 심장 미오신, 라미닌, 비멘틴, 트로포미오신 등 다양한 숙주 단백질과 구조적 유사성을 공유합니다(43, 46, 47). 이러한 세포 반응 중에 연쇄상구균 항원은 MHC 클래스 II 분자를 통해 제시되어 자가 반응성 T 세포를 활성화합니다(40). 실제로 RF 환자의 T 세포는 연쇄상구균 M 단백질, 미오신, 라미닌, 트로포마이오신과 같은 다양한 알파(α)- 나선형 코일 코일 단백질을 인식하며 연쇄상구균 M 단백질과 심장 미오신 모두의 N 말단 부분에서 동일한 에피토프가 확인되었습니다(45). 또한 RF 환자의 판막 조직과 심근에서 1) 심장 미오신과 판막 유래 단백질, 2) 심장 미오신과 연쇄상구균 M 펩티드, 3) 심장 미오신, 연쇄상구균 M 펩티드 및 판막 유래 단백질의 3가지 교차 반응성을 가진 T 세포가 발견되었습니다(48). 인간 심장 미오신 펩타이드의 S2 및 라이트 메로미오신(LMM) 영역과 연쇄상구균 M 단백질의 B 반복 영역(펩타이드 B2 및 B3A)에서 잠재적 모방 부위가 밝혀졌습니다(45). RF의 발병 기전에 관여하는 다른 메커니즘으로는 에피토프 확산과 TCR 변성이 있습니다. Ellis 등은 인간 심장 미오신, 라미닌, 트로포미오신, 연쇄상구균 M 단백질과 같은 서로 다른 α- 나선형 단백질에 대한 교차 반응성 T 세포 반응의 변성을 조사하여 서로 다른 정도의 교차 반응성을 나타내는 최소 6개의 서로 다른 α- 나선형 단백질에 반응하는 서로 다른 T 세포 클론의 모자이크를 관찰했습니다(45). 또한 자가 항체가 내피 세포와 상호 작용할 때 T 세포는 RF에서 활성화되어 혈관 세포 부착 분자 1(VCAM-1)의 상향 조절을 유도하고 심장 판막으로의 T 세포 침윤을 촉진합니다(49). 이렇게 활성화된 자가 반응성 T 세포는 염증성 사이토카인을 생성하여 판막 손상을 유발할 뿐만 아니라 교차 반응성 Abs를 생성하는 B 세포의 활성화도 촉진합니다. 판막 조직의 파괴로 인해 에피토프 확산이 발생하여 체액성 및 세포성 자가 면역 반응이 강화될 수 있습니다.
또 다른 중요한 연쇄상구균 항원은 박테리아 세포벽의 탄수화물 모오이티인 N-아세틸 ß D-글루코사민(GlcNac)입니다(43). RF의 신경학적 증상인 시덴햄 무도병에서 이 박테리아 항원을 인식하는 T세포와 Abs는 뇌세포 항원인 리소강글리오사이드 및 튜불린과 교차 반응하는 것으로 나타났습니다(39, 50, 51). 체액 반응은 임상 증상과 상관관계가 있으며 신경세포 신호를 매개합니다(52).
2.2 Celiac disease (CeD)
Celiac disease is highly interesting in view of the fact that autoimmune reactions are induced by a food component, i.e. dietary gluten (gliadin in wheat, hordein in barley, and secalin in rye are the most prominent examples). Antibodies against gliadin-peptides and the enzyme transglutaminase-2 (TG2) are highly-specific diagnostic markers of CeD, and a CD4+ T cell response towards post-translationally modified gluten peptides has been described. The disease shows a clear genetic association to the MHC-class II allelic products HLA-DQ2 (DQ2.5: DQA1*05:01-DQB1*02:01 or DQ2.2: DQA1*02:01-DQB1*02:02, approx. 95% of the patients) and HLA-DQ8 (DQA1*03:01-DQB1*03:02, approx. 5% of the patients) (53).
Interestingly, gliadin is a substrate for the TG2 enzyme which catalyses deamination at glutamine residues. The conversion of Q to E aa leads to increased binding affinity of peptides to the HLA-DQ2.5/2.2/8 molecules and enhanced recognition by gluten-specific CD4+ T cells (54–56). Hence, CeD-associated T cells preferably react with “self-produced mimotopes” that result from the deamidation of gliadin-derived peptides. Another level of cross-reactivity that has been documented in CeD is the recognition by a single DQ2.5-restricted TCR of peptides of similar, but not identical, aa sequences derived from various gliadins (i.e. α1a and ω1) (57). To which extend this cross-reactivity participates in the immune response against various gliadins and/or hordein or secalin is still not fully investigated, but is starting to be explored at large-scale (57–59). Altogether, the strong anti-gluten CD4+ T cell response present in CeD is providing help to B cells that bind TG2-gliadin complexes and deaminated gluten peptides to mature into plasma cells in the gut that in turn produce deaminated gluten-specific, as well as autoreactive, TG2-specific, Abs (60–62). In addition, gluten-specific CD4+ T cells are consistently found in the small intestine of celiac disease patients, where they activate intraepithelial CD8+ T cells (IELs) via the production of IFN-γ, IL-21 and IL-2 (62). Although these IELs are thought to largely contribute to disease pathogenesis, the link between the gliadin-specific CD4+ T cell response and the recruitment and activation of IELs in the gut remains obscure, especially because these IELs have not been shown to recognise gluten.
Even if there is ample evidence that HLA-DQ2.5, HLA-DQ2.2, or HLA-DQ8 molecules present gluten-derived peptides, expression of these allelic products alone is insufficient to cause disease. Other risk factors which may induce increased expression and activity of TG2 may also be involved. For instance, in vivo and in vitro studies support an association between gut microbiota alterations and celiac disease (63). First, the microbiota composition differs between individuals with active celiac disease, patients on a gluten-free diet, and normal controls in both oral, duodenal and faecal samples, with an increase in virulent strains noted in patients with active CeD (64). Bacteria can modify immunogenic food antigens resulting in an increase or decrease in antigenicity, and also utilise undigested particles as substrates, producing metabolites such as short-chain fatty acids that affect intestinal homeostasis. For instance, Pseudomonas aeruginosa, an opportunistic pathogen isolated from CeD patients, processes gluten to T cell reactive epitopes whereas bacterial species from healthy controls inactivate these reactive epitopes by further proteolytic breakdown (65). Second, and more relevant in the context of T cell cross-reactivity, peptides from common commensal and pathogenic bacteria, especially from several Pseudomonas and Bordetella species can mimic gliadin-derived peptides and activate gliadin-specific, HLA-DQ2.5-restricted T cells from CeD patients (66). It has been, therefore, hypothesised that celiac disease may be induced not only by gluten ingestion but also by infectious processes inducing pathogen-specific T cells that cross-react with gluten epitopes (66).
셀리악병은 식품 성분, 즉 식이 글루텐(밀의 글리아딘, 보리의 호르데인, 호밀의 세칼린이 가장 대표적인 예입니다)에 의해 자가 면역 반응이 유발된다는 점에서 매우 흥미롭습니다.
글리아딘 펩티드와 트랜스글루타미나제-2(TG2) 효소에 대한 항체는 CeD의 매우 특이적인 진단 마커이며, 번역 후 변형된 글루텐 펩티드에 대한 CD4+ T 세포 반응이 설명되어 있습니다. 이 질환은 MHC 클래스 II 대립 유전자 제품인 HLA-DQ2(DQ2.5: DQA1*05:01-DQB1*02:01 또는 DQ2.2)와 명확한 유전적 연관성을 보입니다: DQA1*02:01-DQB1*02:02, 약 95%의 환자) 및 HLA-DQ8(DQA1*03:01-DQB1*03:02, 약 5%의 환자)(53)에 해당합니다.
흥미롭게도 글리아딘은 글루타민 잔기에서 탈아미노화를 촉매하는 TG2 효소의 기질입니다. Q가 E aa로 전환되면 펩타이드의 HLA-DQ2.5/2.2/8 분자에 대한 결합 친화력이 증가하고 글루텐 특이적 CD4+ T 세포에 의한 인식이 향상됩니다(54-56).따라서 CeD 관련 T 세포는 글리아딘 유래 펩타이드의 탈아미드화로 인해 생성되는 "자체 생성 미모토프"와 반응하는 것이 바람직합니다.CeD에서 문서화된 또 다른 수준의 교차 반응성은 다양한 글리아딘(즉, α1a 및 ω1)에서 유래한 유사하지만 동일하지는 않은 aa 서열의 펩타이드를 단일 DQ2.5 제한 TCR이 인식하는 것입니다(57). 이 교차 반응성이 다양한 글리아딘 및/또는 호르데인 또는 세칼린에 대한 면역 반응에 어느 정도까지 관여하는지는 아직 완전히 규명되지 않았지만 대규모로 연구되기 시작했습니다(57-59). 전체적으로 CeD에 존재하는 강력한 항글루텐 CD4+ T 세포 반응은 TG2-글리아딘 복합체와 탈아민화된 글루텐 펩티드에 결합하여 장에서 혈장 세포로 성숙하고 탈아민화된 글루텐 특이성 및 자가 반응성 TG2 특이성 Abs를 생성하는 B 세포에 도움을 줍니다(60-62). 또한 글루텐 특이적 CD4+ T 세포는 셀리악병 환자의 소장에서 지속적으로 발견되며, 이 세포는 IFN-γ, IL-21 및 IL-2를 생성하여 상피 내 CD8+ T 세포(IEL)를 활성화합니다(62). 이러한 IEL이 질병 발병에 크게 기여하는 것으로 생각되지만, 글리아딘 특이적 CD4+ T 세포 반응과 장내 IEL의 모집 및 활성화 사이의 연관성은 아직 불분명하며, 특히 이러한 IEL이 글루텐을 인식하는 것으로 밝혀지지 않았기 때문입니다.
HLA-DQ2.5, HLA-DQ2.2 또는 HLA-DQ8 분자가 글루텐 유래 펩타이드를 존재한다는 충분한 증거가 있더라도 이러한 대립 유전자 생성물의 발현만으로는 질병을 유발하기에는 불충분합니다. TG2의 발현 및 활성 증가를 유도할 수 있는 다른 위험 요인도 관여할 수 있습니다. 예를 들어, 생체 내 및 시험관 내 연구는 장내 미생물군 변화와 셀리악병 사이의 연관성을 뒷받침합니다(63). 첫째, 구강, 십이지장 및 분변 샘플 모두에서 활성 셀리악병 환자, 글루텐 프리 식단을 따르는 환자, 정상 대조군 간에 미생물 구성이 다르며, 활성 CeD 환자에서 독성 균주의 증가가 관찰되었습니다(64). 박테리아는 면역원성 식품 항원을 변형하여 항원성을 증가 또는 감소시킬 수 있으며, 소화되지 않은 입자를 기질로 활용하여 장 항상성에 영향을 미치는 단쇄 지방산과 같은 대사 산물을 생성할 수도 있습니다. 예를 들어, CeD 환자로부터 분리된 기회주의 병원균인 녹농균은 글루텐을 T세포 반응성 에피토프로 처리하는 반면 건강한 대조군의 박테리아 종은 추가 단백질 분해로 이러한 반응성 에피토프를 비활성화합니다(65). 둘째, T 세포 교차 반응성의 맥락에서 더 관련이 있는 것은 일반적인 공생 및 병원성 박테리아, 특히 여러 슈도모나스 및 보데텔라 종의 펩타이드가 글리아딘 유래 펩타이드를 모방하고 CeD 환자의 글리아딘 특이적 HLA-DQ2.5 제한 T 세포를 활성화할 수 있다는 것입니다(66). 따라서 셀리악병은 글루텐 섭취뿐만 아니라 글루텐 에피토프와 교차 반응하는 병원체 특이 T 세포를 유도하는 감염 과정에 의해서도 유발될 수 있다는 가설이 제기되었습니다(66).
2.3 Multiple sclerosis (MS)
Multiple sclerosis is one of the most prevalent autoimmune disorders of the central nervous system (CNS), and is characterised by the loss of the protective myelin sheath that surrounds the axons of neurons (67, 68). Its pathophysiology has been extensively studied, especially in experimental allergic encephalomyelitis (EAE) which is a generally accepted animal model for the human disease. Nevertheless, the aetiology of MS is still unclear. Its association with an infection has been postulated already in the late 1800s, after it was first described (67). Nowadays, several factors such as genetic susceptibility, environment including infectious agents, obesity, lack of sun exposure and vitamin, have been suggested to be involved (69).
Autoantibodies specific for a variety of CNS proteins, as for instance myelin basic protein (MBP) or myelin oligodendrocyte glycoprotein (MOG), are present in the serum, cerebrospinal fluid (CSF), and brain of MS patients (70). Similarly, CD4+ T cells specific for myelin antigens are found in the blood. Studies on antigen recognition demonstrated that CD4+ autoreactive, MBP-specific, T cells from MS patients cross-react with peptides derived from bacterial or viral proteins (71, 72). As shown by structural analyses performed by Lang et al., the same TCR binds a MBP peptide presented by HLA-DRB1*1501 and an unrelated Epstein Barr virus (EBV)-derived peptide bound to HLA-DRB5*0101, a typical example of molecular mimicry (73, 74). A link between EBV infection and MS had already been suggested by the observation that the infection may precede MS pathology and the identification of cross-reactive Abs in MS patients (67). EBV is a well-investigated candidate for antigenic mimicry, from mimotope peptides recognised by T cells to cross-reactive Abs (75). Also, an altered anti-EBV T cell reaction was suggested in MS (76, 77).
These findings led to the concept that an immune response initially activated and expanded by an infectious agent may, in general, cross-react with autoantigens mediating CNS inflammation and induce destruction of the brain. To date, numerous infectious agents have been described to induce cross-reactive T cells against brain-specific epitopes. As an example, peptides from HSV and Pseudomonas aeruginosa bound to MHC molecules are recognised by cross reactive myelin-specific T cells (78). Furthermore, peptides from M. tuberculosis, S. typhimurium and E. coli lead to strong in vitro proliferation of MBP-specific T cells and induced EAE in mice with the same severity and incidence as the autoantigen peptide of MBP (79).
T cell clones isolated from the blood of patients with MS show high specificity for the immunodominant MBP epitope MBP85–99 (80). However, this specificity is not absolute. Indeed, changing the TCR contact residue lysine at position 93 to an arginine, or even just removing a hydroxyl group by changing a phenylalanine to a tyrosine at position 91, can totally abrogate T cell reactivity. This lysine-to-arginine substitution can also result in a more degenerate pattern of TCR recognition, in that a tyrosine or other aa residues can now be tolerated at positions 91 or even 90 (81). Hence, while a TCR appears to be highly specific in one situation, altering the peptide ligand can change the TCR conformation to yield a higher degree of T cell cross-reactivity. Analysis of a further series of MBP85–99 reactive T cell clones led to a similar conclusion, showing that a number of virus-derived epitopes can trigger autoreactive T cell clones in a manner that would not be predicted by simple algorithms (71). One of the studied MBP-reactive T cell clones recognised an epitope of MOG, an entirely different self-protein. Thus, a significant degree of functional degeneracy exists in the recognition of self-antigens by T cells.
다발성 경화증은 중추신경계(CNS)에서 가장 흔한 자가면역 질환 중 하나로, 신경세포의 축삭을 둘러싸고 있는 보호 수초가 소실되는 것이 특징입니다(67, 68).
그 병태생리는 특히 인간 질환에 대해 일반적으로 인정되는 동물 모델인 실험용 알레르기성 뇌척수염(EAE)에서 광범위하게 연구되어 왔습니다. 그럼에도 불구하고 다발성 경화증의 원인은 아직 명확하지 않습니다. 다발성 경화증이 처음 기술된 후 1800년대 후반에 이미 감염과의 연관성이 가정되었습니다(67). 오늘날에는 유전적 감수성, 감염원을 포함한 환경, 비만, 햇빛 노출 부족, 비타민 등 여러 요인이 관여하는 것으로 추정되고 있습니다(69).
예를 들어 미엘린 기저 단백질(MBP) 또는 미엘린 희소돌기아교세포 당단백질(MOG)과 같은 다양한 CNS 단백질에 특이적인 자가 항체가 다발성 경화증 환자의 혈청, 뇌척수액(CSF) 및 뇌에 존재합니다(70). 마찬가지로 미엘린 항원에 특이적인 CD4+ T 세포도 혈액에서 발견됩니다.항원 인식에 관한 연구에 따르면 다발성 경화증 환자의 CD4+ 자가 반응성 MBP 특이적 T 세포는 박테리아 또는 바이러스 단백질에서 유래한 펩티드와 교차 반응하는 것으로 나타났습니다(71, 72).Lang 등이 수행한 구조 분석에서 볼 수 있듯이, 동일한 TCR이 HLA-DRB1*1501에 의해 제시된 MBP 펩티드와 분자 모방의 전형적인 예인 HLA-DRB5*0101에 결합된 관련 없는 엡스타인바 바이러스(EBV) 유래 펩티드에 결합합니다(73, 74). EBV 감염과 다발성 경화증 사이의 연관성은 이미 감염이 다발성 경화증 병리에 선행할 수 있다는 관찰과 다발성 경화증 환자에서 교차 반응성 Abs의 확인을 통해 제시된 바 있습니다(67). EBV는 T세포가 인식하는 미모토프 펩타이드부터 교차 반응성 Abs에 이르기까지 항원 모방에 대해 잘 연구된 후보입니다(75). 또한, 다발성 경화증에서 변형된 항-EBV T 세포 반응이 제안되었습니다(76, 77).
이러한 발견은 감염원에 의해 처음에 활성화되고 확장된 면역 반응이 일반적으로 CNS 염증을 매개하는 자가 항원과 교차 반응하여 뇌의 파괴를 유도할 수 있다는 개념으로 이어졌습니다. 현재까지 수많은 감염원이 뇌 특이적 에피토프에 대해 교차 반응하는 T 세포를 유도하는 것으로 알려져 있습니다. 예를 들어, MHC 분자에 결합된 HSV 및 녹농균의 펩타이드는 교차 반응성 미엘린 특이적 T 세포에 의해 인식됩니다(78). 또한 결핵균, 결핵균( S. typhimurium ), 대장균의 펩타이드는 MBP 특이적 T 세포의 강력한 시험관 내 증식을 유도하고 MBP의 자가항원 펩티드와 동일한 중증도 및 발생률로 마우스에서 EAE를 유도합니다(79).
다발성 경화증 환자의 혈액에서 분리된 T 세포 클론은 면역 우세한 MBP 에피토프 MBP85-99에 대해 높은 특이성을 보입니다(80). 그러나 이러한 특이성이 절대적인 것은 아닙니다. 실제로 93번 위치의 TCR 접촉 잔류 리신을 아르기닌으로 바꾸거나 91번 위치의 페닐알라닌을 티로신으로 바꾸어 수산기를 제거하면 T 세포 반응성이 완전히 없어질 수 있습니다. 이러한 라이신에서 아르기닌으로의 치환은 또한 티로신 또는 다른 AA 잔기가 91번 또는 90번(81번) 위치에서도 허용될 수 있다는 점에서 TCR 인식 패턴을 더욱 퇴화시킬 수 있습니다. 따라서 TCR은 특정 상황에서 매우 특이적인 것처럼 보이지만 펩타이드 리간드를 변경하면 TCR 형태를 변경하여 더 높은 수준의 T세포 교차 반응성을 나타낼 수 있습니다. 일련의 MBP85-99 반응성 T 세포 클론을 추가로 분석한 결과, 다수의 바이러스 유래 에피토프가 단순한 알고리즘으로는 예측할 수 없는 방식으로 자가 반응성 T 세포 클론을 유발할 수 있다는 유사한 결론이 도출되었습니다(71). 연구된 MBP 반응성 T 세포 클론 중 하나는 완전히 다른 자가 단백질인 MOG의 에피토프를 인식했습니다. 따라서 T 세포의 자가 항원 인식에는 상당한 수준의 기능적 퇴화가 존재합니다.
2.4 Evidence for TCR cross-reactivity in other autoimmune diseases
The link between infection and autoimmunity via molecular mimicry has also been investigated in other inflammatory CNS diseases, particularly in chronic Lyme disease. Following acute infection with Borrelia burgdorferi (Bb), a chronic inflammatory disease can emerge which targets joints or the CNS in the absence of residual bacterial infection. In this condition, an autoimmune response to self-antigens (similarly as described above for RF) may arise from bacterial-specific T cells (82). Indeed, in Lyme arthritis, CD4+ T cells isolated from the synovial fluid of patients were shown to recognise a 9mer peptide from an outer surface antigen from Bb (OspA165–173) and an analogous, but not identical, sequence from the human LFA-1 molecule (CD11a332–340) (83). Similarly, Bb-specific T cells from the CSF of a patient with CNS manifestation of borreliosis cross-reacted with several self-antigens, one of them being a myelin antigen (84).
In uveitis, it has also been shown that peptides with similar structure rather than similar aa sequences can induce cross-reactive T cell responses. For instance, similarities of 6 to 7 aa with the 14mer autoantigen peptide from retinal S-antigen (PDSAg) with peptides of 11 or 12 aa in length from different environmental proteins is sufficient to induce autoreactive CD4+ T cell recognition and experimental anterior uveitis in rats (85). Although the pathogenic cells in uveitis are MHC-class II restricted CD4+ T lymphocytes, statistical associations with HLA-class I molecules (B*27, B*51) are well known. Interestingly, the HLA-class I molecule seems to serve as an autoantigen itself, being presented as a peptide (B27125–138, termed B27PD) on HLA-class II and mimicking the retinal PDSAg peptide (86). Oral administration of B27BP peptide to patients was also shown to improve uveitis symptoms, suggesting that cross-reactivity could be even exploited for inducing oral tolerance to autoimmune antigens (87). In a recent study including patients with acute anterior uveitis and ankylosing spondylitis, an HLA-B27-linked rheumatic disease frequently associated with uveitis, TCRs responding to HLA-B*27-bound peptides derived from microbial antigens or from self-antigens were identified. These peptides shared common TCR binding motifs, supporting the idea that HLA-B*27-presented microbial peptides could act as trigger for autoimmunity by activating anti-self CD8+ T cells (88). Interestingly, the ankylosing spondylitis-associated TCRs showed weaker affinity for the human peptide ligands than for a peptide from a conserved bacterial inner membrane protein. Evaluating the structures of seven of the HLA-B*27:05 peptide-TCR complexes, the authors showed that in all of these structures, the TCRs used a similar solution to interact with the conserved motifs in the self and bacterial peptides (89).
In type I diabetes, a T cell-mediated, HLA-DQ2 (DQA1*05:01-DQB1*02:01) and -DQ8 (DQA1*03:01-DQB1*03:02)-associated autoimmune disease directed at pancreatic β cells, insulin B-chain9-23 (B:9-23) is a key epitope presented by MHC-class II to CD4+ T cells targeting pancreatic β-cells. Lack of an acidic aa residue (i.e. aspartic acid and glutamic acid) at position 57 of the DQ8 β chain of the MHC molecule favours binding of the insulin-B peptide and is associated with increased risk of developing the disease (90). Without this acidic residue, the presented peptide repertoire is typically negatively charged (91, 92). Mimotopes with acidic aa substitutions at P9 have been shown to detect self-reactive, IFNγ-producing T cells much stronger than the wild-type peptide (93, 94). Interestingly, an immune response to this mimotope was also observed in control subjects without diabetes, but in these individuals, rather IL-10 producing, hence, anti-inflammatory CD4+ T cells were activated (93).
분자 모방을 통한 감염과 자가 면역 사이의 연관성은 다른 염증성 중추신경계 질환, 특히 만성 라임병에서도 조사된 바 있습니다. 보렐리아 부르그도르페리 (Bb)에 급성 감염된 후 잔류 박테리아 감염이 없는 상태에서 관절이나 중추신경계를 표적으로 하는 만성 염증성 질환이 나타날 수 있습니다. 이 상태에서는 박테리아 특이 T 세포(82)에서 자가 항원에 대한 자가 면역 반응이 발생할 수 있습니다(RF에 대해 위에서 설명한 것과 유사하게). 실제로 라임 관절염에서 환자의 활액에서 분리된 CD4+ T 세포는 Bb 외부 표면 항원(OspA165-173)의 9머 펩타이드와 인간 LFA-1 분자(CD11a332-340)의 유사하지만 동일하지는 않은 서열을 인식하는 것으로 나타났습니다(83). 마찬가지로, 보렐리증 증상을 보이는 중추신경계 환자의 CSF에서 추출한 Bb 특이적 T 세포는 여러 자가 항원과 교차 반응했으며, 그 중 하나는 미엘린 항원이었습니다(84).
포도막염에서는 유사한 AA 서열이 아닌 유사한 구조를 가진 펩타이드가 교차 반응성 T 세포 반응을 유도할 수 있다는 사실도 밝혀졌습니다. 예를 들어, 망막 S-항원(PDSAg)의 14머 자가항원 펩티드와 다른 환경 단백질의 길이가 11 또는 12 aa인 펩티드와 6~7 aa의 유사성은 쥐에서 자가반응성 CD4+ T 세포 인식과 실험적 전방 포도막염을 유도하기에 충분합니다(85). 포도막염의 병원성 세포는 MHC 클래스 II 제한 CD4+ T 림프구이지만, HLA 클래스 I 분자(B*27, B*51)와의 통계적 연관성은 잘 알려져 있습니다. 흥미롭게도 HLA 클래스 I 분자는 자가 항원 자체로 작용하는 것으로 보이며, HLA 클래스 II에서 펩타이드(B27125-138, B27PD라고 함)로 제시되고 망막 PDSAg 펩타이드를 모방합니다(86). 환자에게 B27BP 펩타이드를 경구 투여하면 포도막염 증상이 개선되는 것으로 나타나 교차 반응성이 자가 면역 항원에 대한 경구 내성을 유도하는 데에도 활용될 수 있음을 시사했습니다(87). 포도막염과 자주 연관되는 HLA-B27 결합 류마티스 질환인 급성 전방 포도막염과 강직성 척추염 환자를 포함한 최근 연구에서 미생물 항원 또는 자가 항원에서 유래한 HLA-B*27 결합 펩티드에 반응하는 TCR이 확인되었습니다. 이러한 펩타이드는 공통된 TCR 결합 모티프를 공유하여 HLA-B*27을 나타내는 미생물 펩타이드가 항-자기 CD8+ T 세포를 활성화하여 자가 면역의 트리거로 작용할 수 있다는 생각을 뒷받침합니다(88). 흥미롭게도 강직성 척추염 관련 TCR은 보존된 박테리아 내막 단백질의 펩타이드보다 인간 펩타이드 리간드에 대한 친화력이 더 약한 것으로 나타났습니다. 저자들은 HLA-B*27:05 펩타이드-TCR 복합체 중 7개의 구조를 평가한 결과, 이 모든 구조에서 TCR이 유사한 솔루션을 사용하여 자기 및 박테리아 펩타이드의 보존된 모티프와 상호 작용하는 것으로 나타났습니다(89).
췌장 베타 세포를 표적으로 하는 T 세포 매개, HLA-DQ2(DQA1*05:01-DQB1*02:01) 및 -DQ8(DQA1*03:01-DQB1*03:02) 관련 자가 면역 질환인 제1형 당뇨병에서 인슐린 B-체인9-23(B:9-23)은 MHC-클래스 II가 CD4+ T 세포에 제공하는 핵심 에피토프이며 췌장 베타 세포를 표적으로 삼습니다. MHC 분자의 DQ8 β 사슬 57번 위치에 산성 aa 잔기(예: 아스파르트산 및 글루탐산)가 부족하면 인슐린-B 펩타이드의 결합을 선호하고 질병 발병 위험 증가와 관련이 있습니다(90). 이 산성 잔기가 없으면 제시된 펩타이드 레퍼토리는 일반적으로 음전하를 띠게 됩니다(91, 92). P9에서 산성 aa 치환이 있는 미모토프는 야생형 펩타이드보다 훨씬 더 강력한 자가 반응성 IFNγ 생성 T 세포를 감지하는 것으로 나타났습니다(93, 94). 흥미롭게도 이 미모토프에 대한 면역 반응은 당뇨병이 없는 대조군 피험자에서도 관찰되었지만, 이들에서는 오히려 IL-10을 생성하여 항염증성 CD4+ T 세포가 활성화되었습니다(93).
2.5 Therapeutic implications in autoimmune diseases
Distinct cytokine patterns of T cell subsets make them unique and define their role in host defence or their contribution in disease pathogenesis. In autoimmune diseases, the role of Th1 and Th2 cells along with their cytokine profiles is well documented. In particular, the priming signal (specificity, affinity and avidity of the pMHC/TCR, APC/T cell interaction) controls the maturation, differentiation and function (i.e. cytokine profile) of the T cell (37). Alteration of peptides and of their binding to MHC may, therefore, influence the strength of the immune response. For the development of therapeutic agents in autoimmune diseases, silencing the armful anti-self T cell activity by either shifting the inflammatory Th1 response towards a Th2 profile, inducing regulatory T cells (Tregs), or even completely inhibiting T cells using strong antagonists are all strategies of interest (Figure 1D, left panel). Many of such “mimotopes” have been meanwhile designed based on in vitro testing of the responsiveness of T cells isolated from patients or in vivo using animal models (38). Especially those reducing pathogenic responses have been tested for therapeutic purposes in clinical trials.
The ability of altered peptide ligands (modified peptide sequences derived from an original antigenic peptide, i.e APLs) to shift an unfavourable Th1- in a more favourable Th2-response in the murine EAE model of MS has first been shown by Nicholson and colleagues (95). The authors used an analogue of the encephalitogenic myelin proteolipid PLP139-151 (the common T cell antigen in EAE) with substitutions at the two main TCR contact residues (L144/R147) which had been shown to be a powerful TCR antagonist for the encephalitogenic PLP-specific T cell clones in vitro (96). Injection of this analogue protected the animals from developing EAE. Kuchroo et al. showed that this APL can activate IL-4 secretion by both encephalitogenic T cells and naive T cell clones that cross-react with self-antigens and inhibit autoimmunity by the induction of Tregs leading to bystander suppression of EAE (96). In further animal models of EAE, APLs have been proven to have a significant therapeutic value (97). Meanwhile, autoreactive human T cell clones have been shown to secrete the anti-inflammatory cytokines IL-4 and TGF-β after TCR engagement by APLs (98). However, application of APLs in MS may be a double-edged sword. On the one hand, it was shown that an altered MBP85–99 peptide induces Th2 cytokine secretion by MBP-reactive T cells isolated from the peripheral blood of MS patients while on the other hand, it can induce disease in some patients by activating these MBP-reactive T cells against the patient’s own tissues (99). Moreover, in a phase II clinical trial with this peptide, two out of seven MS patients developed high frequencies of MBP-reactive T cells, and these responses were associated with significant increases in MRI-detectable lesions (100). In contrast, patients treated with lower doses of the same APL experienced some degree of immune deviation towards increases in IL-4 secretion by MBP-reactive T cells (101, 102).
A mimotope was also developed for patients with diabetes mellitus type 1 in order to preserve pancreatic β cell function. It was modified from the human insulin peptide B:9-23 which binds to HLA-DQ8 and is recognised by CD4+ T cells present in the islets of organ donors with type 1 diabetes ((103) and section 2.4). The substitutions in this modified peptide are known to be important in the diabetes-prone NOD mouse model (104, 105). However, a four-arm phase II clinical study conducted by Walter et al. could not show any clinical improvement (as measured by C-peptide concentrations, a measure of pancreatic β cell function), after subcutaneous administration of the mimotopes over two years compared to the placebo (103).
For celiac disease, and as mentioned in section 2.2, disease-associated T cells preferably react with “naturally produced mimotopes” that result from deamidation of gliadin-derived peptides. Epitope-specific immunotherapies are, therefore, a logical translational step. In HLA-DQ2.5-positive celiac disease patients, clinical trials using a combination of three gluten-derived peptides, which contain at least five gliadin-specific T cell epitopes presented by HLA-DQ2.5 (Nexvax2) were conducted. While the phase I studies showed preferable outcomes in terms of safety and tolerability, the recent Nexvax2 phase II trial had to be discontinued due to lack of protection to gluten challenge.
An alternative approach to the use of a single APL is the administration of peptide mixtures that contain many different antigen specificities. Random copolymers that contain aa commonly used as MHC anchors and TCR contact residues have been proposed as possible “universal APLs.” The synthetic immuno-active copolymer glatiramer acetate (GA) is comprised of four aa in random order with an average length of 40-100 residues which resemble MBP (106). It was first synthesised in 1967 to induce EAE in murine models, but was then unexpectedly found to reduce signs and progression of the disease (107). Rather than inducing an autoimmune disease, GA was found to induce regulatory and protective neuroimmune responses. In most patients, daily injection with GA causes a striking loss of responsiveness to this polymer antigen, accompanied by greater secretion of IL-5 and IL-13 by CD4+ T cells, indicating a shift towards a Th2 response (108, 109). In addition, the GA-reactive T cells exhibit a high degree of degeneracy, as measured by their ability to cross-react with a large variety of peptides represented in a combinatorial library (108). GA-induced migration of those highly cross-reactive Th2 (and perhaps regulatory FoxP3- Th3) cells to the sites of inflammation may allow their highly degenerate TCRs to contact self-antigens, which they recognise as weak agonists. These T cells then apparently secrete suppressive, Th2/Th3 cytokines, thus restricting local inflammation (108). Due to these beneficial effects, GA was approved for therapeutic use in 1996 and is since then a first-line treatment of relapsing remitting MS (110, 111).
T 세포 하위 집합의 독특한 사이토카인 패턴은 이들을 고유하게 만들고 숙주 방어에서의 역할 또는 질병 발병에 대한 기여도를 정의합니다. 자가면역 질환에서 Th1 및 Th2 세포의 역할과 사이토카인 프로파일은 잘 알려져 있습니다. 특히 프라이밍 신호(특이성, 친화성 및 pMHC/TCR, APC/T 세포 상호 작용의 적극성)는 T 세포의 성숙, 분화 및 기능(즉, 사이토카인 프로파일)을 제어합니다(37). 따라서 펩타이드의 변화와 MHC와의 결합은 면역 반응의 강도에 영향을 미칠 수 있습니다. 자가면역질환의 치료제를 개발하기 위해서는 염증성 Th1 반응을 Th2 프로파일로 전환하거나 조절 T세포(Treg)를 유도하거나 강력한 길항제를 사용하여 T세포를 완전히 억제함으로써 강력한 항자신 T세포 활동을 침묵시키는 전략이 모두 관심의 대상입니다(그림 1D, 왼쪽 패널). 한편, 이러한 "모조토프"의 대부분은 환자로부터 분리한 T 세포의 반응성에 대한 시험관 내 테스트 또는 동물 모델을 사용한 생체 내 테스트를 기반으로 설계되었습니다(38). 특히 병원성 반응을 감소시키는 것은 임상 시험에서 치료 목적으로 테스트되었습니다.
변경된 펩타이드 리간드(원래 항원 펩타이드에서 파생된 변형된 펩타이드 서열, 즉 APL)가 MS의 쥐 EAE 모델에서 불리한 Th1- 반응을 보다 유리한 Th2- 반응으로 전환하는 능력은 Nicholson과 동료들에 의해 처음 입증되었습니다(95). 저자들은 시험관 내에서 뇌 유발성 PLP 특이적 T 세포 클론에 대한 강력한 TCR 길항제로 밝혀진 두 개의 주요 TCR 접촉 잔기(L144/R147)에 치환된 뇌 유발성 미엘린 단백질 지질 PLP139-151(EAE의 일반적인 T 세포 항원)의 유사체를 사용했습니다(96). 이 유사체를 주사하면 동물이 EAE에 걸리지 않았습니다. 쿠크루 등은 이 APL이 자기 항원과 교차 반응하는 뇌원성 T 세포와 순진한 T 세포 클론 모두에 의해 IL-4 분비를 활성화하고 Tregs를 유도하여 자가 면역을 억제함으로써 EAE의 방관자 억제를 유도할 수 있음을 보여주었습니다(96). EAE의 추가 동물 모델에서 APL은 상당한 치료적 가치가 있는 것으로 입증되었습니다(97). 한편, 자가 반응성 인간 T 세포 클론은 APL에 의한 TCR 결합 후 항염증성 사이토카인 IL-4와 TGF-β를 분비하는 것으로 나타났습니다(98). 그러나 다발성 경화증에 APL을 적용하는 것은 양날의 검이 될 수 있습니다. 한편으로는 변형된 MBP85-99 펩타이드가 MS 환자의 말초 혈액에서 분리된 MBP 반응성 T 세포에 의해 Th2 사이토카인 분비를 유도하는 반면, 다른 한편으로는 환자 자신의 조직에 대해 이러한 MBP 반응성 T 세포를 활성화하여 일부 환자에서 질병을 유발할 수 있음이 밝혀졌습니다(99). 또한, 이 펩타이드를 사용한 2상 임상 시험에서 7명의 다발성 경화증 환자 중 2명이 높은 빈도의 MBP 반응성 T 세포를 보였으며, 이러한 반응은 MRI로 검출 가능한 병변의 현저한 증가와 관련이 있는 것으로 나타났습니다(100). 반면, 저용량의 동일한 APL로 치료받은 환자들은 MBP 반응성 T 세포에 의한 IL-4 분비 증가로 어느 정도의 면역 편차를 경험했습니다(101, 102).
췌장 베타 세포 기능을 보존하기 위해 제1형 당뇨병 환자를 위한 미모토프도 개발되었습니다. 이는 인간 인슐린 펩타이드 B:9-23을 변형한 것으로, HLA-DQ8에 결합하고 1형 당뇨병을 앓고 있는 장기 기증자의 섬에 존재하는 CD4+ T 세포에 의해 인식됩니다((103) 및 섹션 2.4). 이 변형된 펩타이드의 치환은 당뇨병에 걸리기 쉬운 NOD 마우스 모델에서 중요한 것으로 알려져 있습니다(104, 105). 그러나 월터 등이 실시한 4군 2상 임상 연구에서는 2년에 걸쳐 미모토프를 피하 투여한 후 위약에 비해 임상적 개선(췌장 베타 세포 기능의 척도인 C-펩타이드 농도로 측정)을 보여주지 못했습니다(103).
셀리악병의 경우, 섹션 2.2에서 언급했듯이 질병 관련 T 세포는 글리아딘 유래 펩타이드의 탈아미드화 결과인 "자연적으로 생성된 미모토프"와 반응하는 것이 바람직합니다. 따라서 에피토프 특이적 면역 요법은 논리적인 번역 단계입니다. HLA-DQ2.5 양성 체강 질병 환자를 대상으로 HLA-DQ2.5가 제시하는 최소 5개의 글리아딘 특이 T세포 에피토프를 포함하는 3개의 글루텐 유래 펩타이드 조합을 사용한 임상시험이 진행되었습니다(Nexvax2). 1상 임상시험에서는 안전성과 내약성 측면에서 바람직한 결과가 나타났지만, 최근의 Nexvax2 2상 임상시험은 글루텐 챌린지에 대한 보호 효과가 부족하여 중단해야 했습니다.
단일 APL 사용에 대한 대안으로 다양한 항원 특이성을 포함하는 펩타이드 혼합물을 투여하는 방법이 있습니다. MHC 앵커 및 TCR 접촉 잔기로 일반적으로 사용되는 aa를 포함하는 무작위 공중합체가 가능한 "범용 APL"로 제안되었습니다. 합성 면역 활성 공중합체 글라티라머 아세테이트(GA)는 평균 길이 40~100개의 잔기가 무작위 순서로 배열된 4개의 aa로 구성되어 있으며 MBP(106)와 유사합니다. 1967년 쥐 모델에서 EAE를 유도하기 위해 처음 합성되었지만, 예기치 않게 질병의 징후와 진행을 감소시키는 것으로 밝혀졌습니다(107). GA는 자가면역 질환을 유발하기보다는 조절 및 보호 신경면역 반응을 유도하는 것으로 밝혀졌습니다. 대부분의 환자에서 GA를 매일 주사하면 이 중합체 항원에 대한 반응성이 현저히 감소하고, CD4+ T 세포에 의한 IL-5 및 IL-13의 분비가 증가하여 Th2 반응으로의 전환을 나타냅니다(108, 109). 또한, GA 반응성 T 세포는 조합 라이브러리에서 나타나는 다양한 펩티드와 교차 반응하는 능력으로 측정할 때 높은 수준의 퇴행성을 나타냅니다(108). 교차 반응성이 높은 Th2(및 아마도 조절 FoxP3- Th3) 세포가 염증 부위로 GA에 의해 유도된 이동은 고도로 퇴화된 TCR이 약한 작용제로 인식하는 자기 항원과 접촉할 수 있도록 할 수 있습니다. 그러면 이러한 T 세포는 억제성 Th2/Th3 사이토카인을 분비하여 국소 염증을 제한하는 것으로 보입니다(108). 이러한 유익한 효과로 인해 GA는 1996년 치료용으로 승인되었으며 그 이후로 재발 완화 다발성 경화증의 1차 치료제로 사용되고 있습니다(110, 111).
.
3 TCR cross-reactivity in the context of cancer
With the notable exception of rare antigenic aberrant sequences, e.g. mutated antigens, tumours generally present self-antigens on their MHC molecules and are poorly immunogenic (112). This can be globally seen as the result of the thymic negative selection where highly self-reactive T cells are eliminated to prevent the development of autoimmune diseases, leaving us with a TCR repertoire with only low to moderate affinity to self-antigens (113). Although this is beneficial in a healthy state, it makes tumour targeting by T cells a hard task, as it impairs the mounting of an effective and strong immune response. Hence, in contrast to the situation in autoimmune diseases, cross-reactivity of potential pathogen-specific T cells against self-antigens specifically presented by tumour cells is not only desirable, but would likely result in favourable anti-tumour immunity (114).
An early and staggering example of TCR cross-reactivity was described by the group of P. Romero for the tumour-associated antigen (TAA) Melan-A. While the frequency of any antigen-reactive T cell in the peripheral immune naïve repertoire is generally extremely low (< 1 in 100.000 T cells), up to 1 out of 1000 CD8+ T cells bind the immunodominant peptide from Melan-A26-35 (the modified A27L ligand), when presented by HLA-A*0201, both in healthy donors as in melanoma patients (115). Although numerous T cells were able to bind the pMHC, as assessed by MHC-tetramer staining, a subgroup failed to be significantly activated by the Melan-A peptide in a cytotoxicity assay. In contrast, several other tested peptides, which included proteins of self- or pathogen- origin, generated a strong response in the same assay, hinting at the highly cross-reactive nature of this repertoire of T cells (116). Further supporting this, a following study of the same group showed that a tumour-reactive CD8+ T cell clone, also specific to the same immunodominant peptide mentioned above, was able to cross-recognise numerous peptides and that stimulation of this clone with these peptides drove the expansion of a heterogeneous CD8+ T cell population, with only a fraction actually reacting to the Melan-A peptide (117). Importantly, immunisation with Melan-A peptide through vaccination leads to a reduction on the population of cross-reactive T cells and an enrichment of antigen-restricted T cells that can react with the tumour (118). These early works on TCR cross-reactivity demonstrated its relevance not only in tumour biology but also in the design of effective anti-cancer immunotherapies.
3.1 Evidence for tumour antigen recognition by pathogen-specific T cells
3.1.1 T cell cross-reactivity between virus-derived sequences and tumour antigens
Studies have described viral-specific T cells within the microenvironment of several tumour entities with no prior known viral aetiology (119). Although there is experimental evidence for the presence of intracellular bacteria or viruses in tumour cells (120–122), this local pathogen load might not be the only reason for the presence of pathogen-specific T cells within tumours. After sequencing the TCRs of tumour-infiltrating lymphocytes (TILs) in non-small cell lung carcinoma (NSCLC), Chiou et al. identified a novel TAA derived from the epithelial protein TMEM161A. A TCR recognising this peptide was shown to readily cross-react with epitopes from EBV (and E. coli). Specific T cells were not only found in NSCLC patients, but also in healthy donors, an observation which the authors offer as an explanation to the presence of virus-specific T cells within NSCLCs, but possibly also in other tumours (123). In another in silico-based approach, Ragone et al. examined the cancer peptide database and identified numerous TAAs with shared homology with viral sequences. The viruses whose sequences were most commonly shared with the tumour antigens were HIV type 1 (HIV-1), HSV, and human papillomaviruses (HPV). In addition to sequence homology, the authors also report that these peptides share structural similarities with comparable patterns of contact between the HLA molecule and the TCR (114). A recent case report has also described tumour reduction in three metastatic colorectal cancer patients upon SARS-CoV-2 infection (124). Altogether, these studies point out to the fact that pathogen- and tumour antigen- cross-reactive T cell responses might play an important role in anti-cancer immunity, and that the immune repertoire of each patient, shaped by previous infections, might be a crucial factor in disease control.
In murine melanoma models, Chiaro et al. showed that similarities between tumour- and viral- derived antigens can influence the clearance of tumours upon peptide cancer vaccination as a consequence of cross-reactive T cell activity. Upon immunisation with viral peptide pools previously selected based on their homology to tyrosinase related protein (TRP2180–188) or glycoprotein 100 (gp10025–33), a strong reduction in tumour growth was seen. Interestingly, the authors further argue that viral molecular mimicry is an important factor that dictates immune response also in metastatic human melanoma by showing a direct correlation between pre-existing Abs against CMV, and response to the immune checkpoint inhibitor (ICI) anti-PD-1 (125). TCRβ sequencing experiments further suggested that the same T cell clone recognised similar peptide sequences of MAGE-A10 and CMV. Further studies using pre-clinical murine models suggest the relevance of activating virus-specific T cells for tumour growth control (119, 126). The authors describe the formation of an immune-permissive microenvironment upon in vivo virus-peptide vaccination, whereby cross-reactivity of these viral-specific T cells with tumour antigens, although not tested, could be responsible for the effect observed. Interestingly, another study simulating immunisation of mice with the TAA and homologous viral peptides predicted a similar clearance of tumour cells in both scenarios, suggesting equivalent anti-tumour efficacy of the effector T cell response (114).
3.1.2 T cell cross-reactivity between bacterial-derived sequences and tumour antigens
Cross-reactivity of tumour-specific T cells with bacterial epitopes has also been described. In melanoma, a MAGE-A6-derived peptide (MAGE-A6172-187) was shown to be cross-reactive with its highly immunogenic homolog HF-2216-229. This mycoplasma-derived peptide and MAGE-A6 can drive the formation of memory CD8+ T cells. Interestingly, in vitro priming with dendritic cells loaded with the bacterial-derived peptide resulted in CD8+ T cells with 100-fold higher avidity to the MAGE-A6 peptide compared to that of cells primed with the MAGE-A6 peptide itself (127).
The main in vivo source of bacteria-derived antigens is the microbiota. The human gut is colonised by approximately 1014 microbes (128). The sheer number of colonising microorganisms means that exposure of immune cells to these bacteria throughout life is unavoidable, which results in the generation of an immune response against commensal-derived peptides. In a similar analysis to the one performed earlier, Ragone et al. compared all TAAs from the cancer peptide database against the microbiota species Firmicutes (taxid:1239) and Bacteroidetes (taxid:976) sequences. The authors demonstrated a high level of homology of tumour antigens and peptides derived from these species, which account for 90% of all gut microbiota (129). Flückiger et al. showed that T cell clones that recognise the cancer antigen protein glycerol-3-phosphate dehydrogenase 1-like (GPD1-L) and cross-react with epitopes derived from the tail tape measure protein (TMP) of an Enterococcus hirae (E. hirae) bacteriophage, could be detected in melanoma patients. Importantly, the authors further observed an association between the presence of this prophage in the stools of patients with renal and lung cancer, expression of GPD1-L by tumour cells, and a long-term benefit to PD-1 checkpoint blockade (130). Interestingly, in the same study, cyclophosphamide treatment of tumour-bearing mice, which induces the translocation of E. hirae from the gut lumen to the mesenteric and splenic immune tissues, resulted in improved anti-cancer CD8+ T cell responses. This anti-tumour effect was abrogated once the mice were given antibiotics and rescued by administration of E. hirae isolates. Moreover, lack of expression of the TAA by the tumour cells also abolished any anti-tumour immunity previously observed.
Other studies have also shown a favourable clinical outcome in cancer patients presenting CD4+ and CD8+ T cells specific for E. hirae, Bacteroides fragilis, Ruminococcaceae (131), and Akkermansia muciniphila (131–134). The immune repertoire, namely the frequency of precursor T cells prior to antigen exposure, is a critical factor in determining the magnitude of an immune response. Based on the aforementioned observations of cross-reactivity between numerous pathogen-derived epitopes and tumour antigens, it is plausible that the gut microbiome is an important modulator and dictator of how individuals will mount an immune response to tumours but also how they will respond to immunotherapies.
3.2 Neoantigens and T cell cross-reactivity
In contrast to the demonstrated potential of T cells to be cross-reactive (11), neoantigens generally activate specific T cells that react only very weakly against the wild-type (wt) peptide which often differs only in 1 aa (135–138). This apparent contradiction may be explained when considering the position of the mutated aa in the peptide sequence (e.g. if a novel anchor residue for binding to the MHC molecule is created by the new aa) or its structural properties (e.g. changes in peptide charge which renders the peptide “visible” to the TCR). Still, the large majority of predicted neoantigens probably activate similar TCRs to that specific for the self-peptide and are, therefore, not of interest. If this is the case, these neoantigens do not trigger a strong anti-tumour response as a result of central tolerance. On the other hand, neoantigens can share homology to pathogen-derived antigens. In this case, these neoantigens could elicit an efficient response against tumours by activating cross-reactive pre-existing memory T cells that have been previously generated against such pathogens, as discussed above for wt tumour antigens (139). Bessel et al. identified an epitope (SVYRYYGL (SVY)) derived from the genome of the commensal Bifidobacterium breve (B. breve), homologous to the neoepitope expressed by the murine model B16-SIY (SIYRYYGL (SIY)) (140). They further demonstrate that B. breve promotes the expansion of SVY-specific CD8+ T cells and that these are able of effective tumour control in SIY-expressing tumours, although comparison with SIY-specific T cells was not performed. In pancreatic cancer patients, Balachandran et al. demonstrate that the quality of the tumour neoantigens, namely the similarity to pathogen-derived epitopes, rather than the quantity, greatly associates with long-term survival (141).
Importantly, cross-reactive neoantigens seem to be a critical predictive factor for checkpoint inhibitor therapy efficacy. In the seminal study by Snyder et al. which first identified mutated antigens as T cell targets during checkpoint blockade, the authors observed that patients with long-term benefit to anti-CTLA-4 therapy share neoepitopes homologous to more viral and bacterial antigens, in contrast to patients with minimal or no benefit (142). These intriguing findings strongly suggest that cross-reactive T cells specific for pathogens can get activated upon checkpoint inhibition and participate in a clinically significant anti-tumour response. This is in line with the different studies presented above where the importance of the gut microbiome in checkpoint therapy responsiveness has been highlighted (143).
3.3 The two faces of TCR cross-reactivity in tumour immunotherapy
In addition to being able to dictate the outcome of immunotherapies such as checkpoint inhibition and therapeutic cancer vaccination with tumour-derived antigens, TCR cross-reactivity is currently being exploited for the development of novel and more potent cancer therapies, which we will discuss in more detail below.
3.3.1 Overcoming self-tolerance3.3.1.1 Improving affinity
If numerous T cell clones recognise the same epitope, affinity and avidity for this epitope will be inevitable highly variable. Using checkpoint inhibitors will unleash the inhibition in all lymphocytes present in the tumour microenvironment (TME), high or low functional ones. Differently, the goal of therapeutic vaccination is to selectively drive the recruitment of high-avidity T cells and promote strong and long-lasting anti-tumour responses. As mentioned above, high-affinity T cells against TAAs are usually lacking as a consequence of negative selection in the thymus, which leaves us only with a low-affinity repertoire. This tolerance is observed when A2xneu mice (Her2/neu mice crossed with A2.1/Kb mice) are injected with the immunodominant Her2773-782 peptide, which results in little to no tumour control (144). A similar tolerance was observed when mice were injected with p53-derived peptides. In this case, the authors demonstrated that using the p53261-269 self-epitope led to the expansion of cytotoxic T lymphocytes (CTLs) in p53 wt mice with an avidity more than 10-fold lower than the ones obtained from p53 null mice (145). This nicely shows the importance of circumventing tolerance to achieve an effective cancer vaccination.
One way to improve the immunogenicity of TAAs would be to exploit the cross-reactive nature of TCRs. Identification of peptides that are not naturally processed and presented but that can be used to elicit strong cross-reactive T cell responses against the original TAAs is already an old idea. The design of such heteroclitic peptides, where the stability of interaction between the peptide and MHC molecule is improved by replacement of certain aa was shown to be a powerful strategy for both improving CTL reactivity in vitro and controlling tumor growth in mice (144, 146–150). Importantly, these heteroclitic peptides need to be recognised by T cells that cross-react with the native sequence and can, therefore, drive the killing of tumour cells naturally presenting the original peptide. Despite the encouraging results seen in pre-clinical models, this concept has failed yet to lead to the development of an effective cancer therapeutic vaccine (151, 152). A famous example was the observation by Speiser et al. that immunisation of melanoma patients with the wt Melan-A26-35 (together with CpG as adjuvant) was superior in generating high avidity, tumour-reactive T cells, compared to the Melan-A26-35 modified peptide (152). Since the only difference between the two peptides is one aa substitution at an anchoring position (A27L), it suggests that increasing pMHC binding properties is not the ultimate key for improving T cell reactivity to TAAs.
3.3.1.2 Microorganism antigens (MoAs) molecular mimicry
Recently, a novel concept exploiting TCR cross-reactivity for therapeutic purposes has emerged. It is based on the identification of natural analogue peptides capable of inducing strong T cell responses against the tumour antigen. The shared homology between pathogen-derived peptides and tumour antigens and the aforementioned correlations between cross-reactive T cells and clinical outcome makes this an attractive and promising strategy that is currently being further investigated.
We have introduced in sections 3.1 and 3.2 that tumour antigens share homology with numerous pathogen-derived epitopes which, as a consequence, can drive the activation of T cells that share the same TCR. In other words, T cells that have been activated upon exposure to a certain pathogen can cross-react with tumour antigens (Figures 1A–C). The reasons for exploiting this cross-reactivity in the context of therapeutic cancer vaccination are manifold: first, it allows to overcome the low immunogenicity and affinity of natural TAAs, since TCRs that recognise MoAs have not been depleted from the T cell repertoire. Second, memory T cells can be activated by much lower peptide concentrations as compared to their naïve counterparts (see section 2). Third, recalling T cell responses upon immunisation is obviously easier to achieve than priming new effectors, especially when considering the current lack of gold-standard strong adjuvants. Fourth, exploiting “natural” T cells that were already expanded in the body after infection should present less risk of autoimmunity, although, as exemplified in section 2, autoimmunity cannot be fully excluded.
In summary, activation of viral- or commensal- specific T cells that cross-react to the tumour cells have shown promising results in a couple of pre-clinical models. Furthermore, correlations between the presence of these T cells and clinical outcome in patients have also been drawn. All this is opening a new field of research, to identify tumour antigens and MoAs that share high homology for the developing of novel T cell-based immunotherapies for cancer (Figure 1D). Since the presence of MoAs-specific memory T cells depends on prior infections, the composition of the microbiota, and the MHC-allotype, one could speculate that the development of such strategies should be done in an individualised manner to guarantee a high success rate and decrease the risk of side effects. Combination of such therapeutic vaccinations with ICIs could unleash the expansion of potent effector memory cells that readily target the tumour antigen and are able to control tumour growth.
3.3.2 The dark side of TCR cross-reactivity
TCR cross-reactivity undoubtedly opens large avenues for developing more potent cancer therapies. However, there are important bottlenecks to consider. The possible side effects in immunotherapy, especially in adoptive T cell therapies, where optimised TCRs with high affinity against a certain peptide are administered to patients is a serious issue. Side effects with these engineered TCRs are not rare, due to the strong interaction between the TCR and its target. Very low expression levels of the antigen in healthy tissue, which was initially dismissed as potentially dangerous led to severe consequences (153, 154). This on-target toxicity is not that unexpected (Figure 1D). However, overlooking off-target effects due to TCR cross-reactivity can have similarly severe and fatal adverse effects as it was observed in the case of anti-MAGE-A3 TCR engineered T cells. Due to its restrictive expression to immune privileged sites such as placenta and testis which lack the expression of HLA molecules, MAGE-A3 was considered a genuinely tumour-specific target, since it is found to be overexpressed in multiple tumours. This bona fide target attracted the attention and promptly immunotherapies that target this molecule were developed. Contrary to the expectations, severe cases of toxicity were observed, despite the lack of antigen expression in any of the tissues affected. In the first of two well-known incidents, engineered anti-MAGE-A3112–120 (KVAELVHFL) T cells were adoptively transferred to cancer patients after nonmyeloablative lymphodepletion, who then received high doses of IL-2. This led to severe neurological damages, and even to a patient death. This fatal toxicity was attributed to a cross-reactivity of the effector TCRs with a MAGE-A12 sequence (KMAELVHFL) which has a superior binding affinity for HLA-A*0201 than MAGE-A3112–120. MAGE-A12 was found a posteriori to be expressed in the brain (153). In the second, even less predictable case, engineered lymphocytes with affinity-enhanced TCRs against the HLA-A*01-restricted MAGE-A3168-176 peptide (EVDPIGHLY) drove cardiotoxicity and patient death due to recognition of an unrelated peptide derived from the muscle protein titin (ESDPIVAQY) which is presented by cardiomyocytes (155, 156) (Figure 1D). Experimental and computational tools for prediction of potential toxicities have been improved since then and will be presented in section 4.
In general, therapeutic cancer vaccines are safe and no severe side effects have been observed to date. This may arise from the relatively low affinity of the induced T cells. The potential of MoAs to be used in immunisation approaches against tumour antigens, renders caution to what kind of side effects can arise. In a recent study, Gil-Cruz et al. showed that microbiota-derived peptide mimicry can induce lethal cardiomyopathy through the activation of heart-specific (MYH6-specific TCR) Th17 CD4+ T cells (157). In their mouse model, cross-reactive CD4+ T cells are primed in the intestine and later circulate and infiltrate the myocardium where they can damage myosin-expressing cells. In the context of checkpoint inhibition, it is tempting to speculate that not only self-, but also cross-reactive pathogen-specific T cells could be responsible for driving lethal cases of myocarditis that were observed in some patients (158, 159). The large number of auto-immune diseases that are associated with pathogen infection itself (section 2) demonstrate the delicate balance in the selection of these MoAs for therapeutic intervention.
4 Assessing TCR cross-reactivity: experimental evidence, in silico predictions and the need for high through-put testing platforms
A number of the examples of TCR cross-reactivity discussed so far have been brought to light using in vitro systems based on the testing of T cell activity against synthetic peptides. In early works, epitopic peptides of interest were modified by introducing aa substitutions at various positions. Later advances, supported by increased automatization of peptide synthesis, led to the development of synthetic peptide libraries. One common approach is to generate combinatorial (sub)libraries of peptides with each of the 20 aa fixed at one position while all other positions can be occupied by all other aa (160). Such approach can theoretically generate all possible aa sequences for a given peptide length and allows screening of up to 1012 peptides. In vitro testing of agonists or antagonists´ effects on T cell activity can be performed either by measuring cytokine secretion, killing of loaded target cells (for CD8+ T cells), or proliferation (for CD4+ and CD8+ T cells) (32, 161). Once a library has been shown to activate the T cell of interest, sub-libraries can be consecutively tested until the sequence(s) responsible for cross-reactivity is (are) identified. Subsequent database search can finally reveal whether the random peptide is indeed part of a known protein.
Together with the development of TCR engineering and adoptive transfer therapies, currently most advanced in the oncology clinical setting, high through-put and comprehensive approaches for testing TCR cross-reactivity have become mandatory for pre-clinical development. The main interest here is to assess TCR-mediated toxicity, i.e. the potential of transferred T cells to exert deleterious effects in vivo via recognition of non-related pMHC expressed on healthy tissues (Figure 1D). This is particularly relevant when the TCR has been manipulated for increasing its affinity to the cognate pMHC or has been obtained from HLA-unmatched donors (allorestricted). Challenges for the safe use of engineered TCRs in solid tumours have been very recently reviewed (162), and we have presented examples of fatal toxicities in section 3.3. In the context of clinical development, in vitro testing of T cell reactivity against random peptide sequences, as mentioned above, is the most straight-forward approach to assess cross-reactivity. DNA-tagged pMHC multimers, which allow to address TCR-pMHC affinity more easily is an elegant alternative method (163, 164). From the point of view of experimental feasibility, all these assays require high amount of material (e.g. T cell clones), which might be circumvented by modern methods. TCR cloning and subsequent transfer in reporter cells or MHC-matched PBMCs, and possibly the use of soluble TCRs and yeast pMHC libraries can overcome the aforementioned limitations (21). By titrating the peptide concentrations, TCR affinities can be more precisely assessed.
Which threshold of reactivity will lead to in vivo toxicity is likely impossible to predict with high accuracy and might even vary between individuals. One weakness of synthetic peptide testing is that recognition of a particular sequence by a certain TCR as measured in vitro cannot ultimately predict in vivo reactivity, since it is unknown whether this aa sequence is indeed processed and to which extent it is presented on body tissues. More sophisticated platforms try to overcome these limitations. First, testing primary normal cells from a range of organs representing essential human tissues (e.g. cardiovascular, gastrointestinal, brain, liver, among other systems) and/or a panel of tumour cell lines will assess potential off-target recognition (22, 165). Second, alloreactivity against MHC-mismatched cell lines can also be assessed (22, 166). As an example, reactivity of a TCR specific for a MAGEA4-derived epitope presented by HLA-A*0201 was found to recognise HLA-A*0205 (in the absence of MAGEA4), indicating alloreactivity; hence, patients bearing the HLA-A*0205 allelic product should be excluded from the clinical study using this TCR (22). Third, recognition of similar, but not identical synthetic peptides (containing aa substitutions), can also be tested in vitro, and the occurrence of potentially recognised sequences in the human proteome predicted. This combined approach could advantageously replace combinatorial peptide libraries (167).
Lastly, a comprehensive view of all peptides presented by MHC molecules in normal cells is needed and of utmost importance. The typical experimental setting for assessing the MHC ligand “landscape”, is to perform peptide immunoprecipitation followed by mass spectrometry analysis. First milestones steps have been engaged, with the Human Immunopeptidome Project (HIPP) and the HLA ligand atlas which both aim at deciphering the entire MHC-ligandome landscape of human healthy tissues (168, 169). In addition, quantitative analysis of peptide presentation by mass spectrometry has become possible. Using this method, it was recently shown that a peptide derived from collagen type VI A3 is present in 41% of the tumour samples analysed at an average of 228 (max 1928) copies per cell, but only in 6% of the normal tissues with an average of 28 copies (max of 49) per cell (165).
All these approaches are so far imperfect, since it cannot be excluded that an organ subpart, or specialised cells at a certain stage of differentiation or activation, may be targeted by cross-reactive T cells. As discussed earlier, the recognition by MAGE-A3 specific T cells of a titin-derived peptide expressed only in beating cardiomyocytes showed to be fatal for treated patients (155, 156). However, combining and refining them will decrease the chance of unexpected in vivo TCR cross-reactivity and toxicity. Possibly, tissue engineering and the development of 3D in vitro culture systems which better recapitulate the complexity of human organs and can be used in T cell assays might become a relevant addition to the testing pipelines.
In complement to experimental approaches, many efforts are ongoing for developing reliable in silico pipelines for predicting T cell cross-reactivity. It should be noted that many of such tools are not developed specifically for addressing cross-reactivity, but more generally to predict peptide immunogenicity (170). In principle, two aspects can be investigated: on the one hand, the probability for a peptidic sequence to be presented by various MHC allelic products, and on the other hand, the interaction of a specific TCR with a pMHC complex.
Regarding peptide MHC binding, the simplest strategy would be to start from the original peptide and deduce which altered sequence could or not bind to the presenting MHC allelic product. NetMHC and syfpeithi, which are essential publicly available tools, can deliver robust MHC-binding predictions, but they cannot directly interrogate TCR cross-reactivity. In addition, immunogenicity prediction tools based on aa properties (size, charge, aromaticity, gravy score) are also being developed (171, 172). In the tool available at the Immune Epitope Database (IEDB) (173), TCR preferences were deduced from the study of 600 immunogenic and 181 non-immunogenic 9mer peptides: the authors found out that peptides containing aromatic and large side chains aa (in particular phenylalanine) were preferentially recognised by T cells, and that positions 4-6 were the most critical, confirming previous findings. The task is much more complex when addressing the direct binding of a specific TCR to pMHC (see also section 1). Current approaches aiming at modelling such interactions in 3D are based on x-ray crystallography data (174–176). In addition, pMTnet, NetTCR and ERGO are neural networks that predict pMHC-TCR (CDR3 regions of the TCR β, and more recently, α chains) and are in continuous refinement (177–179).
In silico tools rely on the exploitation of experimental data. Hence, in vitro testing of e.g. peptide library scanning is not only useful for current assessment of T cell cross-reactivity, it is also needed for training and improving prediction tools. In this respect, repository of TCR-pMHC interactions and affinities, as well as 3D information, such as those available at the IEDB, Altered TCR Ligand Affinities and Structures (Atlas) (180), Structural T-cell Receptor Database (STCRDab) (181) or TCR associated with pathology conditions (McPAS-TCR) (182) and PMID databases are essential. Implementation of more information, in particular for rare MHC allelic products, is still necessary and will help improving the robustness of these approaches in the next years.
5 Concluding remarks
Cross-reactivity is a very smart property of our adaptive immune system to cope with the large pathogen universe. It also plays a significant role in pathological conditions as different as autoimmunity and cancer. While it has been longer discussed that virus- or bacteria-specific T cells are associated with some autoimmune diseases, more recent research is uncovering their role in cancer. This knowledge can be exploited for therapy in both diseases. Application of mimotopes in the treatment of autoimmune diseases will depend to a large extent upon their ability to suppress immunoreactivity, for instance by stimulating regulatory anti-inflammatory CD4+ T cells, or by directly inhibiting pathogenic cytotoxic CD8+ T cells. This is obviously a complex task, and identification and prediction of self-epitopes and mimotopes recognised by particular TCRs is, therefore, important to make such antigen-specific approaches successful in autoimmune diseases. In cancer immunotherapy, TCR cross-reactivity is becoming an essential consideration, not only for designing more efficient T cell-based treatments, but also for preventing severe side effects. Considering the ongoing personalisation of therapeutic approaches, the upstream TCR selection process needs to be speed up. The development of novel and refined prediction methods is of utmost importance, but is a challenging process due to the numerous aspects that can impact cross-reactivity.
Author contributions
CG, AM, and RK conceived the review and jointly wrote the manuscript. AM prepared the figure which was reviewed by all authors. All authors contributed to the article and approved the submitted version.
Funding
CG and AM are supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy EXC 2180, 390900677.
Acknowledgments
We acknowledge support from the Open Access Publishing Fund of the University of Tübingen.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Schodin BA, Tsomides TJ, Kranz DM. Correlation between the number of T cell receptors required for T cell activation and TCR-ligand affinity. Immunity (1996) 5(2):137–46. doi: 10.1016/s1074-7613(00)80490-2
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Labrecque N, Whitfield LS, Obst R, Waltzinger C, Benoist C, Mathis D. How much TCR does a T cell need? Immunity (2001) 15(1):71–82. doi: 10.1016/s1074-7613(01)00170-4
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Yin Y, Mariuzza RA. The multiple mechanisms of T cell receptor cross-reactivity. Immunity (2009) 31(6):849–51. doi: 10.1016/j.immuni.2009.12.002
PubMed Abstract | CrossRef Full Text | Google Scholar
4. Falk K, Rötzschke O, Stevanović S, Jung G, Rammensee HG. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature (1991) 351(6324):290–6. doi: 10.1038/351290a0
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanović S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics (1999) 50(3-4):213–9. doi: 10.1007/s002510050595
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human alphabeta T cell receptor diversity. Science (1999) 286(5441):958–61. doi: 10.1126/science.286.5441.958
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev Immunol (2004) 4(2):123–32. doi: 10.1038/nri1292
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Zarnitsyna VI, Evavold BD, Schoettle LN, Blattman JN, Antia R. Estimating the diversity, completeness, and cross-reactivity of the T cell repertoire. Front Immunol (2013) 4:485. doi: 10.3389/fimmu.2013.00485
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Hu C, Shen M, Han X, Chen Q, Li L, Chen S, et al. Identification of cross-reactive CD8(+) T cell receptors with high functional avidity to a SARS-CoV-2 immunodominant epitope and its natural mutant variants. Genes Dis (2022) 9(1):216–29. doi: 10.1016/j.gendis.2021.05.006
PubMed Abstract | CrossRef Full Text | Google Scholar
10. Jerne NK. The natural-selection theory of antibody formation. Proc Natl Acad Sci USA (1955) 41(11):849–57. doi: 10.1073/pnas.41.11.849
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Sewell AK. Why must T cells be cross-reactive? Nat Rev Immunol (2012) 12(9):669–77. doi: 10.1038/nri3279
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Lineburg KE, Grant EJ, Swaminathan S, Chatzileontiadou DSM, Szeto C, Sloane H, et al. CD8(+) T cells specific for an immunodominant SARS-CoV-2 nucleocapsid epitope cross-react with selective seasonal coronaviruses. Immunity (2021) 54(5):1055–65.e5. doi: 10.1016/j.immuni.2021.04.006
PubMed Abstract | CrossRef Full Text | Google Scholar
13. Imrie A, Meeks J, Gurary A, Sukhbataar M, Kitsutani P, Effler P, et al. Differential functional avidity of dengue virus-specific T-cell clones for variant peptides representing heterologous and previously encountered serotypes. J Virol (2007) 81(18):10081–91. doi: 10.1128/JVI.00330-07